Abstract
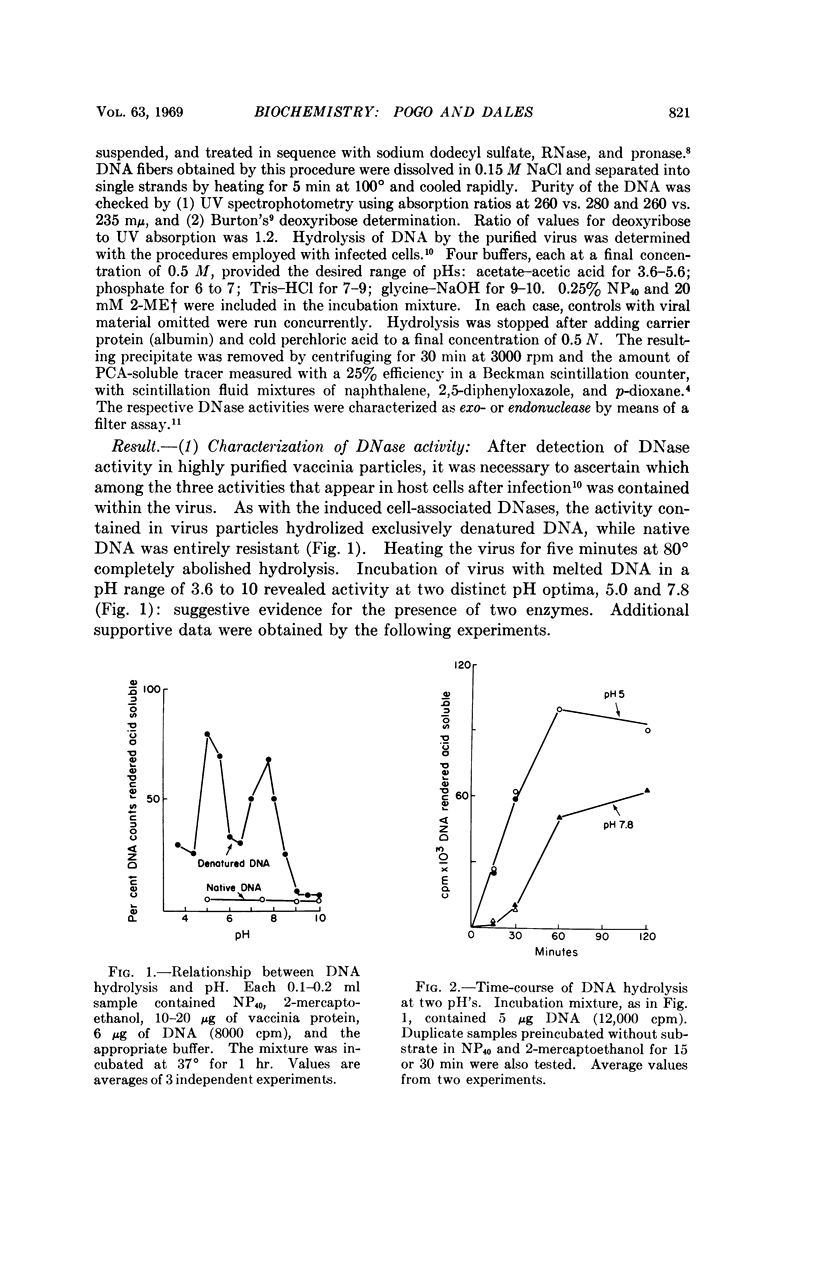
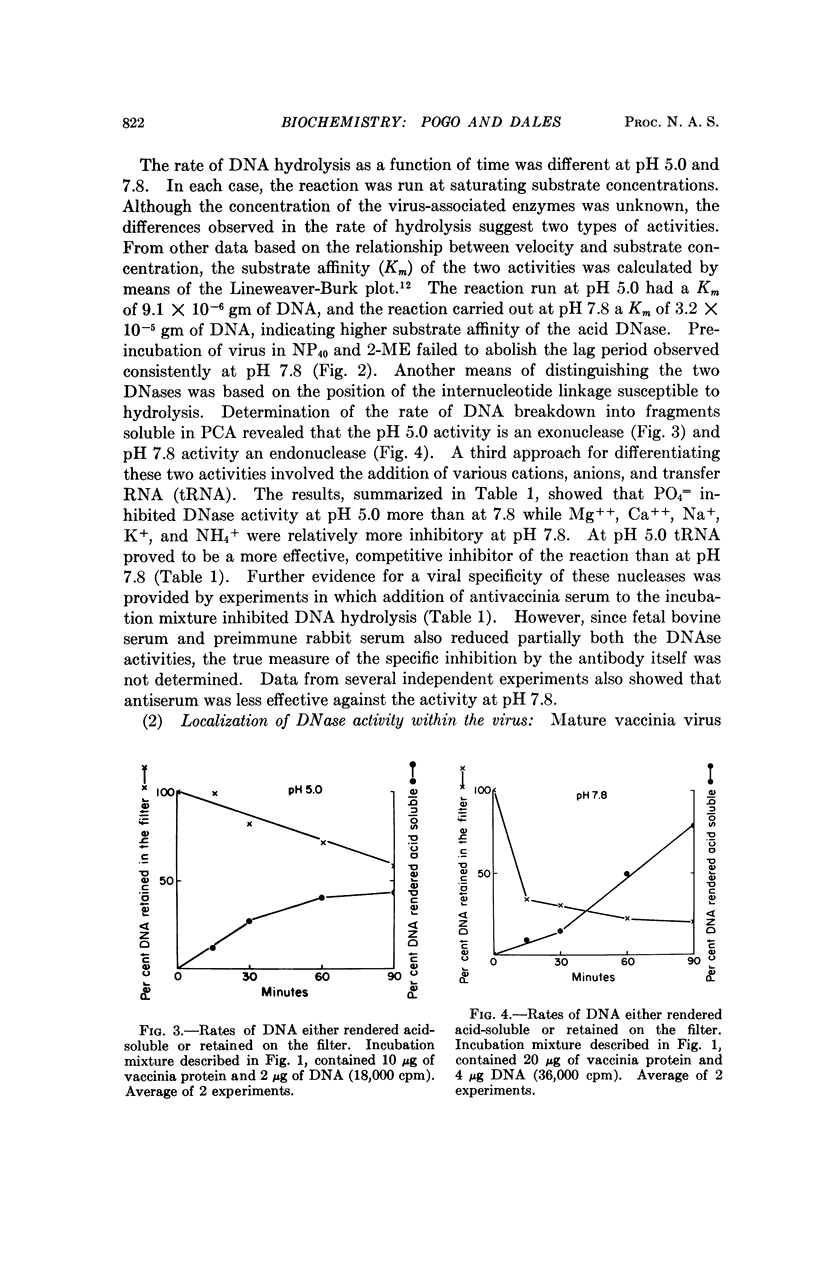
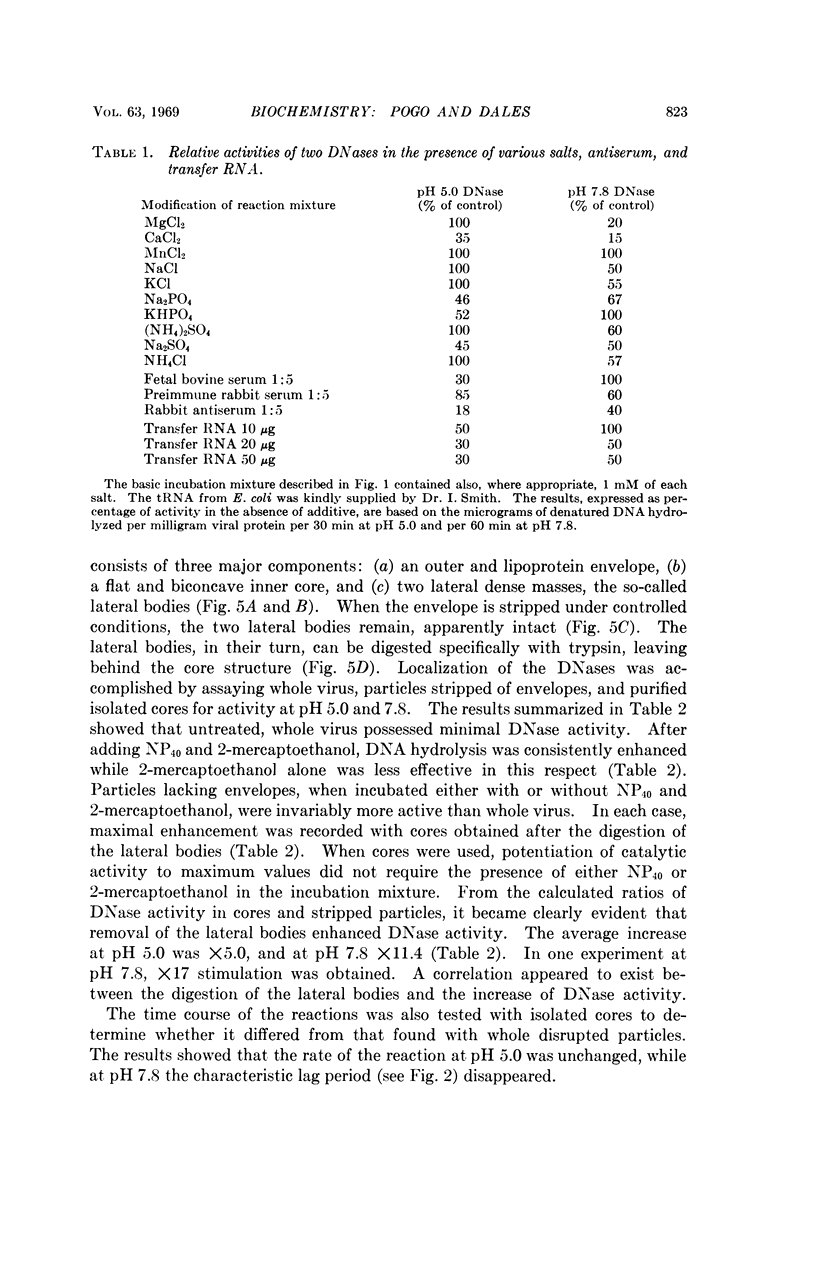
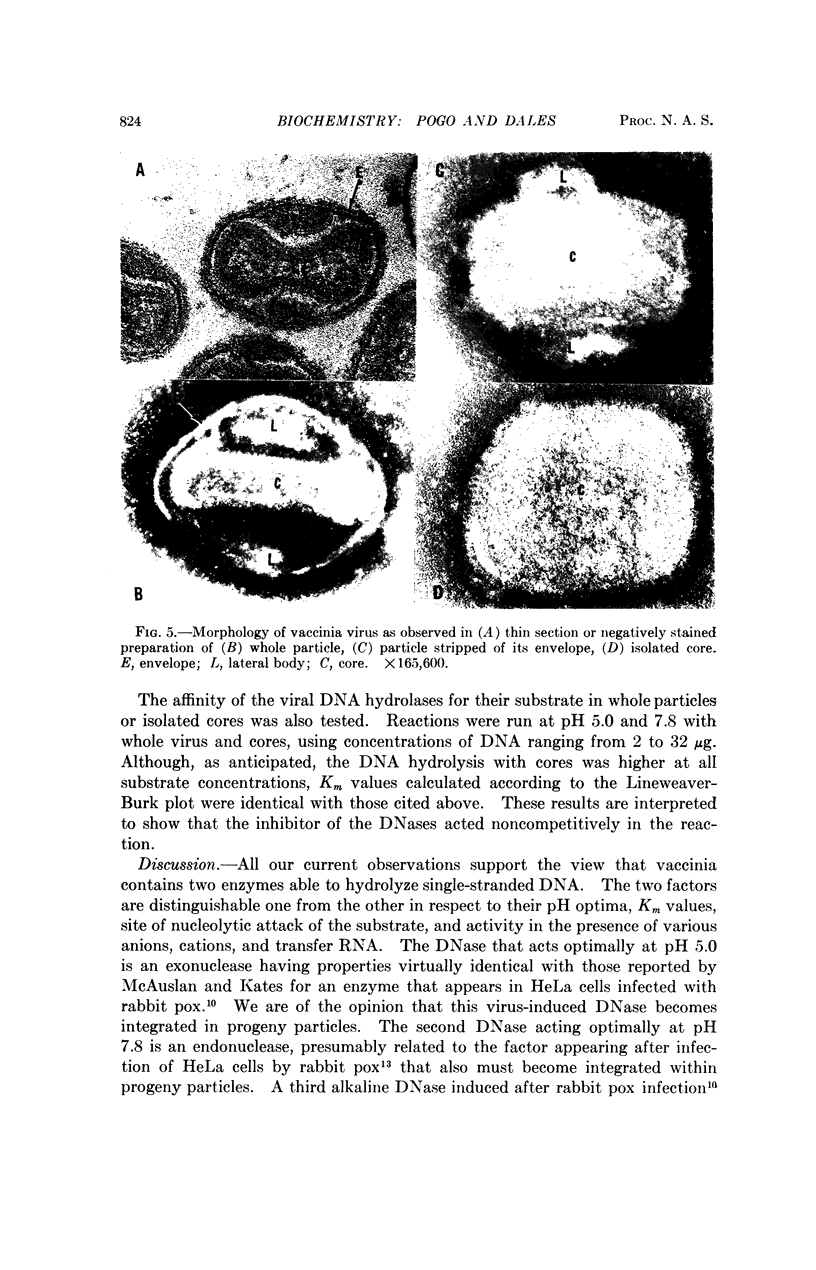
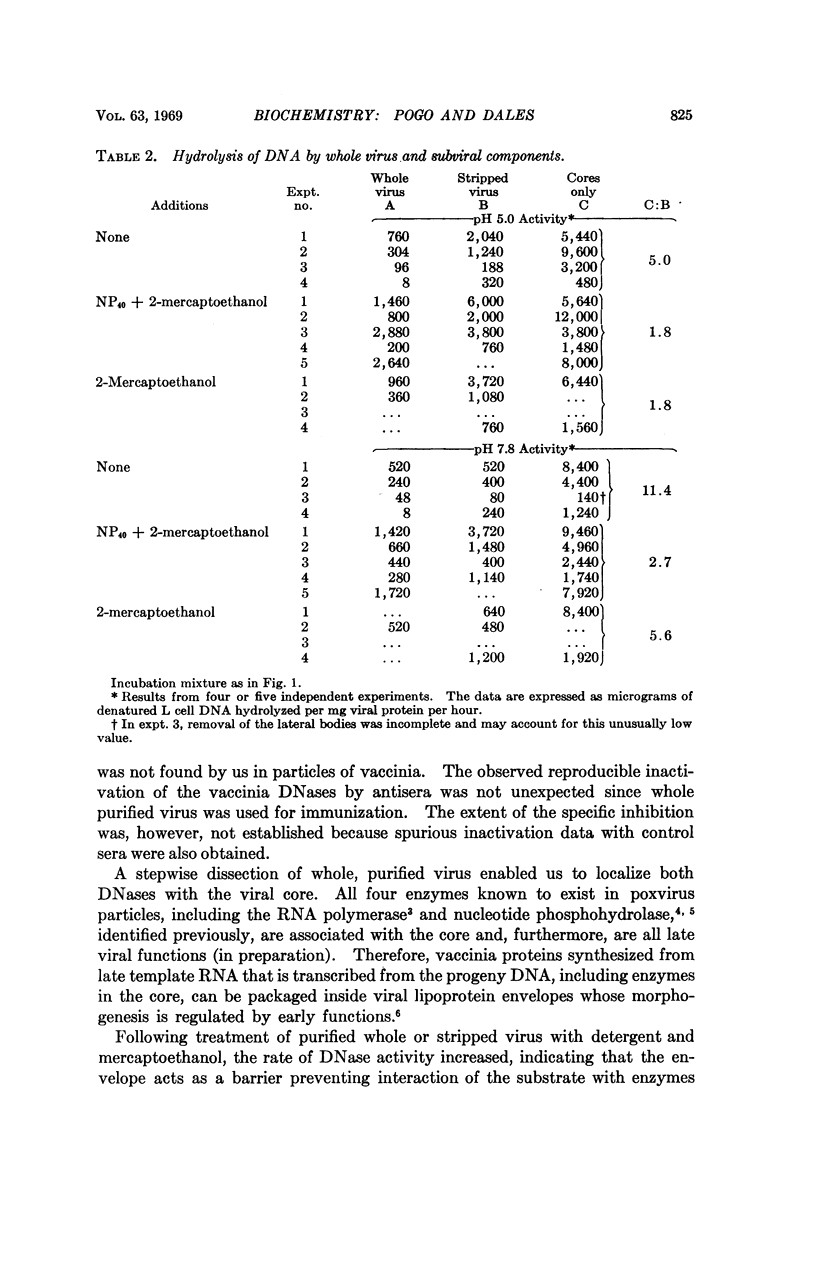
Two DNases, both hydrolizing single-stranded DNA, were identified within highly purified particles of vaccinia virus. One of these, active optimally at pH 5.0, is an exonuclease and the other, most active at pH 7.8, is an endonuclease. These two activities were localized more specifically within the virus cores. Upon elimination of the envelopes, followed by removal of the lateral bodies of vaccinia, both DNases were activated, suggesting that a specific inhibitor of these enzymes may be present in the lateral bodies.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. The uptake and development of vaccinia virus in strain L cells followed with labeled viral deoxyribonucleic acid. J Cell Biol. 1963 Jul;18:51–72. doi: 10.1083/jcb.18.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Mosbach E. H. Vaccinia as a model for membrane biogenesis. Virology. 1968 Aug;35(4):564–583. doi: 10.1016/0042-6822(68)90286-9. [DOI] [PubMed] [Google Scholar]
- GEIDUSCHEK E. P., DANIELS A. A SIMPLE ASSAY FOR DNA ENDONUCLEASES. Anal Biochem. 1965 Apr;11:133–137. doi: 10.1016/0003-2697(65)90052-7. [DOI] [PubMed] [Google Scholar]
- Gold P. H., Dales S. Localization of nucleotide phosphohydrolase activity within vaccinia. Proc Natl Acad Sci U S A. 1968 Jul;60(3):845–852. doi: 10.1073/pnas.60.3.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAFUSA H. Factors involved in the initiation of multiplication of vaccinia virus. Cold Spring Harb Symp Quant Biol. 1962;27:209–217. doi: 10.1101/sqb.1962.027.001.021. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Joklik W. K. Studies on "early" enzymes in HeLa cells infected with vaccinia virus. Virology. 1965 Sep;27(1):80–93. doi: 10.1016/0042-6822(65)90145-5. [DOI] [PubMed] [Google Scholar]
- Jungwirth C., Launer J. Effect of poxvirus infection on host cell deoxyribonucleic acid synthesis. J Virol. 1968 May;2(5):401–408. doi: 10.1128/jvi.2.5.401-408.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski M., Sr DNases and their use in the studies of primary structure of nucleic acids. Adv Enzymol Relat Areas Mol Biol. 1967;29:165–220. doi: 10.1002/9780470122747.ch4. [DOI] [PubMed] [Google Scholar]
- McAuslan B. R., Kates J. R. Regulation of virus-induced deoxyribonucleases. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1581–1587. doi: 10.1073/pnas.55.6.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Inhibition of HeLa cell protein synthesis by the vaccinia virion. J Virol. 1968 Oct;2(10):1028–1037. doi: 10.1128/jvi.2.10.1028-1037.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munyon W., Paoletti E., Ospina J., Grace J. T., Jr Nucleotide phosphohydrolase in purified vaccinia virus. J Virol. 1968 Mar;2(3):167–172. doi: 10.1128/jvi.2.3.167-172.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi M. Studies of DNA replication in vivo. I. Isolation of the first intermediate of DNA replication in bacteria as single-stranded DNA. Proc Natl Acad Sci U S A. 1968 May;60(1):329–336. doi: 10.1073/pnas.60.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki R., Okazaki T., Sakabe K., Sugimoto K., Sugino A. Mechanism of DNA chain growth. I. Possible discontinuity and unusual secondary structure of newly synthesized chains. Proc Natl Acad Sci U S A. 1968 Feb;59(2):598–605. doi: 10.1073/pnas.59.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SALZMAN N. P., SHATKIN A. J., SEBRING E. D. THE SYNTHESIS OF A DNA-LIKE RNA IN THE CYTOPLASM OF HELA CELLS INFECTED WITH VACCINIA VIRUS. J Mol Biol. 1964 Mar;8:405–416. doi: 10.1016/s0022-2836(64)80204-7. [DOI] [PubMed] [Google Scholar]
- Shatkin A. J., Sipe J. D. RNA polymerase activity in purified reoviruses. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1462–1469. doi: 10.1073/pnas.61.4.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]




