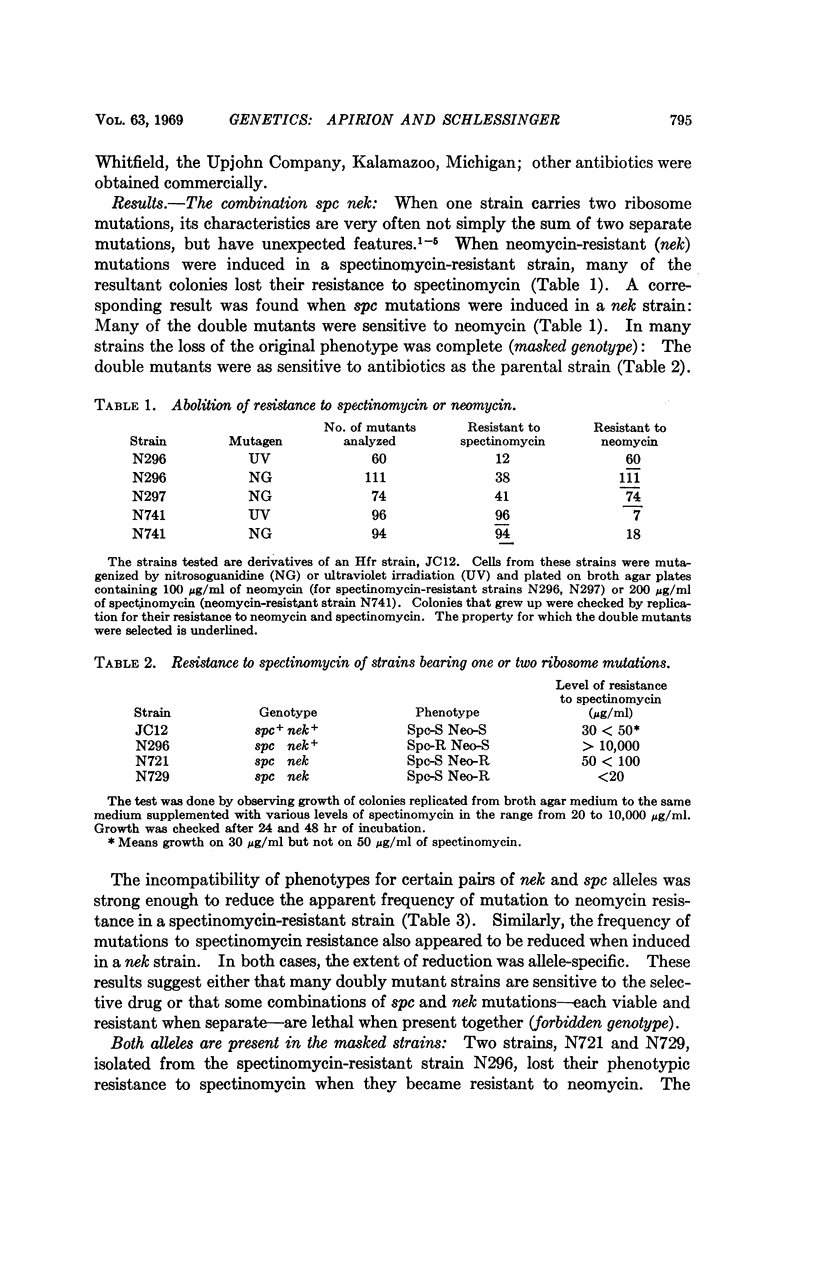
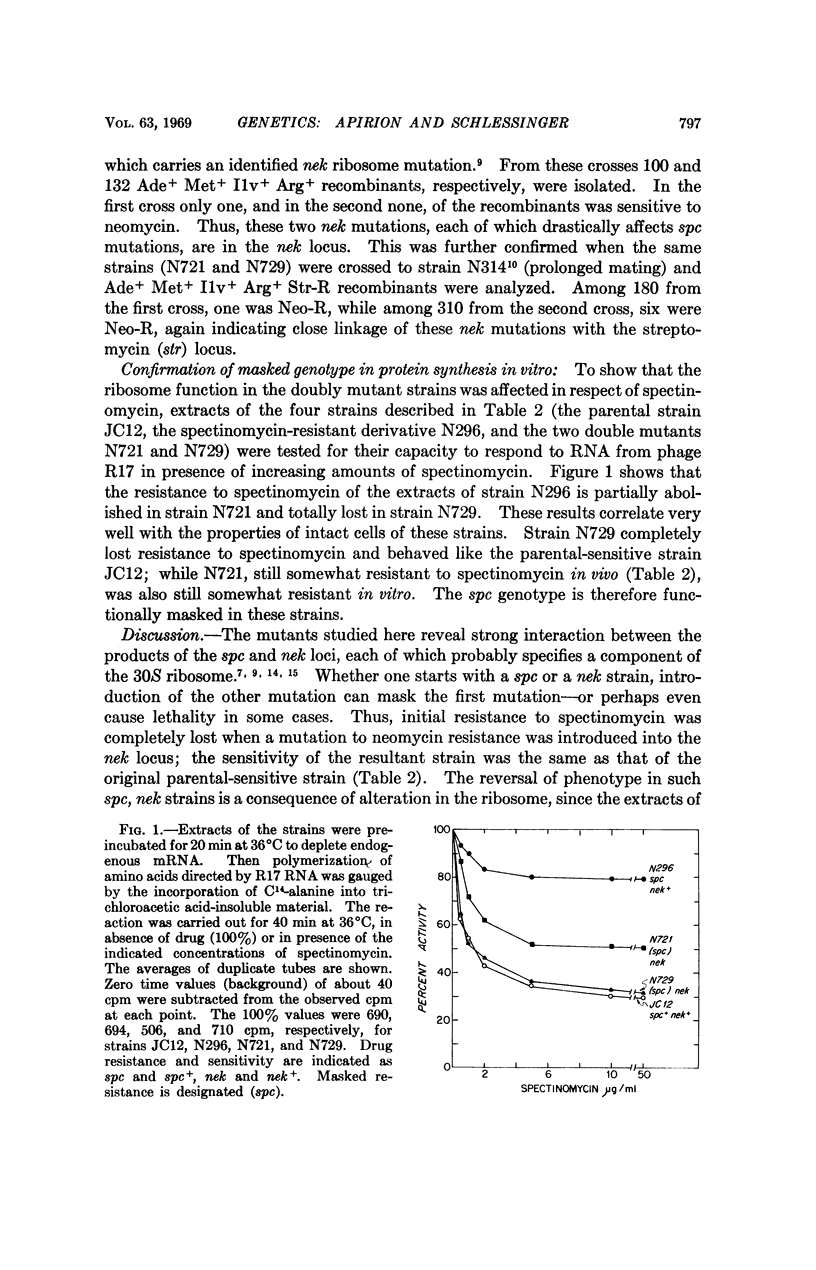
Abstract
Ribosomes are made up of parts that interact so strongly that a mutation in one of them can mask the effect of mutation in another. For example, when a mutation to neomycin resistance, which is a ribosome mutation, is introduced into cells carrying a ribosome mutation to spectinomycin resistance, some of the doubly mutant strains were phenotypically sensitive to spectinomycin, even though the mutation to spectinomycin resistance is still intact and recoverable in appropriate crosses. The neomycin mutant alleles that cause masking were shown by genetic tests to be in an identified locus that affects ribosomes. Protein synthesis in cell-free extracts of a double mutant strain was as sensitive to the action of spectinomycin as was the extract of the doubly sensitive parental strain. Thus, the masking effect of neomycin mutations on the spectinomycin mutation is exerted at the level of the ribosomes.
We conclude that the genetic analysis of an organelle like the ribosome is likely to be severely complicated by pleiotropic effects and by interactions among its component parts and that the ribosomal binding sites for spectinomycin and neomycin are partially interdependent. These results suggest that the function of the ribosome requires a very precise conformation of all its elements, some of which are interdependent. Modification in one element can thus alter the function of another or even render the entire structure nonfunctional.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D., Schlessinger D. Coresistance to neomycin and kanamycin by mutations in an Escherichia coli locus that affects ribosomes. J Bacteriol. 1968 Sep;96(3):768–776. doi: 10.1128/jb.96.3.768-776.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Mapping and complementation of three genes specifying 30S ribosomal components in Escherichia coli. J Bacteriol. 1968 Oct;96(4):1431–1432. doi: 10.1128/jb.96.4.1431-1432.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D., Schlessinger D. Reversion from streptomycin dependence in Escherichia coli by a further change in the ribosome. J Bacteriol. 1967 Oct;94(4):1275–1276. doi: 10.1128/jb.94.4.1275-1276.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- Capecchi M. R. Initiation of E. coli proteins. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1517–1524. doi: 10.1073/pnas.55.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J., Anderson P., Davis B. D. Inhibition of protein synthesis by spectinomycin. Science. 1965 Sep 3;149(3688):1096–1098. doi: 10.1126/science.149.3688.1096. [DOI] [PubMed] [Google Scholar]
- Luzzatto L., Schlessinger D., Apirion D. Escherichia coli: high resistance or dependence on streptomycin produced by the same allele. Science. 1968 Aug 2;161(3840):478–479. doi: 10.1126/science.161.3840.478. [DOI] [PubMed] [Google Scholar]
- Masukawa H., Tanaka N., Umezawa H. Localization of kanamycin sensitivity in the 23S core of 30S ribosomes of E. coli. J Antibiot (Tokyo) 1968 Aug;21(8):517–518. doi: 10.7164/antibiotics.21.517. [DOI] [PubMed] [Google Scholar]
- Phillips S. L., Schlessinger D., Apirion D. Mutants in Escherichia coli ribosomes: a new selection. Proc Natl Acad Sci U S A. 1969 Mar;62(3):772–777. doi: 10.1073/pnas.62.3.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Revised linkage map of Escherichia coli. Bacteriol Rev. 1967 Dec;31(4):332–353. doi: 10.1128/br.31.4.332-353.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



