Abstract
Sex differences are present for all of the phases of drug abuse (initiation, escalation of use, addiction, and relapse following abstinence). While there are some differences among specific classes of abused drugs, the general pattern of sex differences is the same for all drugs of abuse. Females begin regularly self-administering licit and illicit drugs of abuse at lower doses than do males, use escalates more rapidly to addiction, and females are at greater risk for relapse following abstinence. In this review, sex differences in drug abuse are discussed for humans and in animal models. The possible neuroendocrine mechanisms mediating these sex differences are discussed.
Keywords: addiction, cocaine, amphetamine, alcohol, opiates, estradiol, estrogen receptors
Introduction
Drug abuse begins with acquisition or initiation of drug taking and in vulnerable individuals can eventually progress through phases of increased drug taking until an individual is addicted. Sex differences are present for all of the phases of drug abuse, which includes initiation, then escalation of use and the progression to addiction, with subsequent withdrawal followed by relapse [27,82,125,126]. While there are some differences among specific classes of abused drugs, the general pattern of sex differences is the same for all drugs of abuse.
Sex Differences in Drug Abuse in Humans
The rates of drug abuse are currently lower in women than in men. Nevertheless, the number of women using and abusing prescription and illegal drugs is on the rise. Adult men are 2 to 3 times more likely than women to have a drug abuse/dependence disorder, but this current gender difference may reflect differences in opportunity, rather than vulnerability to drug use [125,126]. If one looks at rate of escalation of drug use, however, women tend to increase their rate of consumption of alcohol, marijuana, opioids and cocaine more rapidly than do men [19,54,82,86,99]. Furthermore, once addicted to a drug, women can find it more difficult to quit than men do. This is true for nicotine, as well as many other drugs of abuse [6,21,26,82]. Most of the research on sex differences in drug abuse, in both clinical and pre-clinical studies, has investigated the psychomotor stimulants, so opiates, nicotine, and alcohol are grouped together in this overview for convenience.
Opiates, Nicotine and Alcohol
In humans, whether there is a sex difference in the pattern of opiate use has been questioned [82]. Nevertheless, some studies of addicts indicate that women tend to escalate their use of heroin more rapidly, become addicted in a shorter period of time, and seek treatment earlier than men do [3,59].
Women report shorter intervals between cigarettes, and find it more difficult to quit smoking cigarettes than men (reviewed in [82,109]). In a review of 13 studies that looked at the effect of menstrual cycle on smoking cessation, women tend to have a more difficult time quitting depending on the phase of menstrual cycle, with greater craving and dysphoria during the late luteal phase (when estrogen and progesterone are declining) than during the follicular phase of the cycle (when estradiol is low and increasing and progesterone is low) [26]. Women also exhibit a greater negative affective response to a cue that predicts electric shock during nicotine withdrawal, than do men [55]. One possibility for the menstrual cycle effects on smoking cessation is that during the follicular phase estradiol decreases anxiety and negative affect, thereby alleviating some of the negative consequences of smoking cessation. Support for this idea comes from clinical and pre-clinical research showing that estradiol decreases anxiety and enhances positive affect [131].
Finally, fewer women than men abuse alcohol (7–12% versus 20%). Yet, the frequency that young women are becoming intoxicated on alcohol on a regular basis is rising, and the medical consequences of chronic alcohol consumption are more severe for women than for men. For example, women become addicted to alcohol more rapidly than do men [142], and brain atrophy develops more rapidly in women than in men (other negative medical consequences involve the heart, muscle and liver which are also compromised more rapidly in women than in men [86]).
Cocaine and the Psychomotor Stimulants
Cocaine abuse in particular has increased in the last decade among women so that of the 1.8 million Americans who use cocaine, approximately 39.5% are now female [110]. According to this recent report, among users 12-17 years old 51.5% are women, in the 18-25 age group 42.0% are women, and among cocaine users 26 years and older 38.8% are women [110]. The use and dependence among women of stimulant drug use is a growing public health concern in the USA [27,82,135] and in other countries [22,23]. As with other drugs of abuse, evidence suggests that women are more vulnerable to some aspects of psychostimulant abuse.
Women begin using cocaine or amphetamine (AMPH) and enter treatment at earlier ages than men [51,90] and have more severe cocaine use at intake than men [70]. Thus, the progression to dependence may differ between men and women, with women progressing through the landmark stages from initial use to dependence at a faster rate [18,71]. This “telescoping” effect reflects a briefer time course for the development of medical consequences and behavioral/psychological factors characteristic of a dependence disorder.
In women, the subjective effects of stimulants vary across the menstrual cycle [66-68]. For example, several of the positive subjective effects of d-AMPH such as euphoria, desire, increased energy and intellectual efficiency are potentiated during the follicular phase (when estradiol levels are low at first and rise slowly; progesterone levels are low) relative to the luteal phase (when estradiol levels are moderate and progesterone levels are high). Additionally, administration of estradiol during the follicular phase further increases the subjective effects of d-AMPH [67]. In contrast, the subjective effects of psychomotor stimulant drugs are negatively correlated with salivary progesterone levels in women [136], and progesterone administered during the follicular phase has been reported to attenuate the subjective response to repeated self-administered cocaine [44,45,117].
Abstinent women report higher levels of craving following exposure to cocaine-related cues than do men [103], and women have longer periods of use after abstinence than do men [49]. Such differences may be due to sociocultural factors as well as biological factors. Collectively, these results suggest that women may be more sensitive to the addictive properties of cocaine than men. However, this evidence is based primarily on retrospective reports, and relatively little is known about the neurobiological basis for sex differences in motivational processes in general.
Sex Differences in Animal Models of Drug Use
Basic research on the role of sex and ovarian hormones in the neurochemical and behavioral responses to acute and repeated exposure to drugs of abuse also finds sex differences and may provide insight into the biological causes of sex differences in drug abuse. While there are sex differences in opiates, nicotine and alcohol, most of the pre-clinical research has been done with cocaine and other psychomotor stimulants (primarily AMPH), and so this review will focus on the psychomotor stimulants.
Opiates, Nicotine, and Alcohol
In laboratory animals there are sex differences in acquisition, maintenance, and relapse seen with opiates, nicotine and alcohol. The reader is referred to recent reviews for additional information [82,109]). Not all studies find a sex difference in self-administration of opiates (heroin, morphine, and fentanyl). When there is a sex difference, however, females tend to acquire self-administration more rapidly and take more drug during the maintenance phase (see Table 20-1 in [109]). Female rats also acquire nicotine self-administration more rapidly than males, and will work harder to receive nicotine than males (reviewed in [82,109]). So, acquisition of self-administration of opiates and nicotine occurs more rapidly in females than in males.
In laboratory animals, there are sex differences in the development of and recovery from ethanol dependence. Female rats have decreased seizure threshold following withdrawal and have a more rapid return to the control level of seizure susceptibility [36]. Devaud and colleagues have found that ethanol administration affects GABA-A and NMDA receptor subtypes differently in male and female rat brain, and the direction of the sex difference varies among brain regions [35]. Whether the sex differences in the effects of alcohol and GABA-A and NMDA receptors mediate sex differences in the long-term consequences of alcohol dependence is not known. In mice, sex differences in seizure threshold during ethanol withdrawal is modulated to some extent by ovarian hormones, but there are also inherent sex differences independent of gonadal hormones [1]. It is difficult to characterized sex differences in acquisition of ethanol taking behavior, as rats and mice don't readily self-administer alcohol. Acquisition of ethanol consumption usually involves training animals to self-administer a saccharine sweetened mixture and then fading out the saccharine until animals are taking a pure ethanol solution. This process can take 30 days, making it difficult to parse out factors that influence acquisition. Further research in the clinical setting and the laboratory is needed to clarify the causes and extend our understanding of the nature of these sex differences in alcohol use and abuse.
Psychomotor Stimulants (Cocaine and AMPH)
The acute behavioral response to psychomotor stimulants in rodents can reflect both the sex difference and the modulatory role of gonadal hormones in males and females (e.g., [7,14,17,27,38,39,41,42,50,56,58,65,82,114,128]. With repeated exposure to psychomotor stimulants there is an increase in the psychomotor activating effects of the drug, known as behavioral sensitization. Behavioral sensitization can be different in males and females, and can be differentially affected by gonadal steroid hormones, as we now discuss.
Sensitization of AMPH- or cocaine-induced psychomotor behavior can be defined as the absolute increase in the behavioral response exhibited when two tests are compared. Under such comparisons, intact females exhibit more robust sensitization than do intact males [24,25,46,106,107,127]. Following ovariectomy (OVX) of female rats the expression of sensitization to AMPH is attenuated [24,25,46,106,107] or suppressed all together [116,127]. Estradiol treatments in OVX rats enhance sensitization of locomotor activity induced by AMPH or cocaine [46,95]. These results demonstrate that the neurobiological response to stimulant drugs is sexually dimorphic, but they do not address how this biological difference impacts sex differences in the motivation to take drugs.
Sex Differences in Stimulant Self-Administration in Animals
The animal model of human drug taking behavior that has the most face validity is self-administration. In self-administration studies, animals are trained to bar-press or nose poke in order to receive access to a drug (usually by i.v. infusion). The animal's pattern of drug taking can be studied during acquisition, maintenance and relapse. It is also possible to manipulate the schedule of reinforcement in order to determine motivation to take a drug.
Sex differences have been reported during all phases of the addiction process as assessed using various self-administration paradigms (see [27,82,109]. When a low dose of drug is used, intact or ovariectomized (OVX) female rats acquire cocaine self-administration at a faster rate than do intact of castrated (CAST) males [28,62,78,80]. Estradiol treatment enhances acquisition of cocaine self-administration in OVX female rats [62,83], but not males [64] and the estradiol antagonist, tamoxifen, when given to intact females inhibits acquisition in intact females [78]. So, there are inherent sex differences independent of circulating gonadal hormones in the acquisition of cocaine self-administration, with females being more vulnerable than males. Furthermore, estradiol enhances acquisition in females, but not in males.
During maintenance conditions, when given a choice between two doses of cocaine, female rats in estrus preferred higher doses of cocaine compared with females in other phases of the estrous cycle or male rats [78,79]. A proposed animal model for the transitional process from use/abuse to addiction is a procedure similar to that developed by Roberts et al. [105]. In this model, known as the “discrete trial procedure”, animals are housed in self-administration chambers 24 hours a day, but they only have access to drug during limited times during the day. With this procedure, female rats ‘binge’ for a longer initial period of time, take more cocaine over a 7-day access period, and show a greater loss of diurnal control over cocaine intake than do males [84]. When the role of estradiol in ‘binge’ cocaine intake and subsequent motivational changes is examined, estradiol benzoate (EB) treatment increases the initial binge length and total amount of cocaine self-administered [85]. In one experiment, OVX female rats were tested with and without EB replacement using this procedure (4 trials/hr, 1.5 mg/kg/infusion) over a 7-day period. Results revealed that following a 1-day abstinence period, motivation to obtain cocaine was decreased in OVX rats treated with vehicle, but not in OVX rats treated with EB. In another experiment under extended access conditions, using the discrete trial procedure, OVX rats treated with estradiol consumed more cocaine than vehicle treated controls [78]. These results show that estradiol influences both cocaine self-administration under high access conditions and that there are subsequent motivational changes resulting from such access.
When responding for low doses of cocaine is assessed under a schedule in which the number of responses required in order to obtain a cocaine infusion progressively increases, motivation for access to a drug can be assessed. Under this ‘progressive ratio schedule’ intact female rats reach much higher final ratios than do males, indicating that females are more motivated to obtain cocaine [104]. Females also worked harder for access to cocaine during the phase of the estrous cycle when estradiol was elevated, suggesting that ovarian hormones modulate the motivation to obtain cocaine [104].
In a recent study from our lab we investigated the involvement of ovarian hormones in the motivation to obtain cocaine. We found that estradiol treatment given to OVX rats enhances responding on a progressive ratio schedule at some doses of cocaine (Figure 1). Compared with OIL treated controls, female rats who received 5 μg EB 30 min before the self-administration session worked harder for access to cocaine at 0.4 and 0.5 mg/kg/infusion, but not at 0.3 mg/kg/infusion (Fig. 1). Thus, there are sex differences in the motivation to take cocaine, and estradiol enhances the motivation to take cocaine.
Figure 1. Effect of estradiol benzoate (EB) on the motivation to obtain cocaine.
Ovariectomized (OVX) female rats were prepared for self-administration as previously described [62]. Rats were trained on an FR1 and then an FR2 schedule of reinforcement while receiving 0.4 mg/kg/infusion cocaine, and then were transferred to a progressive ratio (PR) schedule as described by Roberts [104]. Animals were assigned to one of 2 groups: 1) OVX treated with oil 30 min prior to the PR test session for 5 days then no treatment for 2 days (OVX+OIL, N=9); or 2) OVX rats treated with 5 μg EB 30 min prior to the PR session for the first 5 days, and then no treatment for 2 days (OVX+EB, N=12). The Mean +/− SEM number of responses per 4 h progressive ratio test session is illustrated. During the first week the cocaine dose received was 0.3 mg/kg/infusion, during the second week 0.4 mg/kg/infusion, and during the last week 0.5 mg/kg/infusion. Analysis of variance with repeated measures was conducted at each dose. At 0.3 mg/kg/infusion, there was no difference between the groups. At 0.4 mg/kg/infusion, there was a main effect of Group, F1, 19=12.27, p<0.0024; an effect of Day, F4, 19=2.92, p<0.0026; and a Group by Day interaction, F4, 19=3.53, p<0.011. Post hoc pair wise comparisons indicated that OVX+ EB > OVX+OIL group (p<0.001). At 0.5 mg/kg/infusion of cocaine, there was a main effect of Group, F1 , 19=4.84, p<0.04; an effect of Day, F4 ,19=6.08, p<0.0003; but no Group by Day interaction, F4 ,19=0.25. Post hoc pair wise comparisons indicated that OVX+ EB> OVX+OIL group (p<0.001). There were no group differences in the number of nose pokes in the inactive hole during any test sessions. *denotes a significant effect of EB on progressive ratio responding as indicated above.
Sex differences have also been observed under reinstatement testing conditions designed to parallel relapse in humans [69,81,109] but see [47]. In a recent study (Figure 2), we investigated the effect of estradiol in OVX female rats on reinstatement of responding for cocaine. Estradiol (5 μg estradiol benzoate (OVX+EB)) or oil (OVX) was given 30 min prior to each training session when animals were learning to self-administer cocaine and/or prior to testing following withdrawal from cocaine, and responding for cocaine was measured as an animal model of relapse behavior (see legend for Fig. 2 for more details). In this experiment, animals were trained with a dose of cocaine that both OVX and OVX+EB rats would readily self-administer, so the amount of cocaine received during training did not differ among the groups. Then, animals underwent extinction training on scheduled “withdrawal days”, when responding was not reinforced. On days 2, 9, and 16 post-acquisition, animals received an acute dose of OIL or EB (s.c.) to see if hormone treatment or the stress of an injection would re-initiate responding for cocaine (Figure 2A). There was no effect of OIL or EB on responding, which indicated that the interoceptive cues produced by EB (i.e., any internal sensations produced by estradiol) did not induce rats to respond more for access to cocaine. On day 30, after the extinction procedure, animals received an acute dose of OIL or EB (s.c) and 30 min later received 5 mg/kg cocaine (i.p.; Figure 2B). Independent of whether acquisition of cocaine self-administration had occurred with EB treatment, animals that were treated acutely with EB during the reinstatement testing exhibited enhanced responding for cocaine compared with animals treated with OIL (p<0.03; Figure 2B). In a similar experiment, Carroll and colleagues found that acute or chronic estradiol enhanced reinstatement of responding for cocaine [73]. In another experiment, an agonist for estrogen receptor-ß (ERß), but not an agonist for estrogen receptor-α (ERα), was effective at reinstatement of responding when primed with 5 mg/kg cocaine [72]. Only one dose of the agonists were used, so it is possible that higher doses of the ERα agonist might have also been effective. Interestingly, at higher doses of cocaine, responding was re-initiated whether or not the animals were treated the ERß agonist [72]. Thus, estradiol rapidly enhances the subjective effects of cocaine to reinstate responding for access to cocaine, but estradiol does not reinstate responding on its own.
Figure 2. Effect of estradiol on reinstatement of responding for cocaine.
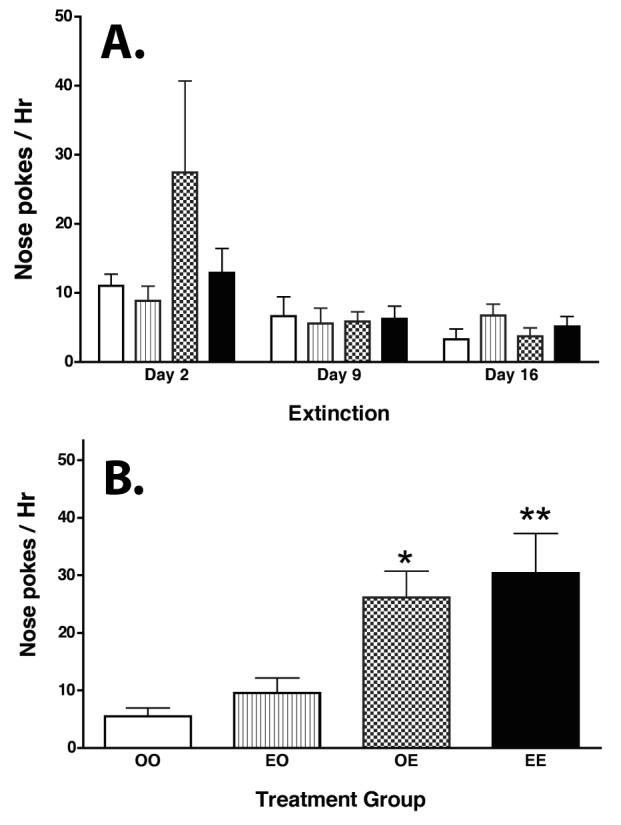
OVX rats were trained to self-administer cocaine with 0.4 mg/kg/infusion cocaine under an FR1 schedule beginning 5 days after catheter surgery using procedures described previously [62]. Animals received 0.1 ml of peanut oil or 5 μg estradiol benzoate (EB, s.c.) in 0.1 μl peanut oil (OIL, s.c.) 30 min before a three-hour self-administration training period. During this three hour training period, the house light and similar paired light + tone conditioned stimuli were present and the syringe was activated to deliver 50 μl of cocaine at the appropriate dose. Daily 3-hour sessions of self-administration training were given for 5 consecutive days followed by 2 days off for 2 weeks, animals were required to earn at least 50 infusions per day for the last two consecutive sessions to continue in the experiment. The extinction training was under an FR1 schedule on days 2, 9, 16 and 30 post-training. During this four hour extinction period, the same house light and similar paired light + tone conditioned stimuli were present and the syringe was activated to deliver 50 μl saline. There were no significant differences in the number of infusions received by the different groups during self-administration training and there were no differences among the groups during extinction.
A. On days 2, 9, and 16 after the extinction session, animals received OIL or EB (half of each group received each treatment for a total of 4 groups: OIL during training + OIL during extinction testing [OIL+OIL]; OIL during training + EB during extinction testing [OIL+EB]; EB during training + OIL during extinction testing [EB+OIL]; EB during training + EB during extinction testing [EB+EB]) and were returned to the test chamber for 90 min. No cues were present during the first 30 min, then nose pokes again activated the light+tone and syringe and nose pokes were recorded for 1 hour. Open bars = OVX Oil+Oil, N=11; Gray bars = OVX Oil+E, N=7; Stippled bars = OVX EB+Oil, N=7; and Black bars = OVX EB+EB N=8. There were no significant differences in the number of active responses made by animals in these groups after EB priming.
B. On day 30 of extinction, animals received OIL or EB (same groups as above) and were returned to the test chamber. Thirty minutes later animals received 5 mg/kg cocaine and nose pokes were recorded for 1 hour. Open bars = OO: OVXOil+Oil, N=11; Gray bars = EO: OVXOil+EB, N=7; Stippled bars = OE: OVXEB+Oil, N=7; and Black bars = EE: OVXEB+EB N=8. There was a significant difference in the number of active responses after treatment with 5 mg/kg cocaine (F 3, 36 =4.523, P<0.0086). Subsequent pair wise comparisons indicated that EB treatment prior to cocaine increased responding for cocaine (OVXOil+EB > OVXOil+Oil (P< 0.0294); OVXEB+EB > OVXEB+Oil (P< 0.0018); and OVXEB+EB > OVXOil+Oil (P< 0.0126).
** OVX EB+EB > OVX Oil+Oil and OVX EB+Oil (P <0.01).
* OVX Oil+EB > OVX Oil+Oil rats (P <0.03)..
In contrast to estradiol, progesterone treatment given concurrently with estradiol counteracts the effect of estradiol on acquisition of cocaine self-administration behavior [64]. We have recently confirmed this finding, and shown that progesterone alone does not affect cocaine self-administration, but that progesterone enhances cocaine intake in EB primed OVX rats [141]. Taken together, a wealth of data now indicate that ovarian hormones contribute to sex differences in cocaine self-administration and that estradiol in particular is a key factor influencing the reinforcing effects of cocaine in female rats. So, over the course of the estrous cycle and menstrual cycle, there are peaks and valleys during which females are more or less susceptible to the reinforcing properties of cocaine. The effect of progesterone to counteract the effects of estradiol in some hormone treatment regimens may reflect hormonal influences on maternal behavior, where estradiol and progesterone are elevated during pregnancy and withdrawal from progesterone is necessary for the rapid onset of maternal behavior at parturition.
Castration (CAST) of males has been reported to enhance sensitization of AMPH- or cocaine-induced psychomotor behavior (e.g., [24,25,106], although this result has not been found consistently [46,127]. It has been hypothesized that if CAST enhances the induction and/or expression of behavioral sensitization, that testosterone treatment should reverse this effect. This is not the case, however, as testosterone treatment has not been found to affect behavioral sensitization in CAST males [46]. Furthermore, there is no effect of CAST on acquisition of cocaine self-administration behavior and a dose of estradiol that enhances self-administration in female rats has no effect on cocaine self- administration behavior in male rats [64]. Thus, the effects of estradiol on the acquisition of cocaine self-administration are sexually dimorphic.
Mechanisms Mediating Sex Differences and Hormone Influences in Rodents on the Response to Psychomotor Stimulants
Female Rat Estrous Cycle
The female rat has a 4-5 day estrous cycle. Circulating estradiol is low during diestrus 1 and increases gradually during diestrus 2 and may persist for a third day of diestrus. The estradiol on these days induces genes needed for initiation of sexual behavior, including induction of progesterone receptors [89]. On the next day, proestrus, there is an endogenous surge of estradiol that occurs around noon, which triggers ovulation about 12 hours later. The surge of estradiol is followed in the afternoon by a surge of progesterone which induces behavioral estrus 4-6 hours later, coincident with ovulation. Progesterone initially acts synergistically with estradiol to induce sexual activity and is subsequently responsible for the termination of sexual receptivity [89].
Female rats are more responsive to AMPH on the evening of behavioral estrus than 24 hr later on diestrus. This is true for the behavioral response to AMPH and for AMPH-stimulated release of dopamine (DA) either in vitro or in vivo [9,11,12,15,17,31,32,107]. Intact female rats also tend to show a greater behavioral response to cocaine on estrus compared to other days of the estrous cycle [113] or to males [132]. The basal extracellular concentrations of DA in the striatum, determined by quantitative microdialysis, are greater on estrus than on diestrus [138]. There is also estrous cycle-dependent variation in striatal DA receptors [37,75]. Following ovariectomy (OVX) the response to AMPH is diminished and estradiol is sufficient to rapidly reinstate the response [14].
Male Rats
In contrast, in males, there are no differences between intact and CAST males in the efficacy of AMPH, cocaine or even stimulation of the nigrostriatal pathway to induce rotational behavior or stereotyped behavior [12,16,108]. In studies with acute and repeated cocaine treatment we do not see an effect of CAST on the behavioral response to cocaine or cocaine self-administration [60,62].
Sex Differences
In addition to sex differences in the behavioral response to psychomotor stimulants discussed above, there are about 10% more D1 DA receptors in the striatum of male rats than in intact female or OVX rats, but no sex difference in the number or binding characteristics of striatal D2 DA receptors [57,74], although in one experiment female rats had fewer D2 receptors than males [92]. In females, however, estradiol rapidly down regulates D2 DA receptor binding in striatum [8]. In vitro, the AMPH-stimulated increase in striatal DA release is comparable for tissue from intact male rats and intact female rats in estrus [17]. There are sex differences, however, in basal and AMPH-stimulated striatal DA release in the absence of gonadal hormones. Following OVX, the AMPH-induced increase in striatal DA release is significantly less than the response of tissue from CAST [9,17]. On the other hand, in experiments with in vivo voltammetry, cocaine or haloperidol induce a greater increase in electrical stimulation evoked extracellular DA in females than in males, possibly due to greater autoreceptor control of the dopamine transporter (DAT) [133,134]. So, there are more D1 DA receptors in striatum of males compared with females, and if there is a sex difference in D2 DA receptors there are more in males than in females, while AMPH-stimulated DA release is comparable in intact males and females during estrus, even though females show a greater behavioral response to AMPH. It is also possible that the ratio of D1/D2 DA receptors plays a role in the behavioral outcome (see Figure 3).
Figure 3. Schematic of Sex Differences and the Effect of Estradiol (E) on the DA system in striatum and NAcc.
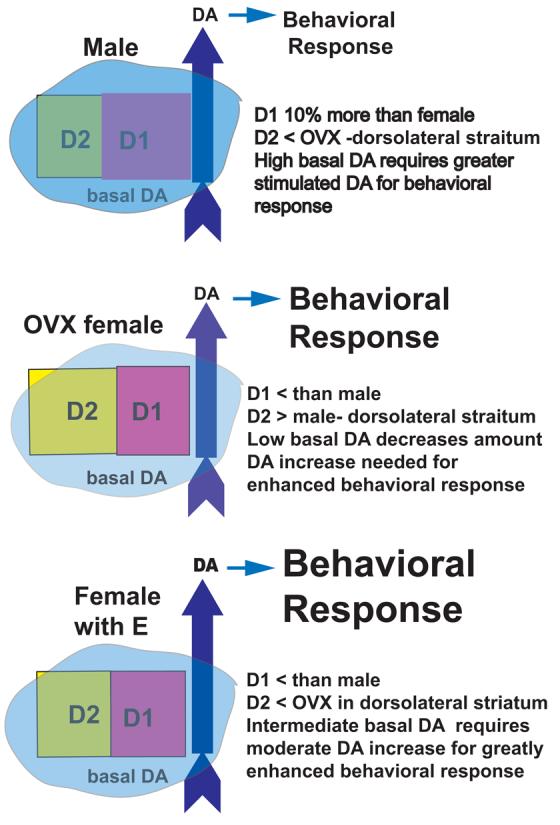
The pink and yellow squares represent the quantity of D1 and D2 DA receptors (respectively). There are 10% more D1 DA receptors (Pink Squares) in the striatum and NAcc of male rats than in intact female rats [2,57]. We find greater D2 binding in the dorsolateral striatum of OVX compared to CAST [8], while others have reported no sex differences in D2 binding when the entire striatum is considered [2,57]. Additionally, in females, estradiol rapidly down-regulates D2 DA receptor binding in dorsolateral striatum of female rats [8]. Research from the pair-bonding literature and the drug abuse literature, suggests that D1 receptors decrease affiliative behavior and addictive behavior, while D2 receptors increase these behaviors [4,112]. These D1/D2 effects are thought to be mediated by DA receptors in the NAcc. Here it is proposed that there is a similar relationship between D1/D2 receptors in dorsolateral striatum, that perhaps in concert with the NAcc, contributes to the behavioral effects and addiction liability of psychomotor stimulant drugs.
The blue overlay represents the basal DA “tone”, the intensity of the color represents the quantity of basal DA, changing the color of DA receptors as their set-point for activation is attenuated by tonic DA stimulation. Results from in vivo microdialysis in freely moving rats have found that the basal extracellular concentrations of DA, as determined by the no net flux method, are twice as high in striatum of CAST males as in OVX females. Additionally, basal DA is greater in estrous females than in diestrous females suggesting that estradiol enhances basal DA “tone” [138].
The stimulated release of DA is represented by the blue arrows, where the darkness of the arrow color is proportional to the amount of release (male=females>OVX in dialysis; females>males). In experiments with in vivo voltammetry, cocaine or haloperidol induce a greater increase in electrical stimulation evoked greater extracellular DA in females than in males, possibly due to greater autoreceptor control of the dopamine transporter (DAT) [133,134]. In vitro, the AMPH-stimulated increase in striatal DA release is comparable for tissue from intact male rats and intact female rats in estrus, and release is attenuated after OVX [17].
The consequence of the coordinated effect of DA stimulation and receptor activation is a behavioral response/ rewarding effect that is greatest in females with OVX+E (or intact females in estrus)>OVX>males.
Results from in vivo microdialysis in freely moving rats have found that the basal extracellular concentrations of DA are twice as high in striatum of CAST males as in OVX females, as determined by the no net flux method [138]. So, it could be that the “tone” and/or basal activity of the striatum and NAcc in males and females are different and this means less behavioral activation induced by AMPH or cocaine in males, thus greater increases in DA release would be necessary to overcome the higher basal DA activity and induce behavioral activation or reinforce responding for cocaine. A schematic representation of this is presented in Figure 3.
Taken with the discussion of the effects of estradiol on cocaine self-administration, it should be pointed out that the addictive properties are not likely to be mediated solely by the effects of estradiol on the ascending DA system. If it were just how much DA activation is produced by a drug, then estradiol should decrease the amount of cocaine consumed, since estradiol enhances DA release. Animals would take less cocaine after estradiol to obtain the optimal amount of DA stimulation, but just the opposite occurs. Estradiol enhances the motivation to take cocaine, so in addition to the enhancement of DA release, the neural systems that respond to the increase in DA must be sexually dimorphic and/or modulated by estradiol so that greater DA stimulation is reinforcing. We suggest that sex-related differences in striatal DA release and receptors reflect an underlying sexual dimorphism in the striatum and NAcc. The concept that activation of the striatum by psychomotor stimulants is sexually dimorphic is supported by the results of a study that examined Fos-immunoreactivity in striatum of CAST and OVX rats after treatment with AMPH [32]. This study found that the pattern of Fos expression differed for males and females, so the areas of the striatum (and presumably down-stream areas) activated by AMPH are sexually dimorphic.
Mechanisms Mediating the Effects of Estradiol on the Striatum of Female Rats
The acute administration of estradiol to OVX rats (but not CAST males) induces a rapid increase in AMPH-induced striatal DA release as detected by in vivo microdialysis [10,30]. Estradiol also induces an increase in striatal DA turnover [40] and, as mentioned above, down-regulates D2 class DA receptors [8]. This sex difference in the effect of estradiol is thought to be due to the direct effect of estradiol on the striatum, as physiological concentrations of estradiol in vitro enhance the AMPH- or K+-induced release of DA from striatal tissue in superfusion [9], and interfere in vitro with the GTP-induced affinity shift of D2 receptors [76]. In cultured striatal neurons from embryonic mouse, estradiol induces changes in adenylate cyclase activity stimulated by D1 and D2 DA receptor agonists by apparently modifying the G-protein coupling process [87,88]. Furthermore, the pulsatile administration of physiological concentrations of estradiol to striatal slices directly stimulates DA release in vitro [9]. Thus, estradiol acts directly on the striatum to induce changes in DA release and DA receptor activity.
Estradiol has also been shown to act directly on the NAcc to enhance K+-stimulated DA release [119,121]. Local injection of 20-50 pg 17ß-estradiol, but not 17α-estradiol, produces a rapid (within 2 min) and dramatic increase in stimulated DA overflow detected by in vivo voltammetry [122]. We have also seen enhanced cocaine-induced DA in dialysate from the striatum of OVX rats 30 min after estradiol treatment [61]. Although there has been less research on estradiol-DA interaction in the NAcc, the work of Thompson and Moss [118-120,123,124,137], and our own work suggests that the mechanism(s) mediating the effects of estradiol in the NAcc and striatum are similar.
Electrophysiological studies have shown that estradiol can induce rapid changes in the response of striatal neurons to D1 and D2 agonists [34]. Results from whole cell clamp studies in acutely dissociated striatal neurons indicate that there are rapid effects of estradiol to decrease current through Ca2+ channels in medium spiny (i.e., GABAergic) striatal neurons [91]. The effects are rapid (within seconds), reverse as soon as estradiol delivery ceases, are sex specific (cells from females respond to much lower doses of estradiol than do cells from males), and are seen at physiologically relevant concentrations of estradiol. Furthermore, estradiol conjugated to bovine serum albumin (E-BSA, prevents estradiol entry onto cells) is also effective, while estradiol applied internally to cells through the electrode is not effective at reducing Ca2+ currents, nor does it block the effect of 1 pM estradiol applied externally. Collectively, these results suggest that the effect of estradiol occurs at the external membrane surface. In the presence of GTPγS (which prevents inactivation of G-protein mediated events) the effect of 17ß-estradiol does not reverse when hormone delivery ceases. Thus, the effect of estradiol is dependent upon a G-protein coupled receptor. Finally, the effect of 17ß-estradiol is stereospecific and sex-specific. We find that 17α-estradiol does not mimic the modulation, and steroid specific as 100 pM estrone and 3-methoxyestriol were ineffective while estriol and 4-hydroxy-estradiol mimic the effect of 17ß-estradiol. Furthermore, in striatal cells from males there was no effect of 17ß-estradiol at concentrations that were effective in females. We conclude that estradiol has rapid stereospecific effects on striatal neurons in females that alter signaling pathways by acting at a receptor on the extracellular membrane [91].
In in vitro superfusion experiments we have found that the effects of estradiol on AMPH-induced striatal DA release from tissue obtained from OVX females is mimicked by the catecholestrogens or E-BSA, but not by diethylstilbesterol (DES), estriol or estrone [139], Furthermore, the effect of estradiol to enhance AMPH-induced DA release from striatal tissue in vitro is blocked by the estradiol receptor antagonist ICI 182,780, but not by tamoxifen [140]. E-BSA mimics the effect of estradiol to enhance AMPH-induced DA release. Thus, the pharmacology of the effects of estradiol in the striatum indicate that it has a steroid-specific and stereo-specific effect. Hydroxylation of the A ring does not inhibit this effect, while modification of the D ring prevents efficacy of a compound in the striatum at mERa. Finally, estradiol need not enter a cell to produce its effect in the striatum.
Due to the results of the studies described above showing effects of estradiol on GABAergic neurons, we hypothesize that estradiol's effect on striatal DA release is mediated indirectly by the effect of estradiol on intrinsic striatal neurons that release GABA. We have recently demonstrated that 30 min following estradiol treatment, the increase in GABA concentrations in dialysate induced by a depolarizing concentration of K+ in the striatum is significantly attenuated, compared with vehicle-treated controls [63]. These results support the idea that estradiol is rapidly inhibiting striatal GABA release, and suggest that enhancement of DA release following estradiol is mediated (at least in part) by a release of inhibition.
Evidence that ERα is also a Membrane Receptor for Estradiol
Evidence that the classical receptors for estradiol (ERα and ERß) may be found in the cell membrane and mediate the rapid responses to estradiol (i.e., mERα and mERß) in female rats come from a variety of sources. A number of immunocytochemical studies have demonstrated that antibodies to ERα will bind to the exterior of the cell. Watson and colleagues have shown that seven different antibodies to ERα bind to the membrane of rat pituitary tumor cells and antibodies that bind to the hormone-binding domain of ERα modulate rapid E-induced prolactin release [94]. In the hippocampus estradiol regulates the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites [143] and there is ultrastructural evidence that there are receptors in axons and synaptic terminals of CA1 [53,93].
When the classical receptors ERα and ERß are transfected into fibroblasts, Chinese hamster ovary (CHO) cells or neuroblasts, a certain proportion of the receptors are found in the cell membrane where they confer rapid responses to estradiol [101,102,129]. In CHO cells in culture, transfection with ERα or ERß results in ER being expressed in the cell membrane and activation of rapid G-protein signaling systems in response to estradiol stimulation [102]. In peripheral cells “” has been shown to be present in association with the protein caveolin, in discrete caveoli domains of the plasma membrane [101]. Caveoli are ’cave-like’ structure in which various receptors and signaling molecules are sequestered. These include G-proteins, tyrosine kinases, and threonine-serine kinases [100]. It is hypothesized that tcaveolin associates with mERα and facilitates its movement to the membrane and anchor the receptor in the caveoli. [101]. This organization of signaling molecules and receptors allows mERs to modulate a variety of signal transduction cascades in target cells. In fibroblasts and neurons in culture, estradiol activation of MAP kinase signaling systems has been found after transfection with ERα or ERß [115,129,130]. Taken as a whole, these results support the idea that the classical ERs (ERα and ERß) are acting at the membrane to confer rapid responses to estradiol in the brain, as has been demonstrated peripherally and in model systems.
Membrane receptors, distinct from ERα or ERß, that respond to estradiol have also been identified in the brain. In the arcuate nucleus of the hypothalamus a G-protein coupled receptor that is involved in energy homeostasis has been reported [96], and the orphan G-protein coupled receptor, GPR30, has been found to mediate rapid responses to estradiol in the hippocampus [48]. Whether these receptors are found in the reward system has not yet been examined. Nevertheless, evidence that estradiol rapidly enhances striatal DA release and the motivation to take cocaine supports the idea that the mER's may play a role in the effects of estradiol on drug taking behavior.
Possible Chromosomal Mechanisms Underlying Sex Differences in Drug Abuse
Most sex differences in the brain are thought to be due to the effects of gonadal hormones during development and/or in the adult mammal [5,20]. Recently, sex differences have also been found that can only be accounted for by the complement of sex chromosomes (XX vs. XY) alone or in combination with gonadal hormone influences [13,29]. Such potential contributions become most evident in cases where sexual phenotype appears to be insensitive to the effects of sex hormones during development or in cases where sex differences develop before the onset of sex-specific patterns of gonadal secretions [13,33,111]. Until recently, determining the influences of gonadal hormones and sex chromosome complement was extremely difficult. However, mouse models are now available in which gonadal hormone status (ovaries vs. testes) is independent of sex chromosome complement (XX vs. XY; [33]). Mice with a deletion of the testis-determining Sry gene from the Y chromosome develop ovaries even when the Y chromosome is present. Absence of the Sry gene in these mice (XY−) as well as in normal females (XX) results in the development of ovaries and a gonadally female phenotype [77]. These mice allow independent assessment of the influences of gonadal hormones and sex chromosome complement on the neurobiology of sex differences in both normal and pathological behavior [33]. Using this model, habit formation has been found to be affected by sex chromosome complement, independent of gonadal hormone status [97] . Specifically, XX mice acquired a food-reinforced habit faster than XY mice, independent of gonadal hormone status. In another study, female mice show greater locomotor sensitization to cocaine compared to males [98], replicating the previous literature [52,127]. Critically, this effect depended upon the gonadal hormone status rather than sex chromosome complement [98]. So, habit formation, but not behavioral sensitization to cocaine, may be influenced by sex chromosomes. Clearly sexual dimorphism in the development of habit formation could have important implications for drug addiction.
In another experiment using this animal model, the effect of sex chromosome complement on brain stimulation reward (BSR) and potentiation of this behavior by AMPH was characterized using a rate-frequency protocol [43]. There were no differences in BSR as measured by the amount of current or frequency required to sustain responding. Interestingly, AMPH potentiated BSR in mice with XY genotypes, but not in mice with XX genotypes, regardless of -sry expression during development. It was concluded that AMPH potentiation of BSR in XY individuals but not XX individuals may reflect the differences in the sensitivity of the DA system neurons caused by group differences in X- or Y-linked genes [43]. An important question remains as to how genetic sex and/or hormonal differences interact and whether differences in the biology of motivational function can explain sex differences that promote uncontrolled and disregulated patterns of intake that are the hallmark of addiction.
Conclusions
From this brief discussion it should be clear that there are sex differences in drug abuse. There are still significant gaps in our knowledge, due to the lack of empirical data that would be generated from a systematic approach to the topic. We know very little about sex differences in marijuana use, for example, in humans or in animal models. The research on sex differences in opioids use and in alcohol are also lacking in sufficient data to draw strong conclusions. Thus, there is substantial need for additional experimental data generated from testing specific hypotheses about the neural bases for sex differences in drug abuse.
Studies of the response to cocaine in gonadectomized male and female rats provide the strongest data regarding the neural evidence for sex differences in drug abuse. These data indicate that there is an underlying sex difference due to sexually dimorphic development of the brain that, in part, mediates the sex difference in drug abuse. Studies from mice in which the testes-determining Sry gene is deleted from the Y chromosome and inserted in an autosome indicate that these sex differences in motivation may, at least in part, be genetic in origin. In addition, there are effects of gonadal hormones that modulate the neural systems that mediate drug taking behaviors. In particular, estradiol enhances the motivation to take drugs, while progesterone can counteract the effect of estradiol. Ultimately research on the neurobiological mechanisms of sex differences in drug abuse will aid in improved treatment and understanding of drug abuse in both females and males.
Acknowledgements
These studies were supported by a grant from the USPHS National Institute on Drug Abuse DA12677 to JBB. Ming Hu was supported by UMSARC training grant. We would like to thank Brandon Luma for excellent technical assistance. All experimental results reported were conducted according to a protocol approved by the University Committee for the Use and Care of Animals at the University of Michigan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Alele PE, Devaud LL. Sex differences in steroid modulation of ethanol withdrawal in male and female rats. Journal of Pharmacology & Experimental Therapeutics. 2007;320:427–36. doi: 10.1124/jpet.106.107896. [DOI] [PubMed] [Google Scholar]
- 2.Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH. Sex differences in dopamine receptor overproduction and elimination. Neuroreport. 1997;8:1495–8. doi: 10.1097/00001756-199704140-00034. [DOI] [PubMed] [Google Scholar]
- 3.Anglin MD, Hser YI, McGlothlin WH. Sex differences in addict careers. 2. Becoming addicted. American Journal of Drug & Alcohol Abuse. 1987;13:59–71. doi: 10.3109/00952998709001500. [DOI] [PubMed] [Google Scholar]
- 4.Aragona BJ, Liu Y, Yu YJ, Curtis JT, Detwiler JM, Insel TR, Wang Z. Nucleus accumbens dopamine differentially mediates the formation and maintenance of monogamous pair bonds.[see comment] Nature Neuroscience. 2006;9:133–9. doi: 10.1038/nn1613. [DOI] [PubMed] [Google Scholar]
- 5.Arnold AP, Gorski RA. Gonadal steroid induction of structural sex differences in the central nervous system. Annu Rev Neurosci. 1984;7:413–42. doi: 10.1146/annurev.ne.07.030184.002213. [DOI] [PubMed] [Google Scholar]
- 6.Back SE, Brady KT, Jackson JL, Salstrom S, Zinzow H. Gender differences in stress reactivity among cocaine-dependent individuals. Psychopharmacology. 2005;180:169–76. doi: 10.1007/s00213-004-2129-7. [DOI] [PubMed] [Google Scholar]
- 7.Bazzett TJ, Albin RL, Becker JB. Malonic acid and the chronic administration model of excitotoxicity. Mitochondrial Inhibitors and Neurodegenerative Disorders. 2000:219–231. [Google Scholar]
- 8.Bazzett TJ, Becker JB. Sex differences in the rapid and acute effects of estrogen on striatal D2 dopamine receptor binding. Brain Res. 1994;637:163–172. doi: 10.1016/0006-8993(94)91229-7. [DOI] [PubMed] [Google Scholar]
- 9.Becker JB. Direct effect of 17ß-estradiol on striatum: sex differences in dopamine release. Synapse. 1990;5:157–164. doi: 10.1002/syn.890050211. [DOI] [PubMed] [Google Scholar]
- 10.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotaional behavior during microdialysis. Neuroscience Letters. 1990;118:169–171. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 11.Becker JB. Estrogen rapidly potentiates amphetamine-induced striatal dopamine release and rotational behavior during microdialysis. Neurosci. Lett. 1990;118:169–71. doi: 10.1016/0304-3940(90)90618-j. [DOI] [PubMed] [Google Scholar]
- 12.Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology Biochemistry and Behavior. 1999;64:803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- 13.Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior.[see comment] Endocrinology. 2005;146:1650–73. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 14.Becker JB, Beer ME. The influence of estrogen on nigrostriatal dopamine activity: behavioral and neurochemical evidence for both pre- and postsynaptic components. Behav Brain Res. 1986;19:27–33. doi: 10.1016/0166-4328(86)90044-6. [DOI] [PubMed] [Google Scholar]
- 15.Becker JB, Cha J. Estrous cycle-dependent variation in amphetamine-induced behaviors and striatal dopamine release assessed with microdialysis. Behav. Brain Res. 1989;35:117–125. doi: 10.1016/s0166-4328(89)80112-3. [DOI] [PubMed] [Google Scholar]
- 16.Becker JB, Molenda H, Hummer DL. Gender differences in the behavioral responses to cocaine and amphetamine. Implications for mechanisms mediating gender differences in drug abuse. Annals of the New York Academy of Sciences. 2001;937:172–87. doi: 10.1111/j.1749-6632.2001.tb03564.x. [DOI] [PubMed] [Google Scholar]
- 17.Becker JB, Ramirez VD. Sex differences in the amphetamine stimulated release of catecholamines from rat striatal tissue in vitro. Brain Res. 1981;204:361–72. doi: 10.1016/0006-8993(81)90595-3. [DOI] [PubMed] [Google Scholar]
- 18.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatr Clin North Am. 1999;22:241–52. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 19.Brady KT, Randall CL. Gender differences in substance use disorders. Psychiatric Clinics of North America. 1999;22:241–52. doi: 10.1016/s0193-953x(05)70074-5. [DOI] [PubMed] [Google Scholar]
- 20.Breedlove SM, Hampson E. Sexual differentiation of the brain and behavior. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press; Cambridge, MA: 2002. pp. 75–115. [Google Scholar]
- 21.Breese GR, Chu K, Dayas CV, Funk D, Knapp DJ, Koob GF, Le DA, O'Dell LE, Overstreet DH, Roberts AJ, Sinha R, Valdez GR, Weiss F. Stress enhancement of craving during sobriety: a risk for relapse. Alcoholism: Clinical & Experimental Research. 2005;29:185–95. doi: 10.1097/01.alc.0000153544.83656.3c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Byqvist S. Drug-abusing women in Sweden: marginalization, social exclusion and gender differences. Journal of Psychoactive Drugs. 2006;38:427–40. doi: 10.1080/02791072.2006.10400582. [DOI] [PubMed] [Google Scholar]
- 23.Byqvist S. Patterns of drug use among drug misusers in Sweden. Gender differences, Substance Use & Misuse. 2006;41:1817–35. doi: 10.1080/10826080601006805. [DOI] [PubMed] [Google Scholar]
- 24.Camp DM, Robinson TE. Susceptibility to sensitization. I. Sex differences in the enduring effects of chronic D-amphetamine treatment on locomotion, stereotyped behavior and brain monoamines. Behav Brain Res. 1988;30:55–68. doi: 10.1016/0166-4328(88)90008-3. [DOI] [PubMed] [Google Scholar]
- 25.Camp DM, Robinson TE. Susceptibility to sensitization. II. The influence of gonadal hormones on enduring changes in brain monoamines and behavior produced by the repeated administration of D-amphetamine or restraint stress. Behav Brain Res. 1988;30:69–88. doi: 10.1016/0166-4328(88)90009-5. [DOI] [PubMed] [Google Scholar]
- 26.Carpenter MJ, Upadhyaya HP, LaRowe SD, Saladin ME, Brady KT. Menstrual cycle phase effects on nicotine withdrawal and cigarette craving: a review. Nicotine & Tobacco Research. 2006;8:627–38. doi: 10.1080/14622200600910793. [DOI] [PubMed] [Google Scholar]
- 27.Carroll M, Lynch W, Roth M, Morgan A, Cosgrove K. Sex and estrogen influence drug abuse. Trends in Pharmacological Sciences. 2004;25:273–279. doi: 10.1016/j.tips.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 28.Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK. Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology. 2002;161:304–313. doi: 10.1007/s00213-002-1030-5. [DOI] [PubMed] [Google Scholar]
- 29.Carruth LL, Reisert I, Arnold AP. Sex chromosome genes directly affect brain sexual differentiation. Nature Neuroscience. 2002;5:933–934. doi: 10.1038/nn922. [DOI] [PubMed] [Google Scholar]
- 30.Castner SA, Becker JB. Estrogen and striatal dopamine release: a microdialysis study. Soc. Neurosci. Abstr. 1990;16 [Google Scholar]
- 31.Castner SA, Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1995 doi: 10.1016/0006-8993(95)01429-2. under revision. [DOI] [PubMed] [Google Scholar]
- 32.Castner SA, Becker JB. Sex differences in the effect of amphetamine on immediate early gene expression in the rat dorsal striatum. Brain Res. 1996;712:245–257. doi: 10.1016/0006-8993(95)01429-2. [DOI] [PubMed] [Google Scholar]
- 33.De Vries GJ, Rissman EF, Simerly RB, Yang LY, Scordalakes EM, Auger CJ, Swain A, Lovell-Badge R, Burgoyne PS, Arnold AP. A model system for study of sex chromosome effects on sexually dimorphic neural and behavioral traits. Journal of Neuroscience. 2002;22:9005–9014. doi: 10.1523/JNEUROSCI.22-20-09005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Demotes-Mainard J, Arnauld E, Vincent JD. Estrogens modulate the responsiveness of in vivo reorded striatal neurons to iontophoretic application of dopamine in rats: role of D1 and D2 receptor activation. J Neuroendocrinol. 1990;2:825–832. doi: 10.1111/j.1365-2826.1990.tb00647.x. [DOI] [PubMed] [Google Scholar]
- 35.Devaud LL, Alele P. Differential effects of chronic ethanol administration and withdrawal on gamma-aminobutyric acid type A and NMDA receptor subunit proteins in male and female rat brain. Alcoholism: Clinical & Experimental Research. 2004;28:957–65. doi: 10.1097/01.alc.0000128225.83916.40. [DOI] [PubMed] [Google Scholar]
- 36.Devaud LL, Chadda R. Sex differences in rats in the development of and recovery from ethanol dependence assessed by changes in seizure susceptibility. Alcoholism: Clinical & Experimental Research. 2001;25:1689–96. [PubMed] [Google Scholar]
- 37.Di Paolo T, Falardeau P, Morissette M. Striatal D-2 dopamine agonist binding sites fluctuate during the rat estrous cycle. Life Sci. 1988;43:665–672. doi: 10.1016/0024-3205(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 38.Di Paolo T, Levesque D, Daigle M. A physiological dose of progesterone affects rat striatum biogenic amine metabolism. Eur J Pharmacol. 1986;125:11–16. doi: 10.1016/0014-2999(86)90077-4. [DOI] [PubMed] [Google Scholar]
- 39.Di Paolo T, Poyet P, Labrie F. Effect of chronic estradiol and haloperidol treatment on striatal dopamine receptors. Eur J Pharmacol. 1981;73:105–6. doi: 10.1016/0014-2999(81)90153-9. [DOI] [PubMed] [Google Scholar]
- 40.Di Paolo T, Rouillard C, Bedard P. 17 beta-Estradiol at a physiological dose acutely increases dopamine turnover in rat brain. Eur J Pharmacol. 1985;117:197–203. doi: 10.1016/0014-2999(85)90604-1. [DOI] [PubMed] [Google Scholar]
- 41.Dluzen DE, Ramirez VD. Bimodal effect of progesterone on in vitro dopamine function of the rat corpus striatum. Neuroendocrinol. 1984;39:149–155. doi: 10.1159/000123971. [DOI] [PubMed] [Google Scholar]
- 42.Dluzen DE, Ramirez VD. In vitro progesterone modulation of amphetamine-stimulated dopamine release from the corpus striatum of ovariectomized estrogen-treated female rats: response characteristics. Brain Res. 1990;517:117–122. doi: 10.1016/0006-8993(90)91016-a. [DOI] [PubMed] [Google Scholar]
- 43.Elmer GI, Pieper JO, Hamilton L, Wise RA, Becker JB, Arnold AP. Society for Neuroscience. Washington, D.C.: 2005. SEX-Chromosome genes influence ampheatmine potentiation of brain stimulation reward independently of gonadal secretions in mice; p. 541.5. Online. [Google Scholar]
- 44.Evans SM, Foltin RW. Exogenous progesterone attenuates the subjective effects of smoked cocaine in women, but not in men. Neuropsychopharmacology. 2006;31:659–74. doi: 10.1038/sj.npp.1300887. [DOI] [PubMed] [Google Scholar]
- 45.Evans SM, Haney M, Foltin RW. The effects of smoked cocaine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 2002;159:397–406. doi: 10.1007/s00213-001-0944-7. [DOI] [PubMed] [Google Scholar]
- 46.Forgie ML, Stewart J. Sex difference in amphetamine-induced locomotor activity in adult rats: role of tetosterone exposure in the neonatal period. Pharmacol, Biochem, Behav. 1994;46 doi: 10.1016/0091-3057(93)90555-8. [DOI] [PubMed] [Google Scholar]
- 47.Fuchs RA, Evans KA, Mehta RH, Case JM, See RE. Influence of sex and estrous cyclicity on conditioned cue-induced reinstatement of cocaine-seeking behavior in rats. Psychopharmacology (Berl) 2005;179:662–72. doi: 10.1007/s00213-004-2080-7. [DOI] [PubMed] [Google Scholar]
- 48.Funakoshi T, Yanai A, Shinoda K, Kawano MM, Mizukami Y. G protein-coupled receptor 30 is an estrogen receptor in the plasma membrane. Biochemical & Biophysical Research Communications. 2006;346:904–10. doi: 10.1016/j.bbrc.2006.05.191. [DOI] [PubMed] [Google Scholar]
- 49.Gallop RJ, Crits-Christoph P, Ten Have TR, Jacques P. Barber JP, Frank A, Griffin ML. Differential Transitions Between Cocaine Use and Abstinence for Men and Women. Journal of Consulting and Clinical Psychology. 2007;75:95–103. doi: 10.1037/0022-006X.75.1.95. [DOI] [PubMed] [Google Scholar]
- 50.Gordon JH. Modulation of apomorphine-induced stereotypy by estrogen: time course and dose response. Brain Res. Bull. 1980;5:679–682. doi: 10.1016/0361-9230(80)90205-1. [DOI] [PubMed] [Google Scholar]
- 51.Griffin ML, Weiss RD, Lange U. A comparison of male and female cocaine abuse. Arch Gen Psychiatry. 1989;46:122–6. doi: 10.1001/archpsyc.1989.01810020024005. [DOI] [PubMed] [Google Scholar]
- 52.Harrod SB, Booze RM, Welch M, Browning CE, Mactutus CF. Acute and repeated intravenous cocaine-induced locomotor activity is altered as a function of sex and gonadectomy. Pharmacol Biochem Behav. 2005;82:170–81. doi: 10.1016/j.pbb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 53.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. Journal of Neuroscience. 2007;27:2102–11. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hernandez-Avila CA, Rounsaville BJ, Kranzler HR. Opioid-, cannabis- and alcohol-dependent women show more rapid progression to substance abuse treatment. Drug & Alcohol Dependence. 2004;74:265–72. doi: 10.1016/j.drugalcdep.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Hogle JM, Curtin JJ. Sex differences in negative affective response during nicotine withdrawal. Psychophysiology. 2006;43:344–56. doi: 10.1111/j.1469-8986.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 56.Hruska RE. 17ßEstradiol regulation of DA receptor interactions with G-proteins. Soc. Neurosci. Abstr. 1988;14:454. [Google Scholar]
- 57.Hruska RE, Ludmer LM, Pitman KT, De Ryck M, Silbergeld EK. Effects of estrogen on striatal dopamine receptor function in male and female rats. Pharmacol Biochem Behav. 1982;16:285–291. doi: 10.1016/0091-3057(82)90162-9. [DOI] [PubMed] [Google Scholar]
- 58.Hruska RE, Silbergeld EK. Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science. 1980;208:1466–1468. doi: 10.1126/science.7189902. [DOI] [PubMed] [Google Scholar]
- 59.Hser YI, Anglin MD, Booth MW. Sex differences in addict careers. 3. Addiction. American Journal of Drug & Alcohol Abuse. 1987;13:231–51. doi: 10.3109/00952998709001512. [DOI] [PubMed] [Google Scholar]
- 60.Hu M, Becker JB. Effects of sex and estrogen on behavioral sensitization to cocaine in rats. J Neurosci. 2003;23:693–699. doi: 10.1523/JNEUROSCI.23-02-00693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hu M, Becker JB. Society for Neuroscience. Atlanta: 2006. Rapid effect of estradiol on cocaine-induced dopamine in striatum and nucleus accumbens. (Program No. 661.10/EE3). [Google Scholar]
- 62.Hu M, Crombag HS, Robinson TE, Becker JB. Biological basis of sex differences in the propensity to self-administer cocaine. Neuropsychopharmacology. 2004;29:81–5. doi: 10.1038/sj.npp.1300301. [DOI] [PubMed] [Google Scholar]
- 63.Hu M, Watson C, Kennedy R, Becker J. Estradiol attenuates the K+-induced increase in extracellular GABA in rat striatum. Synapse. 2006;59:122–124. doi: 10.1002/syn.20221. [DOI] [PubMed] [Google Scholar]
- 64.Jackson LR, Robinson TE, Becker JB. Sex differences and hormonal influences on acquisition of cocaine self-administration in rats. Neuropsychopharmacology. 2006;31:129–138. doi: 10.1038/sj.npp.1300778. [DOI] [PubMed] [Google Scholar]
- 65.Joyce JN, Smith RL, Van Hartesveldt C. Estradiol suppresses then enhances intracaudate dopamine-induced contralateral deviation. Eur J Pharmacol. 1982;81:117–122. doi: 10.1016/0014-2999(82)90608-2. [DOI] [PubMed] [Google Scholar]
- 66.Justice AJH, De Wit H. Acute effects of d-amphetamine during the early and late follicular phases of the menstrual cycle in women. Pharmacology Biochemistry and Behavior. 2000;66:509–515. doi: 10.1016/s0091-3057(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 67.Justice AJH, de Wit H. Acute effects of estradiol pretreatment on the response to d-amphetamine in women. Neuroendocrinology. 2000;71:51–59. doi: 10.1159/000054520. [DOI] [PubMed] [Google Scholar]
- 68.Justice AJH, de Witt H. Acute effects of d-amphetamine during the follicular and luteal phases of the menstrual cycle in women. Psychopharmacology. 1999;145:67–75. doi: 10.1007/s002130051033. [DOI] [PubMed] [Google Scholar]
- 69.Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during estrus. Psychopharmacology (Berl) 2005;182:245–52. doi: 10.1007/s00213-005-0071-y. [DOI] [PubMed] [Google Scholar]
- 70.Kosten TA, Gawin FH, Kosten TR, Rounsaville BJ. Gender differeces in cocaine use and treatment response. J Subst Abuse Treat. 1993;10:63–6. doi: 10.1016/0740-5472(93)90100-g. [DOI] [PubMed] [Google Scholar]
- 71.Kosten TR, Rounsaville BJ, Kleber HD. Ethnic and gender differences among opiate addicts. Int J Addict. 1985;20:1143–62. doi: 10.3109/10826088509056356. [DOI] [PubMed] [Google Scholar]
- 72.Larson E, Carroll M. Estrogen Receptor beta, but not alpha, Mediates Estrogen's Effect on Cocaine-Induced Reinstatement of Extinguished Cocaine-Seeking Behavior in Ovariectomized Female Rats. Neuropharmacology. 2006 doi: 10.1038/sj.npp.1301249. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 73.Larson EB, Roth ME, Anker JJ, Carroll ME. Effect of short- vs. long-term estrogen on reinstatement of cocaine-seeking behavior in female rats. Pharmacology, Biochemistry & Behavior. 2005;82:98–108. doi: 10.1016/j.pbb.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 74.Levesque D, Di Paolo T. Rapid conversion of high into low striatal D2-dopamine receptor agonist binding states after an acute physiological dose of 17 beta- estradiol. Neurosci Lett. 1988;88:113–118. doi: 10.1016/0304-3940(88)90324-2. [DOI] [PubMed] [Google Scholar]
- 75.Levesque D, Di Paolo T. Effect of the rat estrous cycle at ovariectomy on striatal D-1 dopamine receptors. Brain Res Bull. 1990;24:281–4. doi: 10.1016/0361-9230(90)90216-m. [DOI] [PubMed] [Google Scholar]
- 76.Levesque D, Di Paolo T. Modulation by estradiol and progesterone of the GTP effect on striatal D-2 dopamine receptors. Biochem Phamacol. 1993;45:723–733. doi: 10.1016/0006-2952(93)90148-p. [DOI] [PubMed] [Google Scholar]
- 77.Lovell-Badge R, Robertson E. XY female mice resulting from a heritable mutation in the primary testis-determining gene, Tdy. Development. 1990;109:635–46. doi: 10.1242/dev.109.3.635. [DOI] [PubMed] [Google Scholar]
- 78.Lynch WJ. Sex differences in vulnerability to drug self-administration. Experimental & Clinical Psychopharmacology. 2006;14:34–41. doi: 10.1037/1064-1297.14.1.34. [DOI] [PubMed] [Google Scholar]
- 79.Lynch WJ, Arizzi MN, Carroll ME. Effects of sex and the estrous cycle on regulation of intravenously self-administered cocaine in rats. Psychopharmacology. 2000;152:132–139. doi: 10.1007/s002130000488. [DOI] [PubMed] [Google Scholar]
- 80.Lynch WJ, Carroll ME. Sex differences in the acquisition of intravenously self-administered cocaine and heroin in rats. Psychopharmacology (Berl) 1999;144:77–82. doi: 10.1007/s002130050979. [DOI] [PubMed] [Google Scholar]
- 81.Lynch WJ, Carroll ME. Reinstatement of cocaine self-administration in rats: sex differences. Psychopharmacology (Berl) 2000;148:196–200. doi: 10.1007/s002130050042. [DOI] [PubMed] [Google Scholar]
- 82.Lynch WJ, Roth ME, Carroll ME. Biological basis of sex differences in drug abuse: preclinical and clinical studies. Psychopharmacology. 2002;164:121–137. doi: 10.1007/s00213-002-1183-2. [DOI] [PubMed] [Google Scholar]
- 83.Lynch WJ, Roth ME, Mickelberg JL, Carroll ME. Role of estrogen in the acquisition of intravenously self-administered cocaine in female rats. Pharmacology Biochemistry and Behavior. 2001;68:641–646. doi: 10.1016/s0091-3057(01)00455-5. [DOI] [PubMed] [Google Scholar]
- 84.Lynch WJ, Taylor JR. Sex differences in the behavioral effects of 24-h/day access to cocaine under a discrete trial procedure. Neuropsychopharmacology. 2004;29:943–51. doi: 10.1038/sj.npp.1300389. [DOI] [PubMed] [Google Scholar]
- 85.Lynch WJ, Taylor JR. Decreased motivation following cocaine self-administration under extended access conditions: effects of sex and ovarian hormones. Neuropsychopharmacology. 2005;30:927–35. doi: 10.1038/sj.npp.1300656. [DOI] [PubMed] [Google Scholar]
- 86.Mann K, Ackermann K, Croissant B, Mundle G, Nakovics H, Diehl A. Neuroimaging of gender differences in alcohol dependence: are women more vulnerable? Alcoholism: Clinical & Experimental Research. 2005;29:896–901. doi: 10.1097/01.alc.0000164376.69978.6b. [DOI] [PubMed] [Google Scholar]
- 87.Maus M, Bertrand P, Drouva S, Rasolonjanahary R, Kordon C, Glowinski J, Premont J, Enjalbert A. Differential modulation of D1 and D2 dopamine-sensitive adenylate cyclases by 17ß-estradiol in cultures styriatal neurons and anterior pituitary cells. J. Neurochem. 1989;52:410–418. doi: 10.1111/j.1471-4159.1989.tb09136.x. [DOI] [PubMed] [Google Scholar]
- 88.Maus M, Cordier J, Glowinski J, Premont J. 17ß-Oestradiol pretreatment of mouse striatal neurons in culture enhances the responses to adenylate cyclase sensitive tobiogenic amines. Eur. J. Neurosci. 1989;1:1. doi: 10.1111/j.1460-9568.1989.tb00783.x. [DOI] [PubMed] [Google Scholar]
- 89.McCarthy MM, Becker JB. Neuroendocrinology of sexual behavior in the female. In: Becker JB, Breedlove SM, Crews D, McCarthy MM, editors. Behavioral Endocrinology. MIT Press/ Bradford Books; Cambridge, MA: 2002. pp. 117–151. [Google Scholar]
- 90.Mendelson JH, Weiss R, Griffin M, Mirin SM, Teoh SK, Mello NK, Lex BW. Some special considerations for treatment of drug abuse and dependence in women. NIDA Res Monogr. 1991;106:313–327. [PubMed] [Google Scholar]
- 91.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons through a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Miller JC. Sex differences in dopaminergic and cholinergic activity and function in the nigrostriatal system of the rat. Psychneuroendocrinol. 1983;8:225–236. doi: 10.1016/0306-4530(83)90059-8. [DOI] [PubMed] [Google Scholar]
- 93.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. Journal of Comparative Neurology. 2001;429:355–371. [PubMed] [Google Scholar]
- 94.Norfleet AM, Thomas ML, Gametchu B, Watson CS. Estrogen receptor-alpha detected on the plasma membrane of aldehyde-fixed GH(3)/B6/F10 rat pituitary tumor cells by enzyme-linked immunocytochemistry. Endocrinology. 1999;140:3805–3814. doi: 10.1210/endo.140.8.6936. [DOI] [PubMed] [Google Scholar]
- 95.Peris J, Decambre N, Coleman-Hardee M, Simpkins J. Estradiol enhances behavioral sensitization to cocaine and amphetamine-stimulated [3H]dopamine release. Brain Res. 1991;566:255–264. doi: 10.1016/0006-8993(91)91706-7. [DOI] [PubMed] [Google Scholar]
- 96.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis.[see comment] Journal of Neuroscience. 2006;26:5649–55. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Quinn JJ, Hitchcott PK, Arnold AP, Taylor JR. Society for Neuroscience. Abstract Viewer/Itinerary Planner; Washington, DC: 2006. Chromosomal sex determines habit formation: relevance to addiction. Online. [Google Scholar]
- 98.Quinn JJ, Hitchcott PK, Pesquera FR, Arnold AP, Taylor JR. Third Annual Interdisciplinary Women's Health Research Symposium. National Institutes of Health; 2006. Sex differences in habit formation and sensitization to cocaine: Independent contributions of chromosomal sex and gonadal sex. [Google Scholar]
- 99.Randall CL, Roberts JS, Del Boca FK, Carroll KM, Connors GJ, Mattson ME. Telescoping of landmark events associated with drinking: a gender comparison. Journal of Studies on Alcohol. 1999;60:252–60. doi: 10.15288/jsa.1999.60.252. [DOI] [PubMed] [Google Scholar]
- 100.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Molecular and Cellular Biology. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Razandi M, Oh P, Pedram A, Schnitzer J, Levin E. ERs associate with and regulate the production of caveolin: implications for signaling and cellular actions. Molecular Endocrinology. 2002;16:100–15. doi: 10.1210/mend.16.1.0757. [DOI] [PubMed] [Google Scholar]
- 102.Razandi M, Pedram A, Greene G, Levin E. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Molecular Endocrinology. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 103.Robbins SJ, Ehrman RN, Childress AR, O'Brien CP. Comparing levels of cocaine cue reactivity in male and female outpatients. Drug Alcohol Depend. 1999;53:223–30. doi: 10.1016/s0376-8716(98)00135-5. [DOI] [PubMed] [Google Scholar]
- 104.Roberts DC, Bennett SA, Vickers GJ. The estrous cycle affects cocaine self-administration on a progressive ratio schedule in rats. Psychopharmacology (Berl) 1989;98:408–11. doi: 10.1007/BF00451696. [DOI] [PubMed] [Google Scholar]
- 105.Roberts DCS, Brebner K, Vincler M, Lynch WJ. Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug and Alcohol Dependence. 2002;67:291–299. doi: 10.1016/s0376-8716(02)00083-2. [DOI] [PubMed] [Google Scholar]
- 106.Robinson TE. Behavioral sensitization: characterization of enduring changes in rotational behavior produced by intermittent injections of amphetamine in male and female rats. Psychopharmacology (Berlin) 1984;84:466–75. doi: 10.1007/BF00431451. [DOI] [PubMed] [Google Scholar]
- 107.Robinson TE, Becker JB, Presty SK. Long-term facilitation of amphetamine-induced rotational behavior and striatal dopamine release produced by a single exposure to amphetamine: sex differences. Brain Res. 1982;253:231–241. doi: 10.1016/0006-8993(82)90690-4. [DOI] [PubMed] [Google Scholar]
- 108.Robinson TE, Camp DM, Becker JB. Gonadectomy attenuates turning behavior produced by electrical stimulation of the nigrostriatal dopamine system in female but not male rats. Neurosci Lett. 1981;23:203–208. doi: 10.1016/0304-3940(81)90041-0. [DOI] [PubMed] [Google Scholar]
- 109.Roth M, Cosgrove K, Carroll M. Sex differences in the vulnerability to drug abuse: a review of preclinical studies. Neuroscience & Biobehavioral Reviews. 2004;28:533–546. doi: 10.1016/j.neubiorev.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 110.SAMHSA. e. Services DoHaH . Substance Abuse & Mental Health Services Administration. 2005. Results of the 2005 National Survey on Drug Use & Health. [Google Scholar]
- 111.Scordalakes EM, Shetty SJ, Rissman EF. Roles of estrogen receptor alpha and androgen receptor in the regulation of neuronal nitric oxide synthase. Journal of Comparative Neurology. 2002;453:336–344. doi: 10.1002/cne.10413. [DOI] [PubMed] [Google Scholar]
- 112.Self DW, Barnhart WJ, Lehman DA, Nestler EJ. Opposite modulation of cocaine-seeking behavior by D1- and D2-like dopamine receptor agonists.[see comment] Science. 1996;271:1586–9. doi: 10.1126/science.271.5255.1586. [DOI] [PubMed] [Google Scholar]
- 113.Sell SL, Dillon AM, Cunningham KA, Thomas ML. Estrous cycle influence on individual differences in the response to novelty and cocaine in female rats. Behavioural Brain Research. 2005;161:69–74. doi: 10.1016/j.bbr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 114.Sell SL, Thomas ML, Cunningham KA. Influence of estrous cycle and estradiol on behavioral sensitization to cocaine in female rats. Drug Alcohol Depend. 2002;67:281–290. doi: 10.1016/s0376-8716(02)00085-6. [DOI] [PubMed] [Google Scholar]
- 115.Singer CA, Figueroa-Masot XA, Batchelor RH, Dorsa DM. The mitogen-activated protein kinase pathway mediates estrogen neuroprotection after glutamate toxicity in primary cortical neurons. Journal of Neuroscience. 1999;19:2455–2463. doi: 10.1523/JNEUROSCI.19-07-02455.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sircar R, Kim D. Female gonadal hormones differentially modulate cocaine-induced behavioral sensitization in Fischer, Lewis and Sprague-Dawley rats. J Pharmacol exp Ther. 1999;289:54–65. [PubMed] [Google Scholar]
- 117.Sofuoglu M, Babb DA, Hatsukami DK. Effects of progesterone treatment on smoked cocaine response in women. Pharmacol Biochem Behav. 2002;72:431–5. doi: 10.1016/s0091-3057(02)00716-5. [DOI] [PubMed] [Google Scholar]
- 118.Thompson TL. Attenuation of dopamine uptake in vivo following priming with estradiol benzoate. Brain Research. 1999;834:164–167. doi: 10.1016/s0006-8993(99)01508-5. [DOI] [PubMed] [Google Scholar]
- 119.Thompson TL, Bridges SR, Weirs WJ. Alteration of dopamine transport in the striatum and nucleus accumbens of ovariectomized and estrogen-primed rats following N-(p-isothiocyanatophenethyl) spiperone (NIPS) treatment. Brain Research Bulletin. 2001;54:631–638. doi: 10.1016/s0361-9230(01)00472-5. [DOI] [PubMed] [Google Scholar]
- 120.Thompson TL, Moore CC, Smith B. Estrogen priming modulates autoreceptor-mediated potentiation of dopamine uptake. European Journal of Pharmacology. 2000;401:357–363. doi: 10.1016/s0014-2999(00)00432-5. [DOI] [PubMed] [Google Scholar]
- 121.Thompson TL, Moss RL. Estrogen regulation of dopamine release in the nucleus accumbens: genomic- and nongenomic-mediated effects. J Neurochem. 1994;62:1750–6. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- 122.Thompson TL, Moss RL. Estrogen Regulation of Dopamine Release in the Nucleus- Accumbens - Genomic-Mediated and Nongenomic-Mediated Effects. Journal of Neurochemistry. 1994;62:1750–1756. doi: 10.1046/j.1471-4159.1994.62051750.x. [DOI] [PubMed] [Google Scholar]
- 123.Thompson TL, Moss RL. In vivo stimulated dopamine release in the nucleus accumbens: Modulation by prefrontal cortex. Brain Research. 1995;686:93–8. doi: 10.1016/0006-8993(95)00429-t. [DOI] [PubMed] [Google Scholar]
- 124.Thompson TL, Moss RL. Modulation of mesolimbic dopaminergic activity over the rat estrous cycle. Neuroscience Letters. 1997;229:145–148. doi: 10.1016/s0304-3940(97)00450-3. [DOI] [PubMed] [Google Scholar]
- 125.Van Etten ML, Anthony JC. Male-female differences in transitions from first drug opportunity to first use: searching for subgroup variation by age, race, region, and urban status. Journal of Womens Health & Gender-Based Medicine. 2001;10:797–804. doi: 10.1089/15246090152636550. [DOI] [PubMed] [Google Scholar]
- 126.Van Etten ML, Neumark YD, Anthony JC. Male-female differences in the earliest stages of drug involvement. Addiction. 1999;94:1413–9. doi: 10.1046/j.1360-0443.1999.949141312.x. [DOI] [PubMed] [Google Scholar]
- 127.van Haaren F, Meyer M. Sex differences in the locomotor activity after acute and chronic cocaine administration. Pharmacol. Biochem. Behav. 1991;39:923–927. doi: 10.1016/0091-3057(91)90054-6. [DOI] [PubMed] [Google Scholar]
- 128.Van Hartesveldt C, Cottrell GA, Meyer ME. Effects of intrastriatal hormones on the dorsal immobility response in male rats. Pharmacol. Biochem. Behav. 1989;35:307–310. doi: 10.1016/0091-3057(90)90160-j. [DOI] [PubMed] [Google Scholar]
- 129.Wade CB, Robinson S, Shapiro RA, Dorsa DM. Estrogen receptor (ER)alpha and ER beta exhibit unique pharmacologic properties when coupled to activation of the mitogen-activated protein kinase pathway. Endocrinology. 2001;142:2336–2342. doi: 10.1210/endo.142.6.8071. [DOI] [PubMed] [Google Scholar]
- 130.Wade CNB, Dorsa DM. Estrogen activation of cyclic adenosine 5 ′-monophosphate response element-mediated transcription requires the extracellularly regulated kinase/mitogen-activated protein kinase pathway. Endocrinology. 2003;144:832–838. doi: 10.1210/en.2002-220899. [DOI] [PubMed] [Google Scholar]
- 131.Walf AA, Frye CA. A review and update of mechanisms of estrogen in the hippocampus and amygdala for anxiety and depression behavior. Neuropsychopharmacology. 2006;31:1097–111. doi: 10.1038/sj.npp.1301067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walker QD, Cabassa J, Kaplan KA, Li ST, Haroon J, Spohr HA, Kuhn CM. Sex differences in cocaine-stimulated motor behavior: Disparate effects of gonadectomy. Neuropsychopharmacology. 2001;25:118–130. doi: 10.1016/S0893-133X(00)00248-7. [DOI] [PubMed] [Google Scholar]
- 133.Walker QD, Ray R, Kuhn CM. Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology. 2006;31:1193–202. doi: 10.1038/sj.npp.1300915. [DOI] [PubMed] [Google Scholar]
- 134.Walker QD, Rooney MB, Wightman RM, Kuhn CM. Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience. 2000;95:1061–70. doi: 10.1016/s0306-4522(99)00500-x. [DOI] [PubMed] [Google Scholar]
- 135.Wetherington CL, Roman AR, editors. Drug Addiction Research and the Health of Women. U. S. Department of Health and Human Services; Rockville, MD: 1995. [Google Scholar]
- 136.White FJ. A behavioral/systems approach to the neuroscience of drug addiction. The Journal of Neuroscience. 2002;22:3303–5. doi: 10.1523/JNEUROSCI.22-09-03303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wong M, Thompson TL, Moss RL. Nongenomic actions of estrogen in the brain: physiological significance and cellular mechanisms. Crit Rev Neurobiol. 1996;10:189–203. doi: 10.1615/critrevneurobiol.v10.i2.30. [DOI] [PubMed] [Google Scholar]
- 138.Xiao L, Becker JB. Quantitative microdialysis determination of extracellular striatal dopamine concentrations in male and female rats: effects of estrous cycle and gonadectomy. Neuroscience Letters. 1994;180:155–158. doi: 10.1016/0304-3940(94)90510-x. [DOI] [PubMed] [Google Scholar]
- 139.Xiao L, Becker JB. Steroid-specific effects of estrogen agonists and antagonists on amphetamine-induced striatal dopamine released from superfused striatal tissue. Soc. Neurosci Abst. 1997;23:403. [Google Scholar]
- 140.Xiao L, Jackson LR, Becker JB. The effect of estradiol in the striatum is blocked by ICI 182,780 but not tamoxifen: pharmacological and behavioral evidence. Neuroendocrinology. 2003;77:239–245. doi: 10.1159/000070279. [DOI] [PubMed] [Google Scholar]
- 141.Yang H, Zhao W, Hu M, Becker JB. Interactions among ovarian hormones and time of testing on behavioral sensitization and cocaine self-administration. Behavioural Brain Research. 2007 doi: 10.1016/j.bbr.2007.07.005. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zilberman M, Tavares H, el-Guebaly N. Gender similarities and differences: the prevalence and course of alcohol- and other substance-related disorders. Journal of Addictive Diseases. 2003;22:61–74. doi: 10.1300/j069v22n04_06. [DOI] [PubMed] [Google Scholar]
- 143.Znamensky V, Akama K, McEwen BS, Milner TA. Estrogen levels regulate the subcellular distribution of phosphorylated Akt in hippocampal CA1 dendrites. J Neurosci. 2003;23:2340–7. doi: 10.1523/JNEUROSCI.23-06-02340.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]



