Abstract
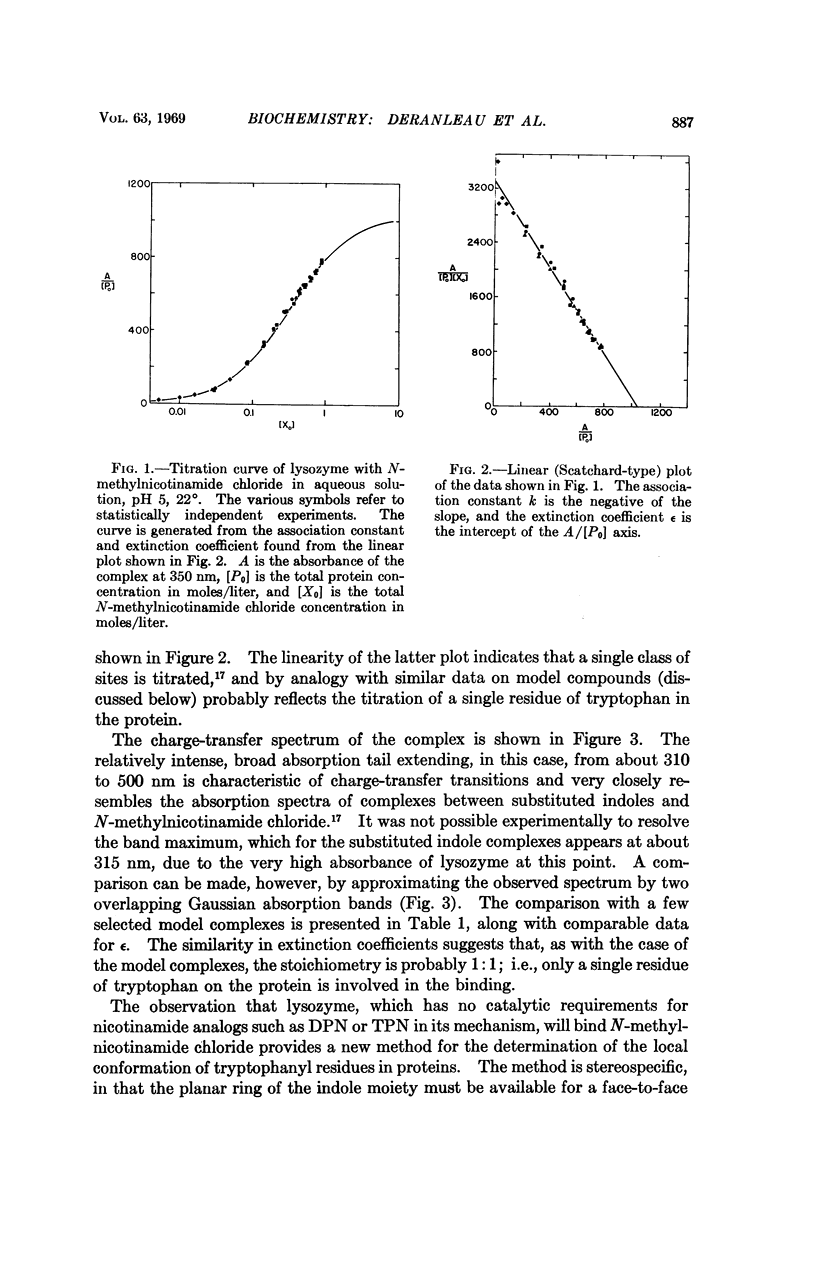
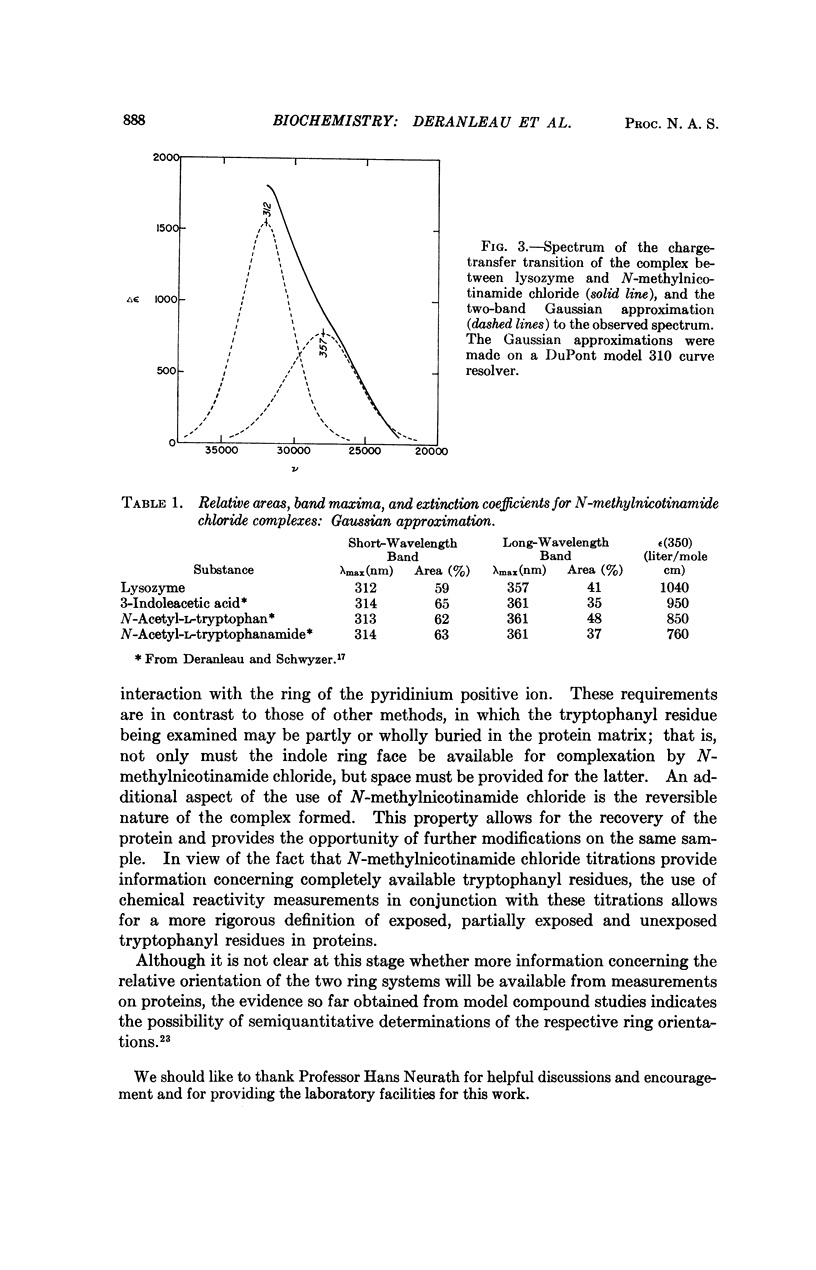
Chicken egg-white lysozyme forms a yellow complex with N-methylnicotinamide chloride. Titration studies utilizing the appearance of the yellow color as a measure of complex formation indicate that the weak complex (association constant k = 3.2 liter mole-1) involves a single class of binding sites on the lysozyme molecule. By analogy with similar titration studies on model compounds containing the indole moiety, the site for N-methylnicotinamide binding is probably the indole ring of a single, solvent-available tryptophan residue. The yellow color itself apparently arises from a charge transfer transition, with the indole ring system serving as the donor and N-methylnicotinamide as the acceptor. Complete resolution of the charge transfer spectrum of the lysozyme-N-methylnicotinamide complex was not achieved due to the very high absorbance of the protein near the short-wavelength absorption edge of the band. However, it is possible to consider the spectrum as the sum of two Gaussian bands whose positions and relative intensities agree remarkably well with the positions and relative intensities obtained by Gaussian fitting of the charge transfer spectra of several model complexes between substituted indoles and N-methylnicotinamide. The geometry for such complex formation requires that the ring faces of both donor and acceptor be more or less completely available for complexation. The possible use of N-methylnicotinamide as a molecular probe for tryptophan residues having at least one indole ring face freely available to the solvent is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CILENTO G., TEDESCHI P. Pyridine coenzymes. IV. Charge transfer interaction with the indole nucleus. J Biol Chem. 1961 Mar;236:907–910. [PubMed] [Google Scholar]
- GREEN N. M., WITKOP B. OXIDATION STUDIES OF INDOLES AND THE TERTIARY STRUCTURE OF PROTEINS. Trans N Y Acad Sci. 1964 Apr;26:659–669. doi: 10.1111/j.2164-0947.1964.tb01933.x. [DOI] [PubMed] [Google Scholar]
- HACHIMORI Y., HORINISHI H., KURIHARA K., SHIBATA K. STATES OF AMINO ACID RESIDUES IN PROTEINS. V. DIFFERENT REACTIVITIES WITH H2O2 OF TRYPTOPHAN RESIDUES IN LYSOZYME, PROTEINASES AND ZYMOGENS. Biochim Biophys Acta. 1964 Nov 8;93:346–346. doi: 10.1016/0304-4165(64)90385-x. [DOI] [PubMed] [Google Scholar]
- HAYASHI K., IMOTO T., FUNATSU M. THE ENZYME-SUBSTRATE COMPLEX IN A MURAMIDASE CATALYZED REACTION. I. DIFFERENCE SPECTRUM OF COMPLEX. J Biochem. 1963 Nov;54:381–387. doi: 10.1093/oxfordjournals.jbchem.a127803. [DOI] [PubMed] [Google Scholar]
- Hartdegen F. J., Rupley J. A. The oxidation by iodine of tryptophan 108 in lysozyme. J Am Chem Soc. 1967 Mar 29;89(7):1743–1745. doi: 10.1021/ja00983a044. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Imoto T., Funatsu G., Funatsu M. The position of the active tryptophan residue in lysozyme. J Biochem. 1965 Sep;58(3):227–235. doi: 10.1093/oxfordjournals.jbchem.a128190. [DOI] [PubMed] [Google Scholar]
- KAUZMANN W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- Kronman M. J., Robbins F. M., Andreotti R. E. Reaction of N-bromosuccinimide with lysozyme. Biochim Biophys Acta. 1967 Dec 12;147(3):462–472. doi: 10.1016/0005-2795(67)90006-2. [DOI] [PubMed] [Google Scholar]
- Moser P. Elektronen-Donator-Acceptor-Komplexe bei Polypeptiden. 3. Optische Aktivität von intra- und intermolekularen Charge Transfer-Absorptionsbanden. Helv Chim Acta. 1968;51(8):1831–1845. doi: 10.1002/hlca.19680510804. [DOI] [PubMed] [Google Scholar]
- Previero A., Coletti-Previero M. A., Jollès P. Localization of non-essential tryptophan residues for the biological activity of lysozyme. J Mol Biol. 1967 Mar 14;24(2):261–268. doi: 10.1016/0022-2836(67)90331-2. [DOI] [PubMed] [Google Scholar]
- RAO G. J., RAMACHANDRAN L. K. The role of tryptophan residues in the enzymic activity of lysozyme. Biochim Biophys Acta. 1962 May 21;59:507–508. doi: 10.1016/0006-3002(62)90213-5. [DOI] [PubMed] [Google Scholar]
- SHIFRIN S. NONCOVALENT INTERACTIONS BETWEEN AMINO ACID SIDE CHAINS AND A COENZYME MODEL. Biochemistry. 1964 Jun;3:829–833. doi: 10.1021/bi00894a018. [DOI] [PubMed] [Google Scholar]
- SOPHIANOPOULOS A. J., VANHOLDE K. E. PHYSICAL STUDIES OF MURAMIDASE (LYSOZYME). II. PH-DEPENDENT DIMERIZATION. J Biol Chem. 1964 Aug;239:2516–2524. [PubMed] [Google Scholar]
- Takahashi T., Hamaguchi K., Hayashi K., Imoto T., Funatsu M. Structure of lysozyme. X. On the structural role of tryptophan residues. J Biochem. 1965 Oct;58(4):385–387. doi: 10.1093/oxfordjournals.jbchem.a128215. [DOI] [PubMed] [Google Scholar]
- WEIL L., BUCHERT A. R., MAHER J. Photoöxidation of crystalline lysozyme in the presence of methylene blue and its relation to enzymatic activity. Arch Biochem Biophys. 1952 Oct;40(2):245–252. doi: 10.1016/0003-9861(52)90108-2. [DOI] [PubMed] [Google Scholar]
- Williams E. J., Herskovits T. T., Laskowski M., Jr Location of chromophoric residues in proteins by solvent perturbation. 3. Tryptophyls in lysozyme and in alpha-chymotrypsinogen and its derivatives. J Biol Chem. 1965 Sep;240(9):3574–3579. [PubMed] [Google Scholar]


