Abstract
The nucleoside 2′,3′-dideoxyadenosine is lethal to E. coli and blocks DNA synthesis irreversibly. The hypothesis that a derived dideoxynucleoside triphosphate is incorporated terminally into the cellular DNA has been tested in an in vitro system. The triphosphate of dideoxyadenosine was synthesized and shown to inhibit the in vitro synthesis of DNA by purified E. coli DNA polymerase. The kinetics of inhibition of nucleotide incorporation and pyrophosphate exchange were studied. Early in synthesis the dideoxynucleotide is a competitive inhibitor of the enzyme. Subsequently, synthesis is almost completely arrested.
Radioactive dideoxyadenosine triphosphate was prepared. The compound was shown to be incorporated enzymatically into dAT copolymer to the extent of about one molecule per molecule of template. It is released from such templates by DNA polymerase at less than 1 per cent of the rate of release of other natural nucleotides. The label is released by snake venom phosphodiesterase far more rapidly than total nucleotides. This nucleoside triphosphate has thus been shown to be a competitive inhibitor of DNA polymerase and a terminator of polydeoxynucleotide chains.
Full text
PDF

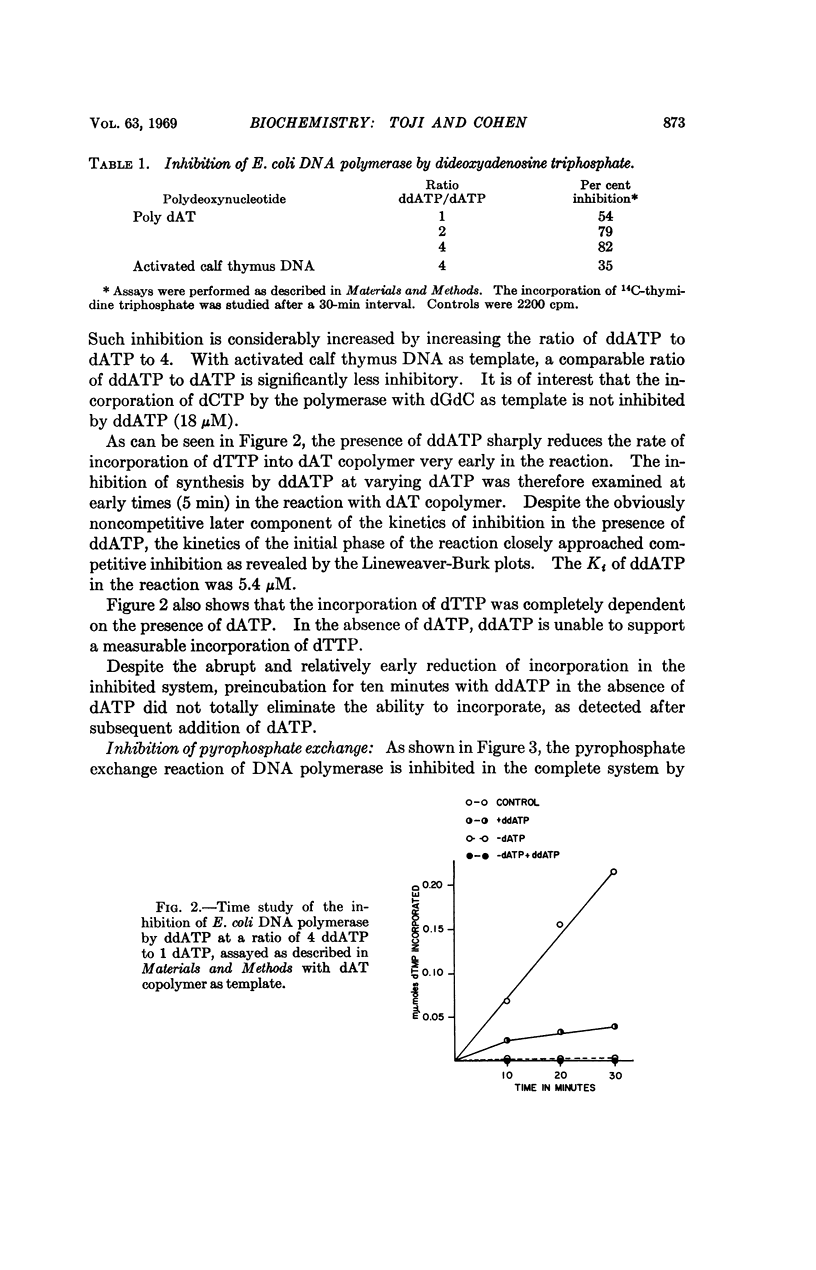
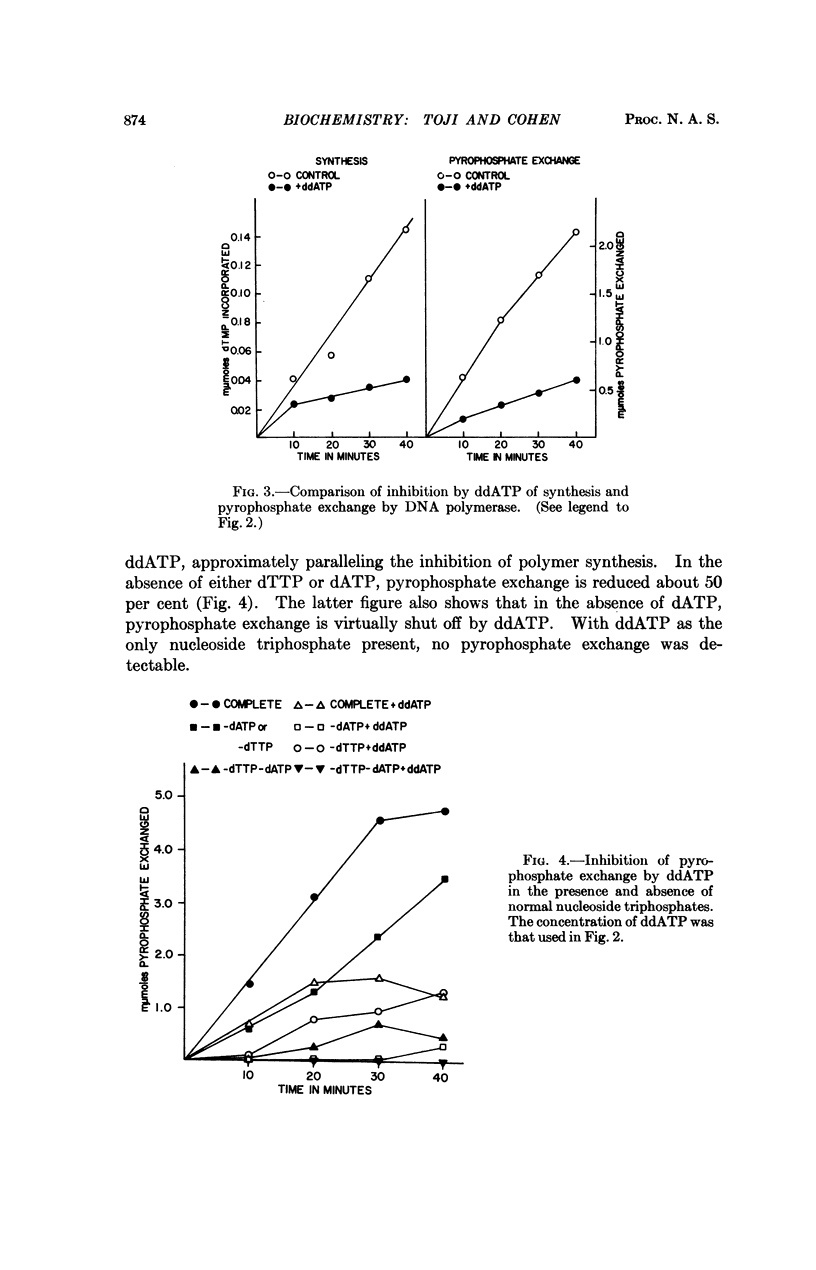
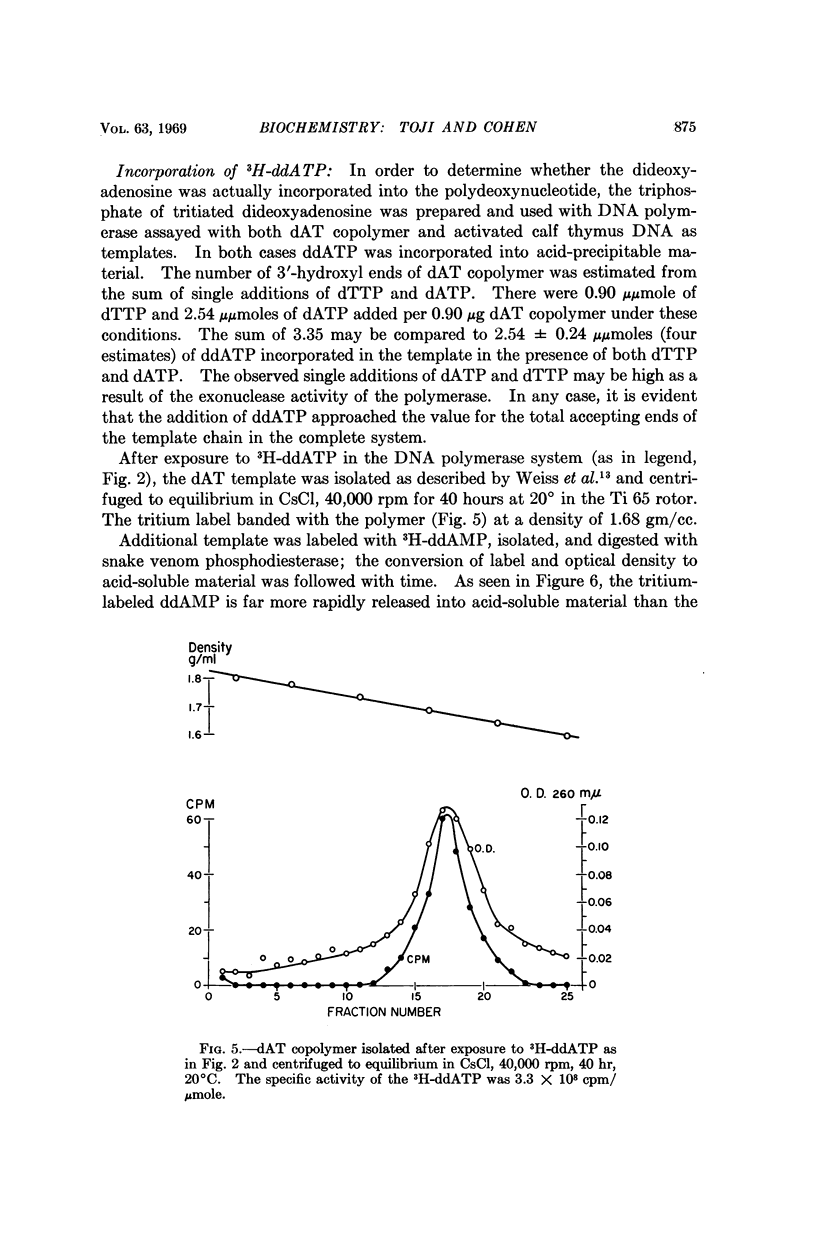
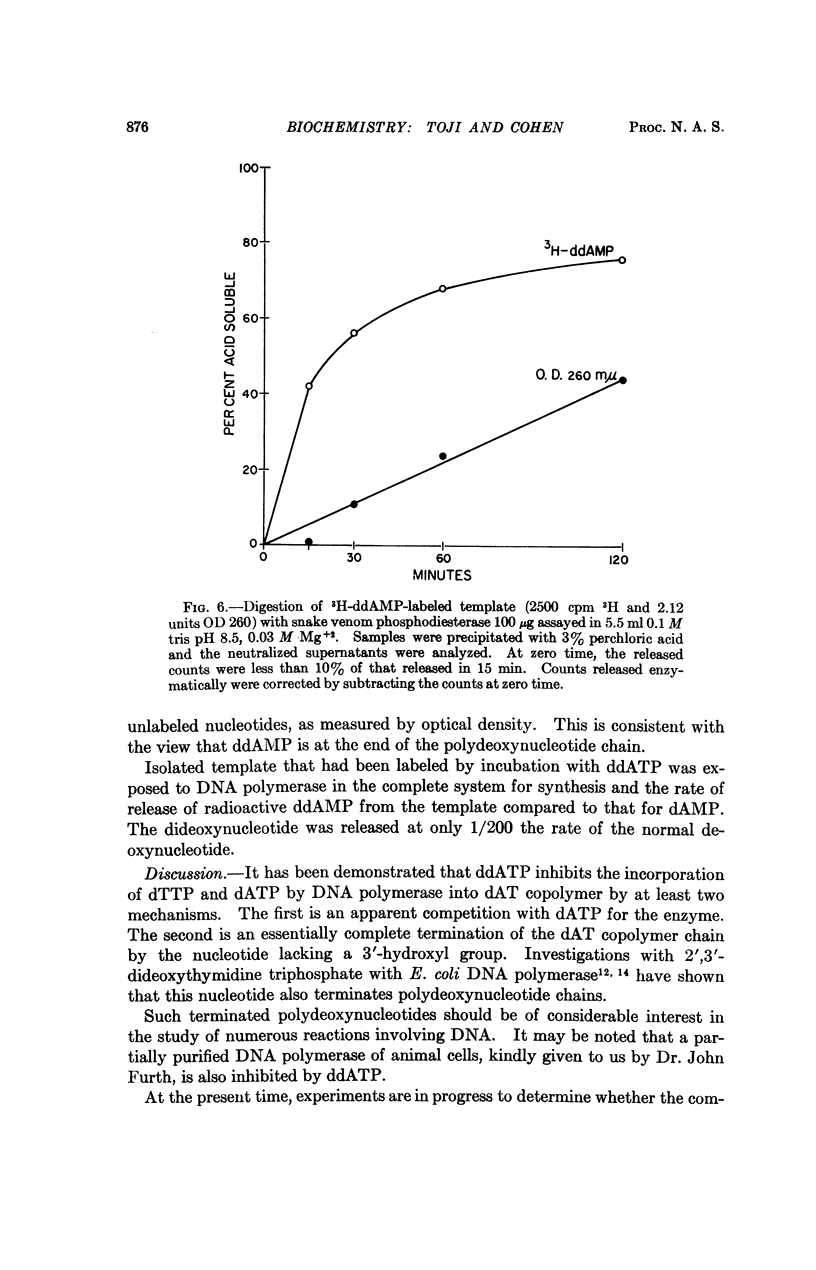

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BESSMAN M. J., LEHMAN I. R., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. II. General properties of the reaction. J Biol Chem. 1958 Jul;233(1):171–177. [PubMed] [Google Scholar]
- Doering A. M., Jansen M., Cohen S. S. Polymer synthesis in killed bacteria: lethality of 2',3'-dideoxyadenosine. J Bacteriol. 1966 Sep;92(3):565–574. doi: 10.1128/jb.92.3.565-574.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg A. Active center of DNA polymerase. Science. 1969 Mar 28;163(3874):1410–1418. doi: 10.1126/science.163.3874.1410. [DOI] [PubMed] [Google Scholar]
- LEHMAN I. R., BESSMAN M. J., SIMMS E. S., KORNBERG A. Enzymatic synthesis of deoxyribonucleic acid. I. Preparation of substrates and partial purification of an enzyme from Escherichia coli. J Biol Chem. 1958 Jul;233(1):163–170. [PubMed] [Google Scholar]
- McCarthy J. R., Jr, Robins M. J., Townsend L. B., Robins R. K. Purine nucleosides. XIV. Unsaturated furanosyl adenine nucleosides prepared via base-catalyzed elimination reactions of 2'-deoxyadenosine derivatives. J Am Chem Soc. 1966 Apr 5;88(7):1549–1553. doi: 10.1021/ja00959a043. [DOI] [PubMed] [Google Scholar]
- NEU H. C., HEPPEL L. A. NUCLEOTIDE SEQUENCE ANALYSIS OF POLYRIBONUCLEOTIDES BY MEANS OF PERIODATE OXIDATION FOLLOWED BY CLEAVAGE WITH AN AMINE. J Biol Chem. 1964 Sep;239:2927–2934. [PubMed] [Google Scholar]
- Robins M. J., McCarthy J. R., Jr, Robins R. K. Purine nucleosides. XII. The preparation of 2',3'-dideoxyadenosine, 2',5'-dideoxyadenosine, and 2',3',5'-trideoxyadenosine from 2'-deoxyadenosine. Biochemistry. 1966 Jan;5(1):224–231. doi: 10.1021/bi00865a029. [DOI] [PubMed] [Google Scholar]
- Weiss B., Live T. R., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid. V. End group labeling and analysis of deoxyribonucleic acid containing single straned breaks. J Biol Chem. 1968 Sep 10;243(17):4530–4542. [PubMed] [Google Scholar]



