Abstract
The ventrolateral region of the ventromedial hypothalamus (VMHvl) plays an essential role in female sexual behavior. Oxytocin (OT) is released from the paraventricular nucleus to downstream sites such as the VMHvl to facilitate female sexual behavior and shows characteristics of a prolactin (PRL)-releasing factor. During mating, vaginal cervical stimulation (VCS) received from a vasectomized male triggers twice-daily PRL surges that persist up to 12+ d, a period known as pseudopregnancy (PSP). To determine whether OT is involved in PSP by acting within the VMHvl, female rats were infused bilaterally with an oxytocin receptor antagonist (OTR-A), a vasopressin receptor-1a antagonist (V1a-A), or artificial cerebral spinal fluid 30 min before mating. All females received a sufficient amount of VCS, 15 intromissions, to induce PSP. Females infused with OTR-A (20 ng/0.4 μl) with implants targeting the VMHvl showed only a 22% induction of PSP, as measured using vaginal diestrus and serum PRL concentrations. In contrast, controls and V1a-A (80 ng/0.4 μl) infused females exhibited 100% induction of PSP. Females infused with OTR-A returned to estrus after 5 d, whereas females infused with either artificial cerebral spinal fluid or V1a-A remained in diestrus for 12–13 d in both the correct and missed placement groups. Although OT can act as a PRL releasing factor, the PRL surge does not begin until 18–24 h after mating. Together, our results suggest that OT release in the VMHvl mediates the effects of VCS on the induction of the PRL secretion needed to establish PSP.
THE VENTROMEDIAL HYPOTHALAMUS (VMH) is widely known as the site at which steroids act to facilitate female sexual behavior in the female rat. The ventrolateral division of the ventromedial hypothalamus (VMHvl) is the predominant site in the control of the lordosis reflex and paracopulatory sexual behaviors. Within the VMHvl, it is predominantly the activation of estrogen receptors and progesterone receptors that facilitates sexual receptivity (1). However, administration of a norepinephrine (NE) antagonist, prazosin, into the VMH can attenuate sexual receptivity, suggesting that NE also plays a role in sexual behavior (2,3,4).
In addition to its role in female rat sexual behavior, the VMHvl plays a facilitative role in mating-induced pseudopregnancy (PSP). When a female rat receives sufficient vaginal cervical stimulation (VCS) during mating, bi-circadian surges of prolactin (PRL) are triggered within 48 h and persist for 10–12 d (5,6,7). Upon penile intromission, a rapid release of NE occurs 20 min after mating in the anterior VMH (8). We have previously shown that noradrenergic receptor activation is important in the VMHvl for the induction of PSP (9). This mating-induced PSP can be blocked by an α-1 noradrenergic receptor antagonist, prazosin, when administered into the VMHvl 30 min before mating in gonad-intact animals. Interestingly, prazosin blocks the VCS input received during mating and prevents PSP but has no effect on the lordosis posture (9).
It is possible that oxytocin (OT) actions within the VMH at the time of mating stimulate NE release during the initiation of PSP and also facilitate the lordosis reflex (2). In steroid-primed female rats, administration of an OT dose [via intracerebroventricular (icv) infusion] capable of facilitating lordosis resulted in an increased release of NE within the VMH in the absence of mating. Therefore, we chose to determine whether activation of oxytocin receptors (OTRs) within the VMHvl is required for mating-induced PSP.
Both NE and OT facilitate sexual receptivity when bound to their target receptors, with their release and/or receptor binding in the VMHvl being modulated by sex steroids (4,10,11). The lordosis posture of a female may be artificially facilitated by infusion of OT into the VMHvl or abolished by administration of an OTR antagonist (OTR-A) when given in synchrony with a progesterone injection 4 h before testing (12,13). OT, acting as a PRL-releasing factor, appears to play a role in expression of the PRL surges that cause PSP (14,15,16,17,18,19). OT shows direct actions either in pituitary lactotrophs or within the hypothalamus to provoke PRL surges shown in response to artificial, cervical stimulation (14,17,20). For example, a single injection of OT into the periphery is capable of stimulating rhythmic PRL surges in ovariectomized rats that are similar to the PRL surges induced by artificial VCS (15). Moreover, OT shows rhythmic release into the blood stream in synchrony with mating-induced diurnal and nocturnal PRL (N-PRL) surges in the female rat, and these surges can be blocked by infusion of an OTR-A via the carotid artery (19,20). However, new evidence shows that administration of an OT antagonist over a 24-h period into the periphery has no long-term effect on cervically induced PRL surges (17), suggesting that OT control of mating-induced PRL surge production results from hypothalamic actions of this peptide. Finally, female rats given mating stimulation sufficient to induce PSP show an increase in c-fos expression (neural activation marker) in OT neurons within the paraventricular nucleus (PVN) but do not show increased circulating OT at the time of the crucial N-PRL surge (21). Overall, when the OT system is activated, parvocellular neurons, which send projections to the VMH (22), release OT for further downstream action on sexual behavior and possibly the induction of PSP.
In addition to the involvement of OT in sexual receptivity, arginine vasopressin (AVP) also modulates lordosis. However, administration of AVP inhibits lordosis, whereas an AVP antagonist enhances sexual receptivity (23). AVP is able to bind OTRs (24). In the present study, we sought to verify selectivity of OTR-A actions in the induction of PSP by infusing a selective vasopressin receptor-1a antagonist (V1a-A) into the VMHvl before mating.
We hypothesized that OT, released in the VMHvl in response to genitosensory inputs during mating, provokes the PRL surges needed to establish PSP.
Materials and Methods
Two separate experiments were conducted. The following procedures were common to both experiments. In experiment 1, an OTR-A was administered, and in experiment 2, a V1a-A was administered into the VMHvl under the same protocol.
Animals
Experimental animals (n = 41) were Long-Evans female rats weighing 200–225 g obtained from Charles River Laboratories (Wilmington, MA). Sexually experienced, vasectomized males (350–400 g) were used to provide mating stimulation. All animals were housed individually in suspended metal cages on a reversed light cycle (lights on 2100–0900 h). All animals were given access to food and water ad libitum. Throughout all experiments, the estrous cycles of females were monitored by daily vaginal lavage (25). Only females that exhibited two complete estrous cycles (4–5 d) were used. All experimental procedures were approved by The Institutional Animal Use and Care Committee of Boston University in accordance with National Institutes of Health guidelines.
Brain cannulation surgery
Animals were anesthetized with an isoflurane/oxygen gas mixture; isoflurane gas was set at 2.5–3% and the oxygen gas to 2.5 liter/min, during surgery. Animals were placed in a Kopf stereotaxic apparatus using the flat skull position. Bilateral guide cannulae were implanted and aimed toward the ventrolateral aspect of the ventromedial nucleus of the hypothalamus using the following coordinates: anterior-posterior (AP): −3.14 mm from bregma, medial-lateral (ML): ±1.0 mm and dorsal-ventral (DV): −7.8 mm in accordance with the atlas of Paxinos and Watson (50). The guide cannulae were fixed to the skull using Kentac Cem radiopaque cement (3M ESPE, St. Paul, MN) and flat stainless steel screws. The incision was closed with sutures and treated with antiseptic cream. At drug treatment, insert needles protruded 2 mm from the guide cannulae to a final depth of DV −9.8 mm.
Drug infusion
In experiment 1, each female was infused with either the oxytocin antagonist [d(CH2)51, Tyr(Me)2, Thr4, Orn8, Tyr-NH29-vasotocin (OTR-A; Bachem, King of Prussia, PA)], or artificial cerebral spinal fluid (aCSF). Thirty minutes before mating, bilateral infusion needles (Kendall, Mansfield, MA) attached to a 1.0-μl Hamilton syringe (7000 series; Reno, NV) by PE20 polyethylene tubing (50 cm; Becton Dickinson and Co., Sparks, MD) were inserted into the guide cannulae. OTR-A (20 ng/0.4 μl; n = 21) or aCSF (0.4:l μl; n = 10) was infused for 2 min through the infusion needles. The needles were left in place for an additional minute to allow for diffusion of the drug away from the needle tip. Six females infused with OTR-A did not receive any mating stimulation and were left in their home cage (HC) after infusion.
In experiment 2, each female was infused with either a highly selective V1a antagonist [d (CH2)5 [Tyr(Me)2]AVPa (V1a-A; kindly provided by Dr. Maurice Manning, Department of Biochemistry and Cancer Biology, Medical College of Ohio, Toledo, OH)] or aCSF. Thirty minutes before mating, bilateral infusion needles (Kendall) attached to a 1.0-μl Hamilton syringe (7000 series) by PE20 polyethylene tubing (50 cm; Becton Dickinson) were inserted into the guide cannulae. V1a-A (20 ng/0.4 μl; n = 5) or aCSF (0.4:l μl; n = 5) was infused for 2 min through the infusion needles as described previously.
Behavioral testing
OTR-A, V1a-A, and aCSF females received 15 intromissions from sexually experienced vasectomized males under nonpaced mating conditions. This amount of VCS is capable of inducing PSP in 100% of females (9). A separate group of animals infused with OTR-A were not mated (n = 6) and remained within their HC after infusion to show that the drug is ineffective of inducing PSP alone. Mating was conducted in a dimly illuminated room between 1200 and 1400 h. Females were selected for mating based on the characteristic proestrus vaginal smear (25). Females were placed into a Plexiglas testing chamber (37.5 × 75 × 30 cm) with fresh bedding and remained until they received 15 intromissions from the male. All groups received uncontrolled numbers of mounts-without-intromission and ejaculations when they occurred. The lordosis quotient (LQ) (LQ = number of lordosis responses/number of mounts and intromissions × 100) was derived as a measure of sexual receptivity. The intensity of each lordosis (lordosis rating) was scored on a scale of 0–3 as previously described (26). The frequency of female paracopulatory solicitations (ear wiggling, hopping, and darting) was also recorded.
Plasma PRL measurement after mating
Blood was collected from females on d 6 after mating at the time of the N-PRL surge (1900 h, 2 h before lights on). Serum collection occurred at a time that the PRL surges of PSP are well established (16). Blood samples (50–100 μl) were obtained from anesthetized females through the saphenous vein, as previously described (27). After collection, blood samples were centrifuged for 15 min at 1000 g, and serum was stored at −20 C before assay. All samples were assayed by Dr. A. Parlow at the National Hormone and Peptide Program at the Harbor-University of California Los Angeles Medical Center, Los Angeles, CA.
Histological verification of cannulae placements
After the completion of post-mating PSP or completion of two post-mating estrous cycles, animals were killed. Animals were deeply anesthetized with sodium pentobarbital (ip; Henry Schein Inc., Melville, NY) and were perfused transcardially with 0.9% saline, followed by 10% formalin. Brains were collected and postfixed in 10% formalin/25% sucrose until histological processing. Fixed brains were sliced in 30-μm sections on a cryostat, dried onto gel-coated slides, stained with cresyl violet, dehydrated, and placed under coverslips using Permount (Sigma Chemical Co., St. Louis, MO). Sections containing the VMHvl were examined under a light microscope, and cannulation tracts were identified. Cannulae placement was considered correct if the tip of the tract was present along the VMHvl at AP −3.14 and ML ±1.0 mm. These cells within the VMHvl were targeted due to previous data showing a high density of OTRs and OT fibers within this area (11). Data from animals with correct placements were compared with animals with incorrect placements to demonstrate site-specific actions of drug infusion.
Statistical analysis
Animals were considered PSP if they showed 10–12+ consecutive days of diestrous vaginal cytology after treatment. This length of diestrus is known to be a reliable indicator of PSP (25). The percentage of animals showing PSP and the mean number of days between estrus were used as measures of PSP, as well as the nocturnal serum PRL concentration (ng/ml) measured 6 d after mating (9,28,29). Nonparametric χ2 analysis was used to analyze the effect of drug treatment on the incidence of PSP and paracopulatory behaviors, followed by one-tailed Fisher’s exact probability comparison. A two-way ANOVA was used to compare the mean days of diestrus after treatment in drug-infused and control animals with correct or incorrect cannulae placements, followed by post hoc analysis with Tukey and Bonferroni high significant difference (HSD) tests. The same statistical measurement (two-way ANOVA with post hoc analysis) was used to compare the serum PRL concentration in OTR-A-infused with aCSF-infused females.
Results
Verification of cannula targeting
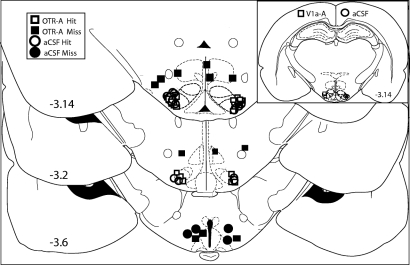
Placements of infusions for each animal in the study are presented in a cartoon photo image (Fig. 1). Placements anterior, posterior, or dorsolateral to this target region (“hits”) were considered incorrect (“misses”). Data from animals with misplaced cannulae were used as a separate control group.
Figure 1.
The diagram illustrates the bilateral infusion sites in individual females rats of either OTR-A or aCSF. The VMHvl is outlined in bold black dotted lines −3.14 mm behind bregma. Drug-treated animals are depicted as squares, and controls are indicated by circles. Open symbols indicate correctly placed VMHvl cannulae (Hit), whereas incorrectly placed cannulae are indicated by closed symbols (Miss). Numbers indicate the distance behind bregma, according to Paxinos and Watson (50). A smaller diagram illustrating infusion sites of individual female rats of either V1a-A or aCSF is presented in the upper-right corner. Drug-treated animals are depicted as open squares, and controls are depicted as open circles. All infusion placements were correctly placed (Hit).
Effects of OTR-A on percent induction of PSP
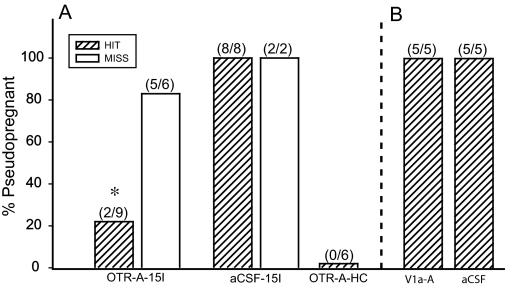
To determine whether OTRs within the VMHvl play a role in PSP induction, we infused an OTR-A before mating (Fig. 2A). Females infused bilaterally with OTR-A into the VMHvl 30 min before receiving 15 intromissions showed a 22% incidence of PSP, whereas the OTR-A-treated females with incorrect placements showed 83% induction of PSP. Females infused bilaterally with aCSF showed 100% induction of PSP, when infused 30 min before mating. OTR-A infusions into the VMHvl significantly decreased the incidence of PSP [χ2 (1) = 7.22, P ≤ 0.008; Fisher’s exact test P ≤ 0.05]. At the same time, specificity of infusion placement showed central OTR actions within the VMHvl [χ2 (1) = 11.52, P ≤ 0.02; Fisher’s exact test P ≤ 0.05] on PSP induction in mated females only. None of the HC females infused with OTR-A became PSP. Thus, OTR-A infusion alone, into the VMHvl, is not able to induce PSP (Fig. 2A).
Figure 2.
A, OTR-A (20 ng/0.4 μl saline) infused bilaterally into the VMHvl 30 min before mating stimulus of 15 intromissions (15I) blocked PSP in 80% of the animals in the Hit group. *, P ≤ 0.05 comparisons with OTR-A-15 intromissions Miss and the two aCSF-15 intromissions groups. B, Infusions of V1a-A (80 ng/0.4 μl saline) infused into the VMHvl 30 min before 15 intromissions resulted in 100% induction of PSP.
Effects of OTR-A on estrous cycling
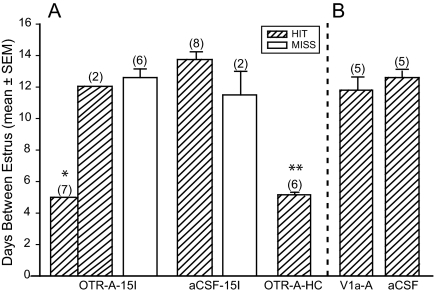
To examine further OTR action within the VMHvl for involvement in mating-induced PSP, estrus cycles were calculated by daily vaginal smearing. The mean days between estrus (Fig. 3A) in the correctly placed OTR-A-infused group showed a normal 5-d estrous cycle (n = 7). However, two females with correct placements showed estrous cycles of 12 d, which was equivalent to females treated with OTR-A with incorrectly placed cannulae and to females given an aCSF infusion with correct and incorrect cannula placement. Infusion of OTR-A into the VMHvl before mating significantly reduced the incidence of a prolonged diestrous state [F (4,27)=28.40; P ≤ 0.001]. Moreover, OTR-A infusion placement showed central effects within the VMHvl on estrous cycle length [F (1,10) = 5.55; P ≤ 0.04]. Both aCSF-infused (correct and incorrect placements) and incorrectly placed OTR-A infusions resulted in a mean diestrus length indicative of PSP. OTR-A-infused females with correct placement had significantly fewer mean days between estrus (Tukey HSD, P ≤ 0.001) than all other groups.
Figure 3.
A, OTR-A (20 ng/0.4 μl saline) infused into the VMHvl 30 min before mating stimulus of 15 intromissions (15I) resulted in an estrous cycle length of 5 (non-PSP) and 12 d (PSP) in the Hit group. *, P ≤ 0.05 comparisons with OTR-A-Miss, both aCSF groups and V1a-A. **, P ≤ 0.05 comparisons with OTR-A Miss, both aCSF groups. There was no statistical difference between OTR-A 15 intromissions and OTR-A HC females. B, V1a-A (80 ng/0.4 μl saline) infused into the VMHvl 30 min before 15 intromissions resulted in an estrous cycle length of 11.8 ± 0.37.
Effects of OTR-A on the N-PRL surge
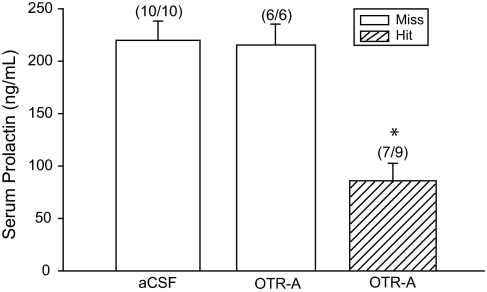
To confirm that the persistent diestrous vaginal cytology we observed was indicative of PSP, blood samples were taken at the expected time of the N-PRL surge, 6 d after mating (16,29). Serum samples were obtained and analyzed for PRL (Fig. 4). Females that showed a prolonged diestrous state in the aCSF group showed a mean serum PRL level of 220 ng/ml, whereas females infused with OTR-A (correct placement) had significantly lower serum PRL levels of 84 ng/ml and no establishment of PSP. Two females that were infused with OTR-A with correct cannula placement and all OTR-A missed cannula placement animals displayed prolonged diestrous cytology and showed N-PRL levels equivalent to aCSF-infused females (217 ng/ml). Infusing OTR-A into the VMHvl before mating significantly blocked the N-PRL surge indicative of PSP [F (3,21) = 12.47; P ≤ 0.001].
Figure 4.
OTR-A (20 ng/0.4 μl) infused into the VMHvl blocked the N-PRL surge in correctly placed, cycling females. Blood was collected at N-PRL surge time, 1900 h, 6 d after mating. Two females that were PSP in the correctly placed OTR-A infusion group are not included in this figure. *, P ≤ 0.05 comparisons with aCSF and OTR-A missed (Miss) groups.
Effects of OTR-A on female sexual behavior
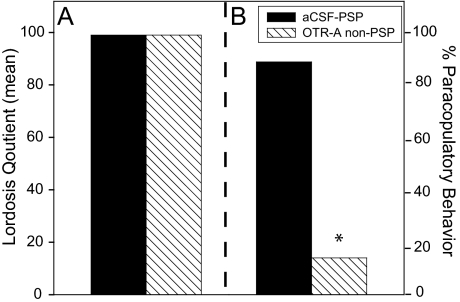
To verify OT involvement in female sexual behavior, behavioral data were assessed in OTR-A-infused females. Data for the LQ and paracopulatory behaviors, including hopping, darting, and ear wiggling, are presented in Fig. 5. There was no difference in the LQ between OTR-A, non-PSP, and the aCSF, PSP females (Fig. 5A). However, there was a significant reduction in the expression of paracopulatory behavior in OTR-A-infused females (Fig. 5B: χ2 = 7.843, P ≤ 0.005; Fisher’s exact test P ≤ 0.05). Whereas, females that were infused with aCSF showed an increased overall display rate of paracopulatory behaviors compared with OTR-A-infused females. Overall, a significant effect was seen on the incidence of PSP induction and the expression of paracopulatory behavior in OTR-A-infused animals (χ2 = 5.03, P ≤ 0.025; Fisher’s exact test P ≤ 0.05).
Figure 5.
OTR-A (20 ng/0.4 μl saline) infused into the VMHvl had no effect on LQ compared with aCSF (0.4:1 μl) infused animals (panel A) but blocked paracopulatory behaviors (hopping, darting, and ear wiggling) in 80% of the animals tested (panel B). *, P ≤ 0.05 comparisons with aCSF groups.
Effects of V1a-A on percent induction of PSP
To determine whether OTR-A acts in a selective manner toward OTR within the VMHvl, a highly selective V1a-A was infused before mating (Fig. 2B). All females infused bilaterally with V1a-A or aCSF 30 min before mating were PSP. All drug infusions were correctly placed. Animals infused with OTR-A showed a significantly reduced incidence of PSP compared with V1a-A infused animals [χ2 (1) = 6.96, P ≤ 0.008; Fisher’s exact test P ≤ 0.05].
Effects of V1a-A on estrous cycling
Correctly placed, bilateral infusions of V1a-A had no effect on mating-induced diestrous state (Fig. 3B). Females infused with V1a-A showed a mean diestrus length of 12 d, which was equivalent to aCSF-infused animals. There was no statistical difference between V1a-A and aCSF-infused animals. However, OTR-A, correctly placed infusions, showed significantly fewer days between estrus compared with V1a-A and aCSF-infused animals [F (2,15) = 13.05; P ≤ 0.001].
V1a-A effects on female sexual behavior
There was no significant effect of V1a-A infusion into the VMHvl before mating stimulation on LQ or paracopulatory behaviors. All females infused with either V1a-A or aCSF 30 min before mating showed an overall 99% LQ, and 100% of subjects displayed paracopulatory behaviors.
Discussion
The present experiments highlight an important role for OTR activation within the VMHvl in the induction of PSP after the receipt of mating stimulation in the female rat. Administration of an OTR-A within the VMHvl 30 min before the receipt of 15 intromissions prevented PSP in approximately 80% of the animals. In addition, OTR-A significantly lowered mating-induced N-PRL release, the crucial surge required for the maintenance of PSP (7,30). Furthermore, paracopulatory behaviors were also reduced in OTR-A-infused females with no change in LQ. No effect was observed in animals infused with either V1a-A or aCSF before mating.
Previous research has shown that the VMHvl is an important region within the hypothalamus for the initiation of PSP (9,30). In particular, activation of the α-1 noradrenergic receptor (9) is required within the VMHvl during mating for PSP to occur. In addition, VCS triggers the release of NE within the VMH, which facilitates both the lordosis reflex as well as the induction of PSP (8,9). The VMH receives a dense innervation via the ventral noradrenergic bundle (VNAB) from the medulla, which helps transmit the somatosensory input received during mating from the pelvic nerve (31,32). Applying an electrolytic lesion to the VNAB has blocked PSP induction in 80% of females given artificial VCS (33). Finally, lesioning of VNAB inputs into the VMHvl with a selective noradrenergic neurotoxin significantly reduced the incidence of mating-induced PSP (our unpublished data).
Previous data (9) point to interactive OT and NE signaling in the VMH that promotes the induction of PSP. Thus, NE acts as the primary excitatory neurotransmitter in regulating OT secretion during lactation and parturition (34). In addition, magnocellular nuclei within the PVN receive dense innervation by noradrenergic fibers from the brainstem (35). At the same time, oxytocinergic fibers from the PVN extend to the spinal cord (35), showing elevated concentrations of OT within spinal cord superfusates after artificial VCS application in intact female rats (36). These findings suggest that OT and NE interact during mating in brain regions that receive VCS input. In the present study, we did not explore whether an interaction exists between OT and NE specifically within the VMHvl in response to mating. However, when considered in light of our previous study (9), the present findings suggest that a cascade of events needed for PSP induction was blocked by preventing the interaction of OT and NE within the VMHvl in processing VCS input.
Evidence suggests that OT acts as a PRL-releasing factor to promote the PRL surges needed for the induction of PSP (14,15,17,19). As described previously, a single infusion of OT into the jugular vein is capable of inducing PRL surges that mimic those induced by cervical stimulation (15). In addition, infusion of an OT antagonist through the jugular vein, before cervical stimulation, abolished cervically stimulated PRL surges. Our data support this hypothesis, in that infusing an OTR-A into the VMH before mating showed long-term effects on PSP. Furthermore, OTR-A infusions showed site specificity within the VMHvl on PSP induction due to the misplaced OTR-A infusions, further suggesting that OT actions on mating-induced PRL surges are specific to the hypothalamus and do not involve actions on pituitary lactotrophs. Finally, in addition to VMHvl actions of OT contributing to the long-term stimulation of PRL release needed for the maintenance of PSP, NE also promotes PSP induction by acting in the VMHvl (9) (our unpublished data). Besides the VMH, there are data that suggest that OT neuronal activity within the PVN aides the VCS input in initiating PRL secretion 24–48 h after mating (21). Using FOS expression as a neural activity marker, OT neurons showed an increased response 1 h after mating with no changes 5 d after mating in accordance with the N-PRL surge. However, OT neurons within the PVN show a daily activity rhythm that coincides with mating-induced, nocturnal and diurnal, PRL release (37,38). Although it is known that OT can act on PRL in the initiation of PSP, there are no previous data connecting OT actions to the VMHvl in this process.
It is well established that OT fibers from parvocellular neurons extend down into the VMH from the PVN (22). In the present study, we were able to block the N-PRL surge necessary for PSP induction through decreased activation of OTRs in the VMHvl at the time of mating. In this instance, OTR-A blocked the N-PRL surge of PSP by affecting PRL directly at the hypothalamic level or influenced the sensory input pathway conveying information to other regions of the brain known to be involved in stimulating PRL release. In this experiment, infusion of OTR-A showed site specificity in that misplaced targets had no effect on PSP, suggesting the OTR-A’s actions were selective to OTRs closely surrounding the VMHvl.
OT and AVP are synthesized in magnocellular and parvocellular nuclei of the PVN (39). AVP and OT exert opposite effects on the expression of female sexual behavior in rats. Administration of a high dose of AVP significantly inhibited the lordosis response, whereas an AVP antagonist facilitated sexual receptivity in response to artificial VCS in nonsteroid primed, ovariectomized rats (23). Paracopulatory behaviors, such as hopping and darting, were inhibited after icv administration of AVP; conversely, these behaviors were stimulated by administering a V1a-A in ovariectomized, steroid-primed rats (40). Interestingly, AVP has a high binding affinity for both OT and AVP receptors, whereas OT has a high affinity only for OTRs (24). However, the VMH is known to contain a dense population of OTRs in an area that includes steroid receptors (41,42), whereas AVP receptors show high density in the supraoptic, paraventricular, and suprachiasmatic nuclei of the hypothalamus (43). In the present study, infusing the V1a-A into the VMHvl before sufficient mating stimulation to induce PSP did not reduce the incidence of PSP, indicating that vasopressin action within the VMHvl is not necessary for PSP induction. These data suggest that our selective OTR-A bound to OTRs only, because if OTR-A bound to vasopressin receptors, an enhanced response of paracopulatory behaviors, rather than the inhibition we observed, and no reduction in PSP would likely have occurred.
An alternative possibility is that the reduced incidence of PSP after infusion of an OTR-A into the VMHvl resulted from a disruption of the ability of OTRs to respond to estradiol and/or progesterone at proestrus. A number of studies have shown that OTRs are steroid dependent within the VMH (10,11,44). Estrogen causes an increase of OTR binding within the VMHvl as well as the sensitivity of VMH neurons to OT facilitation of lordosis (45). Progesterone shifts OTR binding toward the ventrolateral edge of the VMH where OT fibers from the PVN terminate. OTR-A administration (100–1000 ng, icv) into ovariectomized, steroid-primed rats resulted in a reduction in both receptive (lordosis) and paracopulatory (hop/dart) behaviors when administered at the time of progesterone injection, 4 h before testing (13). These effects of OTR-A on sexual behavior may be due to a reduction in OTR binding within the VMH. In contrast, we observed a 60% reduction in paracopulatory behaviors with an OTR-A at a significantly lower dose (20 ng) when directly infused into the VMH of intact, cycling animals 30 min before mating and also saw no effect on LQ. We conclude that there was no effect of OTR-A on lordosis due to the use of naturally cycling females and the infusion of the OTR-A at a time when estrogen and progesterone stimulation of lordosis has already taken place (46).
The present study, combined with our previous findings (9), demonstrate that NE and OT signaling in the VMHvl play an essential role in allowing VCS inputs to stimulate the PRL surges needed to establish PSP. Understanding the exact role of the VMH in mating-induced PRL surges will require further investigation. Lesions of the dorsomedial hypothalamus/VMHare known to reduce the N-PRL surges after receipt of VCS activation (47). There are efferent and afferent connections between the VMH and dorsomedial hypothalamus (48,49), which suggests that communication occurs between the two regions in response to genitosensory input during mating that is needed to induce the twice-daily PRL surges necessary for maintenance of PSP.
Acknowledgments
We thank Drs. Michael Baum and Thomas Gilmore for comments on the manuscript.
Footnotes
Present address for L.E.N.: Reproductive Medicine Associates of New Jersey, Morristown, New Jersey 07960.
This work was supported by National Institutes of Health Grants MH68147 and MH01435 (to M.S.E.). L.E.N. was partially supported by National Institutes of Health predoctoral training Grant T32-HD07387.
Disclosure Statement: The authors have nothing to declare.
First Published Online November 15, 2007
Abbreviations: aCSF, Artificial cerebral spinal fluid; AVP, arginine vasopressin; HC, home cage; icv, intracerebroventricular; LQ, lordosis quotient; NE, norepinephrine; N-PRL, nocturnal prolactin; OT, oxytocin; OTR, oxytocin receptor; OTR-A, oxytocin receptor antagonist; PRL, prolactin; PSP, pseudopregnancy; PVN, paraventricular nucleus; V1a-A, vasopressin receptor-1a antagonist; VCS, vaginal cervical stimulation; VMH, ventromedial hypothalamus; VMHvl, ventrolateral region of the ventromedial hypothalamus; VNAB, ventral noradrenergic bundle.
References
- Rubin BS, Barfield RJ 1983 Progesterone in the ventromedial hypothalamus facilitates estrous behavior in ovariectomized, estrogen-primed rats. Endocrinology 113:797–804 [DOI] [PubMed] [Google Scholar]
- Vincent PA, Etgen AM 1993 Steroid priming promotes oxytocin-induced norepinephrine release in the ventromedial hypothalamus of female rats. Brain Res 620:189–194 [DOI] [PubMed] [Google Scholar]
- Etgen AM 1990 Intrahypothalamic implants of noradrenergic antagonists disrupt lordosis behaviour in female rats. Physiol Behav 48:31–36 [DOI] [PubMed] [Google Scholar]
- Vathy I, Etgen AM 1989 Hormonal activation of female sexual behavior is accompanied by hypothalamic norepinephrine release. J Neuroendocrinol 1:383–388 [DOI] [PubMed] [Google Scholar]
- Adler NT 1969 Effects of male copulatory behaviour on successful pregnancy of the female rat. J Comp Physiol Psychol 69:613–622 [DOI] [PubMed] [Google Scholar]
- Wilson JR, Adler N, Le Boeuf B 1965 The effects of intromission frequency on successful pregnancy in the female rat. Proc Natl Acad Sci USA 53:1392–1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunnet JW, Freeman ME 1983 The mating-induced release of prolactin: a unique neuroendocrine response. Endocr Rev 4:44–61 [DOI] [PubMed] [Google Scholar]
- Etgen AM, Morales JC 2002 Somatosensory stimuli evoke norepinephrine release in the anterior ventromedial hypothalamus of sexually receptive female rats. J Neuroendocrinol 14:213–218 [DOI] [PubMed] [Google Scholar]
- Northrop LE, Shadrach JL, Erskine MS 2006 Noradrenergic innervation of the ventromedial hypothalamus is involved in mating-induced pseudopregnancy in the female rat. J Neuroendocrinol 18:577–583 [DOI] [PubMed] [Google Scholar]
- Johnson AE, Ball GF, Coirini H, Harbaugh CR, McEwen BS, Insel TR 1989 Time course of estradiol dependent induction of oxytocin receptor binding in the ventromedial hypothalamic nucleus of the rat. Endocrinology 125:1414–1419 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Johnson AE, Flanagan LM, Frankfurt M, Pfaff DW, McEwen BS 1993 The oxytocin receptor: a target for steroid hormones. Regul Pept 45:115–119 [DOI] [PubMed] [Google Scholar]
- Schulze HG, Gorzalka BB 1991 Oxytocin effects on lordosis frequency and lordosis duration following infusion into the medial preoptic area and ventromedial hypothalamus of female rats. Neuropeptides 18:99–106 [DOI] [PubMed] [Google Scholar]
- Witt DM, Insel TR 1991 A selective oxytocin antagonist attenuates progesterone facilitation of female sexual behavior. Endocrinology 128:3269–3276 [DOI] [PubMed] [Google Scholar]
- Egli M, Bertram R, Sellix MT, Freeman ME 2004 Rhythmic secretion of prolactin in rats: action of oxytocin coordinated by vasoactive intestinal polypeptide of suprachiasmatic nucleus origin. Endocrinology 145:3386–3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egli M, Bertram R, Toporikova N, Sellix MT, Blanco W, Freeman ME 2006 Prolactin secretory rhythm of mated rats induced by a single injection of oxytocin. Am J Physiol Endocrinol Metab 290:E566–E572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS 1995 Prolactin release following mating and genitosensory stimulation in females. Endocr Rev 16:508–528 [DOI] [PubMed] [Google Scholar]
- McKee DT, Poletini MO, Bertram R, Freeman ME 2007 Oxytocin action at the lactotroph is required for prolactin surges in cervically stimulated ovariectomized rats. Endocrinology 148:4649–4657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram R, Egli M, Toporikova N, Freeman ME 2006 A mathematical model for the mating-induced prolactin rhythm of female rats. Am J Physiol Endocrinol Metab 290:E573–E582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arey BJ, Freeman ME 1990 Oxytocin, vasoactive-intestinal peptide, and serotonin regulate the mating-induced surges of prolactin secretion in the rat. Endocrinology 126:279–284 [DOI] [PubMed] [Google Scholar]
- Arey BJ, Freeman ME 1989 Hypothalamic factors involved in the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology 124:878–883 [DOI] [PubMed] [Google Scholar]
- Polston EK, Centorino KM, Erskine MS 1998 Diurnal fluctuations in mating-induced oxytocinergic activity within the paraventricular and supraoptic nuclei do not influence prolactin secretion. Endocrinology 139:4849–4859 [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE 1983 Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci 6:269–324 [DOI] [PubMed] [Google Scholar]
- Sodersten P, De Vries GJ, Buijs RM, Melin P 1985 A daily rhythm in behavioral vasopressin sensitivity and brain vasopressin concentrations. Neurosci Lett 58:37–41 [DOI] [PubMed] [Google Scholar]
- Barberis C, Tribollet E 1996 Vasopressin and oxytocin receptors in the central nervous system. Crit Rev Neurobiol 10:119–154 [DOI] [PubMed] [Google Scholar]
- Cooper R, Goldman J, Vanderbergh J 1993 Monitoring the estrous cycle in the laboratory rodent by vaginal lavage. In: Heindel J, Chapin R, eds. Methods in toxicology. New York: Academic Press; 45–56 [Google Scholar]
- Hardy DF, Debold JF 1971 The relationship between levels of exogenous hormones and display of lordosis by the female rat. Horm Behav 2:287–297 [Google Scholar]
- Hem A, Smith AJ, Solberg P 1998 Saphenous vein puncture for blood sampling of the mouse, rat, hamster, gerbil, guinea pig, ferret and mink. Lab Anim 32:364–368 [DOI] [PubMed] [Google Scholar]
- Lehmann ML, Erskine MS 2004 Induction of pseudopregnancy using artificial VCS: importance of lordosis intensity and prestimulus estrous cycle length. Horm Behav 45:75–83 [DOI] [PubMed] [Google Scholar]
- Lehmann ML, McKellar H, Erskine MS 2005 Coding for the initiation of pseudopregnancy by temporally patterned activation of amygdalar NMDA receptors. J Neurosci 25:8696–8703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erskine MS, Lehmann ML, Cameron NM, Polston EK 2004 Co-regulation of female sexual behavior and pregnancy induction: an exploratory synthesis. Behav Brain Res 153:295–315 [DOI] [PubMed] [Google Scholar]
- Moore RY, Bloom FE 1979 Central catecholamine neuron systems: anatomy and physiology of the norepinephrine and epinephrine systems. Annu Rev Neurosci 2:113–168 [DOI] [PubMed] [Google Scholar]
- Collins JJ, Lin CE, Berthoud HR, Papka RE 1999 Vagal afferents from the uterus and cervix provide direct connections to the brainstem. Cell Tissue Res 295:43–54 [DOI] [PubMed] [Google Scholar]
- Hansen S, Stanfield EJ, Everitt BJ 1980 The role of ventral bundle noradrenergic neurons in sensory components of sexual behaviour and coitus-induced pseudopregnancy. Nature 288:152–154 [DOI] [PubMed] [Google Scholar]
- Bealer SL, Crowley WR 1998 Noradrenergic control of central oxytocin release during lactation in rats. Am J Physiol 274(3 Pt 1):E453–E458 [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW 1982 Immunohistochemical identification of neurons in the paraventricular nucleus of the hypothalamus that project to the medulla or to the spinal cord in the rat. J Comp Neurol 205:260–272 [DOI] [PubMed] [Google Scholar]
- Sansone GR, Gerdes CA, Steinman JL, Winslow JT, Ottenweller JE, Komisaruk BR, Insel TR 2002 Vaginocervical stimulation releases oxytocin within the spinal cord in rats. Neuroendocrinology 75:306–315 [DOI] [PubMed] [Google Scholar]
- Gainer H, Wray S 1994 Cellular and molecular biology of oxytocin and vasopressin. In: Knobil E, Neill J, eds. The physiology of reproduction. New York: Raven Press; 1099–1129 [Google Scholar]
- Arey BJ, Freeman ME 1992 Activity of oxytocinergic neurons in the paraventricular nucleus mirrors the periodicity of the endogenous stimulatory rhythm regulating prolactin secretion. Endocrinology 130:126–132 [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID 2004 Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol 25:150–176 [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Boccia ML 2006 Vasopressin interactions with oxytocin in the control of female sexual behavior. Neuroscience 139:843–851 [DOI] [PubMed] [Google Scholar]
- Tribollet E, Dubois-Dauphin M, Dreifuss JJ, Barberis C, Jard S 1992 Oxytocin receptors in the central nervous system. Ann NY Acad Sci 652:29–38 [DOI] [PubMed] [Google Scholar]
- Johnson AE 1990 The regulation of oxytocin receptor binding in the ventromedial hypothalamic nucleus by gonadal steroids. Ann NY Acad Sci 652:357–373 [DOI] [PubMed] [Google Scholar]
- Hurbin A, Orcel H, Alonso G, Moos F, Rabie A 2002 The vasopressin receptors colocalize with vasopressin in the magnocellular neurons of the rat supraoptic nucleus and are modulated by water balance. Endocrinology 143:456–466 [DOI] [PubMed] [Google Scholar]
- Insel TR 1992 Oxytocin: A neuropeptide for affiliation-evidence from behavioral, receptor autoradiographic, and comparative studies. Psychoneuroendocrinology 17:3–35 [DOI] [PubMed] [Google Scholar]
- Schumacher M, Coirini H, Frankfurt M, McEwen BS 1989 Localized actions of progesterone in hypothalamus involve oxytocin. Proc Natl Acad Sci USA 86:6798–6801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME 1988 The ovarian cycle of the rat. In: Knobil E, Neill J, eds. The physiology of reproduction. New York: Raven Press; 1893–1926 [Google Scholar]
- Gunnet JW, Mick C, Freeman ME 1981 The role of dorsomedial-ventromedial area of the hypothalamus in the control of prolactin secretion induced by cervical stimulation. Endocrinology 109:1846–1850 [DOI] [PubMed] [Google Scholar]
- Saper CB, Swanson LW, Cowan WM 1975 The efferent connections of the ventromedial nucleus of the hypothalamus of the rat. J Comp Neurol 169:409–442 [DOI] [PubMed] [Google Scholar]
- Canteras NS, Simerly RB, Swanson LW 1994 Organization of projections from the ventromedial nucleus of the hypothalamus: a Phaseolus vulgaris-leucoagglutinin study in the rat. J Comp Neurol 348:41–79 [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C 1998 The rat brain in stereotaxic coordinates. New York: Academic Press [Google Scholar]