Abstract
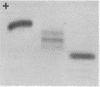
Polymorphism in primate thyroxine-binding prealbumin was investigated with agarose gel electrophoresis at pH 8.6. In the rhesus monkey (Macaca mulatta), three forms of this protein were found in random sera: a single rapidly migrating band similar to that in human and other primate sera, a single slowly migrating band cathodal to rhesus albumin, and a five-banded form, the most rapid and slowest bands of which corresponded to the other two forms. The frequencies of occurrence of these three forms were consistent with the hypotheses that rhesus prealbumin is under the control of two codominant autosomal alleles, PAF and PAS, and that the protein occurs naturally in serum as a tetramer composed of similar subunits.
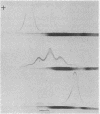
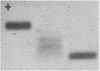
It was possible, by simple mixing in vitro, to produce five-banded prealbumin patterns from rhesus PA SS serum and rhesus PA FF serum, M. arctoides serum, P. hamadryas serum, and human serum. Thyroxine was bound by all the hybrid molecules produced in this fashion.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- APPELLA E., MARKERT C. L. Dissociation of lactate dehydrogenase into subunits with guanidine hydrochloride. Biochem Biophys Res Commun. 1961 Nov 20;6:171–176. doi: 10.1016/0006-291x(61)90123-1. [DOI] [PubMed] [Google Scholar]
- Alper C. A., Propp R. P. Genetic polymorphism of the third component of human complement (C'3). J Clin Invest. 1968 Sep;47(9):2181–2191. doi: 10.1172/JCI105904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLUMBERG B. S. Biochemical polymorphisms in animals: haptoglobins and transferrins. Proc Soc Exp Biol Med. 1960 May;104:25–28. doi: 10.3181/00379727-104-25714. [DOI] [PubMed] [Google Scholar]
- BLUMBERG B. S., ROBBINS J. Thyroxine-serum protein complexes: single dimension gel and paper electrophoresis studies. Endocrinology. 1960 Sep;67:368–378. doi: 10.1210/endo-67-3-368. [DOI] [PubMed] [Google Scholar]
- Bellabarba D., Peterson R. E., Sterling K. An improved method for chromatography of iodothyronines. J Clin Endocrinol Metab. 1968 Feb;28(2):305–307. doi: 10.1210/jcem-28-2-305. [DOI] [PubMed] [Google Scholar]
- DIGIULIO W. D., MICHALAK Z., WEINHOLD P. A., HAMILTON J. R., THOMA G. E. USE OF AGAR GEL ELECTROPHORESIS AND AUTORADIOGRAPHY TO MEASURE THYROXINE-BINDING PROTEIN CAPACITIES. J Lab Clin Med. 1964 Aug;64:349–354. [PubMed] [Google Scholar]
- Hunter W. M., Greenwood F. C. A radio-immunoelectrophoretic assay for human growth hormone. Biochem J. 1964 Apr;91(1):43–56. doi: 10.1042/bj0910043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LAURELL C. B. ANTIGEN-ANTIBODY CROSSED ELECTROPHORESIS. Anal Biochem. 1965 Feb;10:358–361. doi: 10.1016/0003-2697(65)90278-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurell C. B., Niléhn J. E. A new type of inherited serum albumin anomaly. J Clin Invest. 1966 Dec;45(12):1935–1945. doi: 10.1172/JCI105498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. B. PRE-ALBUMIN VARIATIONS IN PRIMATES. Nature. 1963 Nov 23;200:810–810. doi: 10.1038/200810a0. [DOI] [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- OPPENHEIMER J. H., SURKS M. I., SMITH J. C., SQUEF R. ISOLATION AND CHARACTERIZATION OF HUMAN THYROXINE-BINDING PREALBUMIN. J Biol Chem. 1965 Jan;240:173–180. [PubMed] [Google Scholar]
- Rérat C., Schwick H. G. Données cristallographiques sur la préalbumine du plasma sanguin. Acta Crystallogr. 1967 Mar 10;22(3):441–442. doi: 10.1107/s0365110x67000908. [DOI] [PubMed] [Google Scholar]
- SCHEIDEGGER J. J. Une micro-méthode de l'immuno-electrophorèse. Int Arch Allergy Appl Immunol. 1955;7(2):103–110. [PubMed] [Google Scholar]
- WIELAND T., PFLEIDERER G. Nachweis der Heterogenität von Milchsäure-dehydrogenasen verschiedenen Ursprungs durch Trägerelektrophorese. Biochem Z. 1957;329(2):112–116. [PubMed] [Google Scholar]