Abstract
The consumption of bi-valve molluscan shellfish has been associated with outbreaks of viral gastroenteritis and hepatitis A. Investigations were undertaken to determine the heat inactivation conditions necessary to render shellfish such as cockles safe for the consumer. Conditions for the laboratory maintenance of live cockles are described. In preliminary experiments either poliovirus (106 TCID50/ml seawater) or hepatitis A virus (HAV) (approx. 104 RFU/ml seawater) was introduced into the shellfish tank. Following 48 h filter feeding, virus was recovered from cockles using an adsorption-elution extraction procedure. Titres of virus recovered ranged from 104 to 105 TCID50/ml of shellfish extract for poliovirus and from 103 to 105 RFU/ml of shellfish extract for HAV. Active ingestion of the virus from the seawater was demonstrated by recovering virus from within cockle guts.
To quantify recovered HAV, end-point dilutions and an adaptation of a radioimmunofocus assay (RIFA) were compared. The tests were of similar sensitivity but the RIFA has the advantage of being relatively rapid, shortening the time taken to complete an experiment by as much as 4 weeks.
Full text
PDF


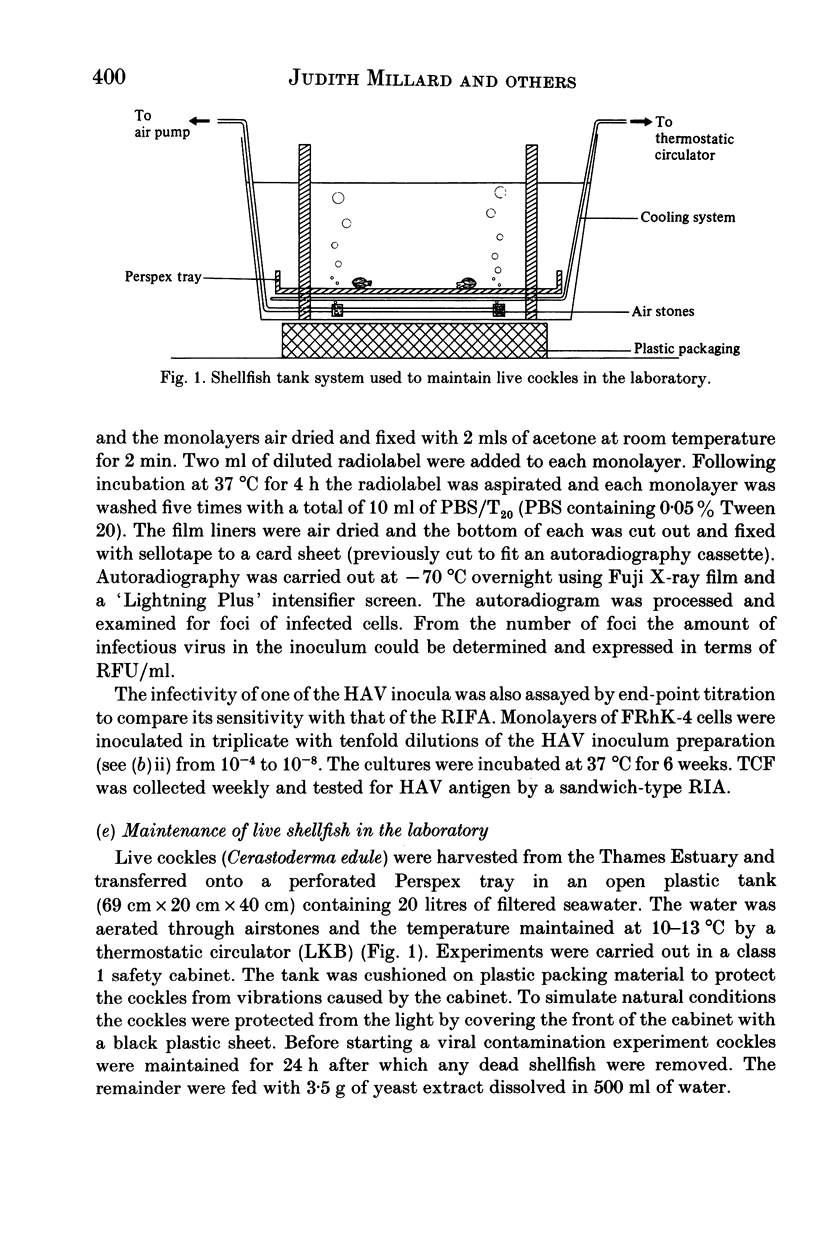

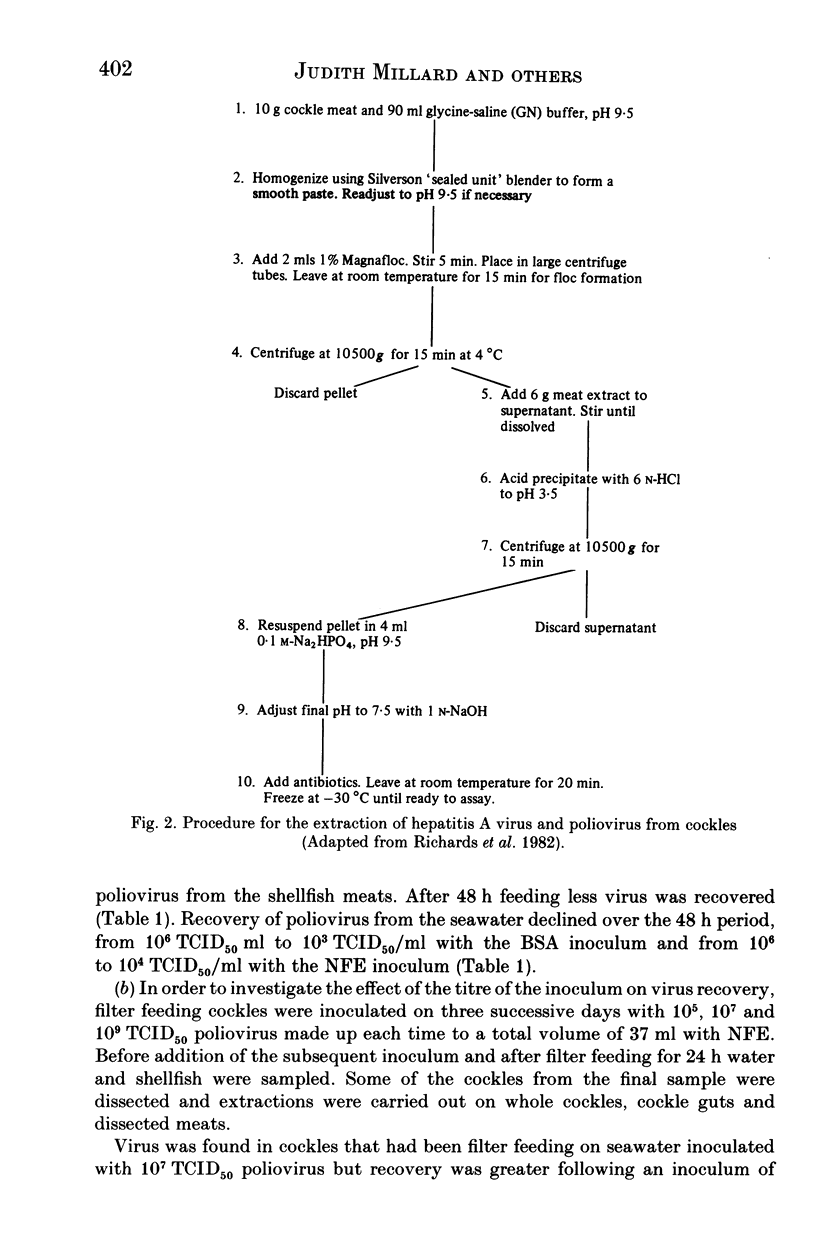
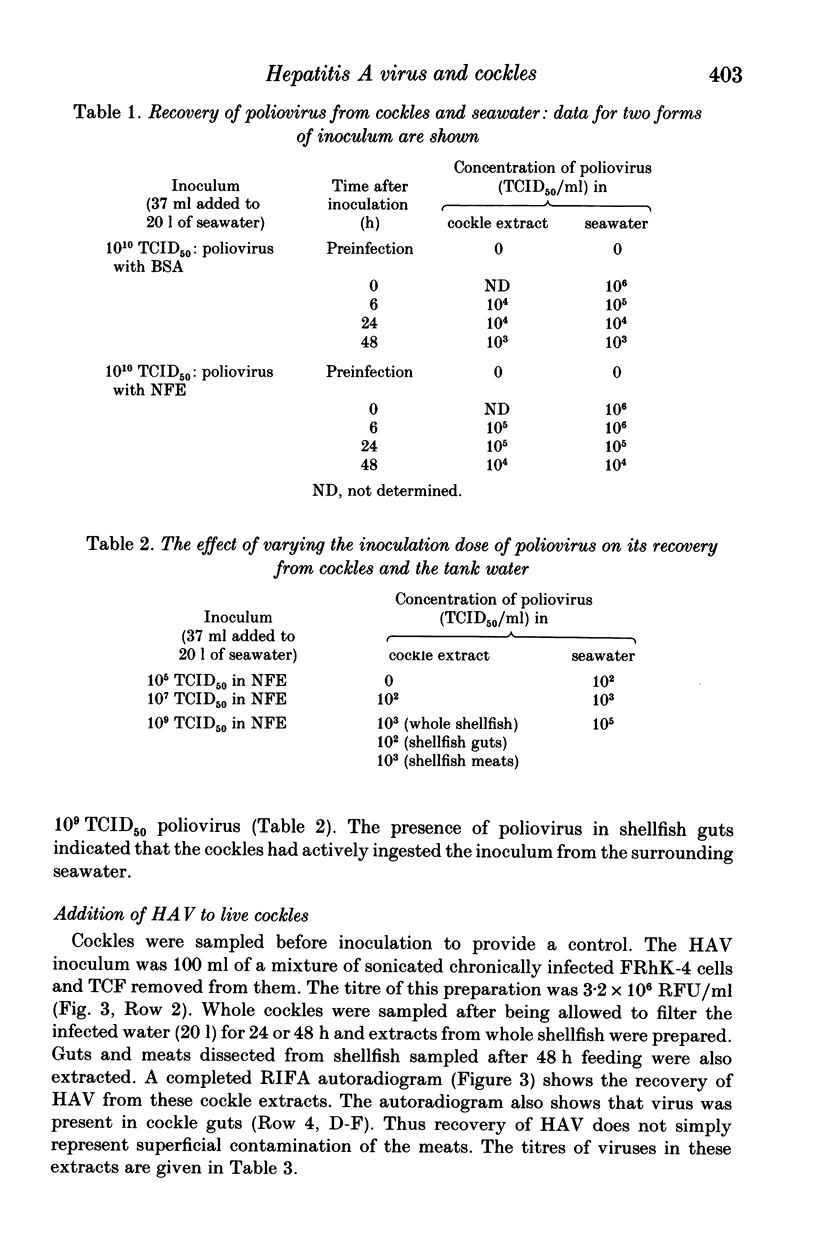
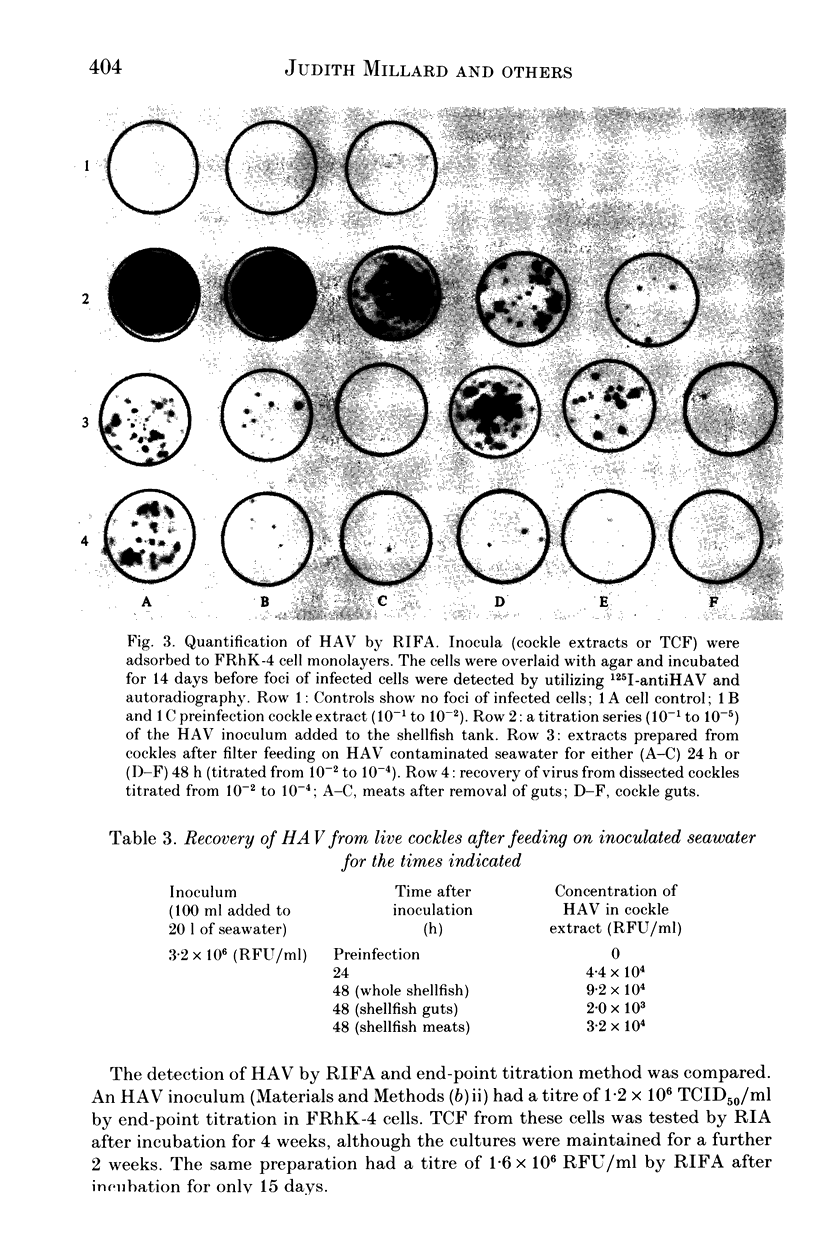






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleton H., Palmer S. R., Gilbert R. J. Foodborne gastroenteritis of unknown aetiology: a virus infection? Br Med J (Clin Res Ed) 1981 May 30;282(6278):1801–1802. [PMC free article] [PubMed] [Google Scholar]
- Appleton H., Pereira M. S. A possible virus aetiology in outbreaks of food-poisoning from cockles. Lancet. 1977 Apr 9;1(8015):780–781. doi: 10.1016/s0140-6736(77)92960-9. [DOI] [PubMed] [Google Scholar]
- Dahling D. R., Berg G., Berman D. BGM, a continuous cell line more sensitive than primary rhesus and African green kidney cells for the recovery of viruses from water. Health Lab Sci. 1974 Oct;11(4):275–282. [PubMed] [Google Scholar]
- Feingold A. O. Hepatitis from eating steamed clams. JAMA. 1973 Jul 30;225(5):526–527. doi: 10.1001/jama.1973.03220320054027. [DOI] [PubMed] [Google Scholar]
- Flehmig B. Hepatitis A-virus in cell culture: I. propagation of different hepatitis A-virus isolates in a fetal rhesus monkey kidney cell line (Frhk-4). Med Microbiol Immunol. 1980;168(4):239–248. doi: 10.1007/BF02121807. [DOI] [PubMed] [Google Scholar]
- Frösner G. G. Züchtung des Hepatitis-A-Virus in Gewebekultur: Möglichkeit zur Virusproduktion für Impfstoffe und Testzwecke, zur Untersuchung von Patienten auf Infektiosität und zur Prüfung von Desinfektionsmitteln. Offentl Gesundheitswes. 1982 Jun;44(6):370–373. [PubMed] [Google Scholar]
- Johnson K. M., Cooper R. C., Straube D. C. Procedure for recovery of enteroviruses from the Japanese cockle Tapes japonica. Appl Environ Microbiol. 1981 Apr;41(4):932–935. doi: 10.1128/aem.41.4.932-935.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPLAN A. S., MELNICK J. L. Effect of milk and cream on the thermal inactivation of human poliomyelitis virus. Am J Public Health Nations Health. 1952 May;42(5 Pt 1):525–534. doi: 10.2105/ajph.42.5_pt_1.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koff R. S., Sear H. S. Internal temperature of steamed clams. N Engl J Med. 1967 Mar 30;276(13):737–739. doi: 10.1056/NEJM196703302761307. [DOI] [PubMed] [Google Scholar]
- Lemon S. M., Binn L. N., Marchwicki R. H. Radioimmunofocus assay for quantitation of hepatitis A virus in cell cultures. J Clin Microbiol. 1983 May;17(5):834–839. doi: 10.1128/jcm.17.5.834-839.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luqman W. A., Matej L. A., Smith M. L. Comparison of prolactin levels in human semen and seminal plasma. J Endocrinol. 1979 Apr;81(1):131–133. doi: 10.1677/joe.0.0810131. [DOI] [PubMed] [Google Scholar]
- O'Mahony M. C., Gooch C. D., Smyth D. A., Thrussell A. J., Bartlett C. L., Noah N. D. Epidemic hepatitis A from cockles. Lancet. 1983 Mar 5;1(8323):518–520. doi: 10.1016/s0140-6736(83)92203-1. [DOI] [PubMed] [Google Scholar]
- Parry J. V., Mortimer P. P. The heat sensitivity of hepatitis A virus determined by a simple tissue culture method. J Med Virol. 1984;14(3):277–283. doi: 10.1002/jmv.1890140312. [DOI] [PubMed] [Google Scholar]
- Peterson D. A., Wolfe L. G., Larkin E. P., Deinhardt F. W. Thermal treatment and infectivity of hepatitis A virus in human feces. J Med Virol. 1978;2(3):201–206. doi: 10.1002/jmv.1890020303. [DOI] [PubMed] [Google Scholar]
- Portnoy B. L., Mackowiak P. A., Caraway C. T., Walker J. A., McKinley T. W., Klein C. A., Jr Oyster-associated hepatitis. Failure of shellfish certification programs to prevent outbreaks. JAMA. 1975 Sep 8;233(10):1065–1068. doi: 10.1001/jama.233.10.1065. [DOI] [PubMed] [Google Scholar]
- Provost P. J., Hilleman M. R. Propagation of human hepatitis A virus in cell culture in vitro. Proc Soc Exp Biol Med. 1979 Feb;160(2):213–221. doi: 10.3181/00379727-160-40422. [DOI] [PubMed] [Google Scholar]
- ROOS B. Hepatitepidemi, spridd genom ostron. Sven Lakartidn. 1956 Apr 20;53(16):989–1003. [PubMed] [Google Scholar]
- Richards G. P., Goldmintz D., Green D. L., Babinchak J. A. Rapid methods for extraction and concentration of poliovirus from oyster tissues. J Virol Methods. 1982 Dec;5(5-6):285–291. doi: 10.1016/0166-0934(82)90019-2. [DOI] [PubMed] [Google Scholar]
- Siegl G., Weitz M., Kronauer G. Stability of hepatitis A virus. Intervirology. 1984;22(4):218–226. doi: 10.1159/000149554. [DOI] [PubMed] [Google Scholar]
- Sobsey M. D., Carrick R. J., Jensen H. R. Improved methods for detecting enteric viruses in oysters. Appl Environ Microbiol. 1978 Jul;36(1):121–128. doi: 10.1128/aem.36.1.121-128.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]





