Abstract
Alanine-based peptides of defined sequence and length show measurable helix contents, allowing them to be used as a model system both for analyzing the mechanism of helix formation and for investigating the contributions of side-chain interactions to protein stability. Extensive characterization of many peptide sequences with varying amino acid contents indicates that the favorable helicity of alanine-based peptides can be attributed to the large helix-stabilizing propensity of alanine. Based on their analysis of alanine-rich sequences N-terminally linked to a synthetic helix-inducing template, Kemp and coworkers [Kemp, D. S., Boyd, J. G. & Muendel, C. C. (1991) Nature (London) 352, 451–454; Kemp, D. S., Oslick, S. L. & Allen, T. J. (1996) J. Am. Chem. Soc. 118, 4249–4255] argue that alanine is helix-indifferent, however, and that the favorable helix contents of alanine-based peptides must have some other explanation. Here, we show that the helix contents of template-nucleated sequences are influenced strongly by properties of the template–helix junction. A model in which the helix propensities of residues at the template–peptide junction are treated separately brings the results from alanine-based peptides and template-nucleated helices into agreement. The resulting model provides a physically plausible resolution of the discrepancies between the two systems and allows the helix contents of both template-nucleated and standard peptide helices to be predicted by using a single set of helix propensities. Helix formation in both standard peptides and template–peptide conjugates can be attributed to the large intrinsic helix-forming tendency of alanine.
The energetic cost of α-helix nucleation generally is considered to originate from the requirement of constraining the conformation of three consecutive amino acids before the first helical hydrogen bond can form (1). In contrast, helix propagation requires only one additional residue to be constrained for the formation of one additional hydrogen bond. The overall extent of helix formation depends on the energetics of both nucleation and propagation, as well as on chain length. To separate the effects of helix nucleation and propagation experimentally, Kemp and coworkers (2, 3) have designed a template (Ac-Hel1; see Fig. 1A) that overcomes the nucleation penalty for helix formation by providing hydrogen-bond acceptors for the otherwise unsatisfied NH groups at the N terminus of the helix. Short peptides attached to this template show significant helix formation that is nucleated preferentially from the template. Because the template efficiently nucleates helical segments, the macroscopic characteristics of the template-nucleated helix depend primarily on the nucleation properties of the template and the propagation propensities of the attached amino acid residues.
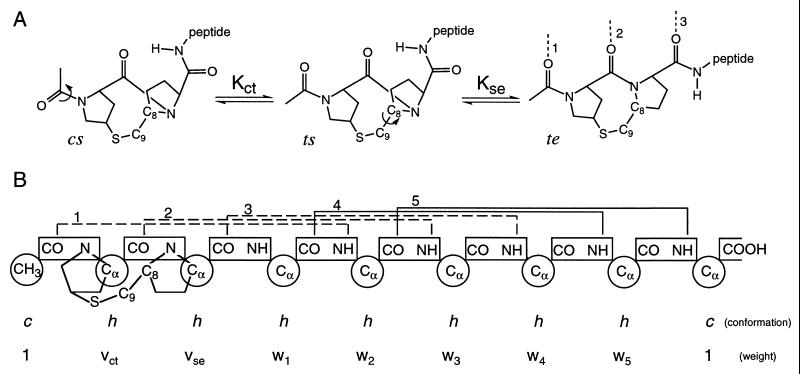
Figure 1.
(A) Schematic representation of the conformational transitions of the template required for helix nucleation. Cis–trans isomerization of the first peptide bond orients two carbonyl groups relative to one another, whereas rotation about the C8—C9 bond aligns the third carbonyl. In the te conformation, three carbonyl groups are aligned to accept helical hydrogen bonds, shown by dotted lines and numbered according to the propagating helical residue with which they are associated. (B) Diagram of unit conformations and weights assigned for the completely helical (te5) state of Ac-Hel1–A6-OH. Chain units are indicated by circles, and peptide bonds are shown in rectangles. The h and c conformations assigned to amino acid units indicate the helical and nonhelical conformations, respectively. Conformations assigned to template units represent the conformational state of the template (see Methods). Statistical weights are assigned based on unit conformations (see Methods and the Supplementary Material). i, i + 4 helical hydrogen bonds are indicated by brackets above the peptide bonds and numbered according to the propagating helical residue with which they are associated. Peptide–template hydrogen bonds are shown as dotted lines. Solid lines indicate intrapeptide hydrogen bonds.
Using the Ac-Hel1 template as both a nucleation site and a reporter of helix content, Kemp and coworkers (2, 4–6) have reported significant helix formation for a number of short alanine-based peptide sequences under a variety of conditions. Quite surprisingly, however, the authors conclude from their data that alanine is helix-indifferent with an equilibrium constant for propagation of approximately one (2, 4). In direct contrast, alanine is observed to be a strong helix former in a variety of standard peptide systems (7–9). To explain this paradox, Kemp and coworkers (2) have suggested that the helix content of standard alanine-based peptides might originate from an anomalously large nucleation propensity of alanine. A large nucleation propensity is inconsistent, however, with the extent of fraying observed in alanine-based peptides (10–12). Furthermore, to model their Ac-Hel1–peptide conjugate data, Kemp and coworkers (2, 4–6) assume that the nucleation propensity of alanine is 1–2 orders of magnitude smaller than that of the template, and the measured values of the nucleation propensity determined with standard peptide helices (8, 10) fall into this range. Kemp and coworkers (5, 6) have also suggested that the helix content of alanine–lysine peptides results from a large helix propensity of lysine. Substantial helix formation is observed, however, in alanine-based peptides solubilized with polar residues other than lysine (8, 13–16).
What then is the origin of differences between template-nucleated helices and standard alanine-based peptides? A basic assumption made by Kemp and coworkers is that residue properties, such as helix propensity, are not affected by the presence of the template. This assumption is likely to be valid for residues distant from the template, but the template–peptide junction may differ both structurally and energetically from an ideal peptide helix. Although explanations for the observed properties of peptide helices must apply equally to standard peptides and to the peptide portion of template–peptide conjugates, properties of the template–peptide junction affect only Ac-Hel1–peptide conjugates. Consequently, such junctional effects offer a possible explanation for differences between the two systems. Here, we test the hypothesis that the low apparent helix propensity of alanine determined from template-nucleated helices is a consequence of the properties of the template–peptide junction. A helix–coil model in which the helix propensities of the junctional residues are treated separately can account for the differences between helix propensities found in alanine-based peptides and Ac-Hel1–peptide conjugates, and the results indicate that helix formation in both standard peptides and template–peptide conjugates can be attributed to the favorable helix propensity of alanine.
METHODS
Properties of Ac-Hel1.
The reporting conformational template, Ac-Hel1, is comprised of two proline residues linked by an —S—CH2— bridge (refs. 2, 3, and references therein). In Ac-Hel1–peptide conjugates, three template conformational states are populated: cs, ts, and te. The c and t identifiers refer to the cis–trans isomerization about the prolyl–prolyl peptide bond, whereas the s and e identifiers indicate the staggered or partially eclipsed orientation of the C8—C9 bond (Fig. 1A). Isomerization of the prolyl–prolyl bond to the trans conformation orients two carbonyl groups to accept hydrogen bonds from the N terminus of an α-helix. Rotation of the C8—C9 bond to the partially eclipsed state positions the third carbonyl group to nucleate a helix. The te conformation, consequently, is the only state that efficiently nucleates helical segments, thereby linking helix formation to the conformational equilibria of the template detected by NMR.
The prolyl cis–trans isomerization is slow on the NMR time scale, and the staggered-eclipsed interconversion is in the fast-exchange regime, leading to two separate sets of NMR resonances for the template: one corresponding to the cs conformation, and the other to the te + ts state average. The relative te vs. ts character of the trans ensemble can be estimated qualitatively from analysis of chemical shifts and coupling constants, but the limiting values of these parameters have not been determined, precluding quantitative estimates of te and ts populations. The overall trans/cis ratio (t/c) of the template can be quantitated by integration of the NMR resonances, and t/c ratios taken from the literature are used here as a quantitative measure of helix formation. From a statistical analysis of measured t/c ratios of a set of 56 template-nucleated peptides, Kemp and coworkers (3) report that uncertainty in integration results in a mean standard deviation of 0.11 for t/c ratios in the range of 2.0–3.9, corresponding to an uncertainty of 2.8–5.5% in reported t/c values. For cases where uncertainties have not been reported, we arbitrarily assumed a 3% uncertainty in t/c ratios, the level resulting from a 1.5% uncertainty in NMR peak volume integration.
Lifson–Roig-Based Formalism.
The Lifson–Roig model (17), a statistical mechanical treatment of the helix–coil transition, describes peptide helix formation in terms of the chain length (Nr), a propagation propensity (w), and a nucleation penalty (v2). The nucleation penalty in the Lifson–Roig model arises because two residues, or three peptide-bond units, must be constrained to the helical conformation before the first helical hydrogen bond can form (17). Fixing the orientation of one pair of dihedral angles positions two carbonyl groups relative to one another, and a third carbonyl group is oriented properly when the second pair of dihedral angles is constrained. Nucleation in standard peptides and in Ac-Hel1–peptide conjugates is formally analogous, and standard helix–coil models can be adapted easily to describe helix formation in template-nucleated helices. To facilitate description of helix nucleation by the template, we have permuted the original Lifson–Roig notation so that both nucleating residues occur on the N-terminal side of a helical segment (18). With this change in convention, a residue, i, is defined as a nucleating residue and assigned weight vi if one or more of its two nearest N-terminal neighbors are in the c conformation. A propagating helical residue, i, whose two nearest N-terminal neighbors are both in the h conformation, is assigned the propagation propensity, wi, and it is associated with a hydrogen bond between the NH of residue i + 1 and the CO of residue i − 3 (Fig. 1B).
The template contains three hydrogen bond acceptors and consequently is described by three units in the Lifson–Roig-based formalism. The conformations accessible to the group of three units are restricted, however, such that only three combinations (ccc, chc, and chh) are allowed. In the case of the three template units, c and h indicate the conformational states available to the template rather than referring to the helical and nonhelical conformations. As in the standard Lifson–Roig model, the terminal unit is in the c state by definition. The conformation of the second unit reflects the cis–trans isomerization, whereas the conformation of the third unit represents the staggered-eclipsed transition. These allowed conformations of the template, consequently, correspond to the cs, ts, and te states, respectively. According to the permuted Lifson–Roig model, these states are assigned weights 1, vct, and vctvse, respectively, where the subscripts ct and se indicate that these statistical weights describe the cis–trans and staggered-eclipsed transitions of the template, rather than the helix–coil transition of an amino acid residue. Details of the implementation of the Lifson–Roig-based model are given in the Supplementary Material.
Application of the Model to Data.
Values for the Lifson–Roig nucleation and propagation parameters determined in standard alanine-based helices are used for residues in the Ac-Hel1–peptide conjugates (8). Helix propensities at 25°C were calculated from those reported at 0°C by assuming an enthalpy of −1 kcal/mol residue (refs. 19 and 20; 1 cal = 4.18 J). To test the hypothesis that differences between template-nucleated and standard peptide helices can be explained by deviations of the template–peptide junction from an ideal peptide helix, we have allowed the propensities of residues adjacent to the template to differ from these standard propensities. Because the overall t/c ratios to which the model is fitted are not sufficient to determine a more complex relation between helix propensity and separation from the template, we assume that the properties of residues i ≥ 4 are unaffected by the presence of the template, whereas properties of the first three residues, which form template–peptide helical hydrogen bonds, are all affected equally (see Discussion). Propagation propensities for residues associated with hydrogen bonds to the template (1 ≥ i ≥ 3) are assumed to be related to propensities measured in standard peptides by
 |
1 |
where the superscript indicates the standard propensity determined in peptide helices, and C describes the reduction in helix propensity of residues associated with hydrogen bonds to the template. The parameter C, as well as the template parameters vct and the product vctvse, were determined from fitting the helix–coil model to the observed t/c ratios using the program nonlin (21). Confidence intervals of 66% reported by nonlin are given for all fitted parameters. Data analysis was also performed by using the mass-action formalism described by Kemp and coworkers (refs. 2 and 4; see Supplementary Material). The fitted parameter values and the predicted t/c ratios obtained with the two models are identical within error, indicating that the results presented here are independent of whether the mass–action or Lifson–Roig-based formalism is used.
RESULTS
The nomenclature of Kemp and coworkers is used to refer to template–peptide conjugates (see, for example, ref. 4). The template name (Ac-Hel1) is followed by the sequence of attached amino acids in the standard one-letter code and the chemical structure of the C-terminal moiety. The nomenclature Ac-Hel1–An-OH, consequently, indicates a sequence of n alanines attached to the template. The C-terminal carboxyl group is unblocked, and the chain terminates with a free acid (-OH). The nomenclature Ac-Hel1–An-NH2 also indicates a sequence of n alanines attached to the template, but the C-terminal amino acid is carboxyamidated, and the chain terminates with an -NH2 moeity.
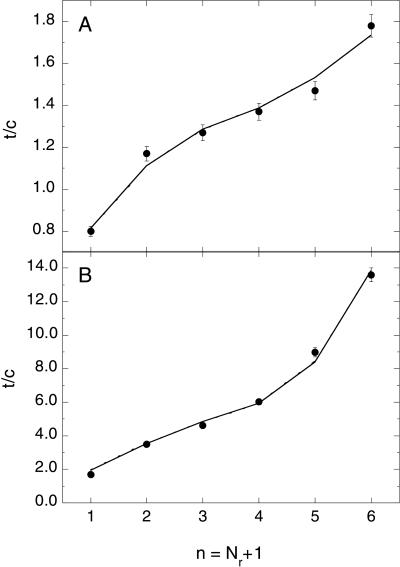
t/c ratios reported by Kemp and coworkers (4) for the series Ac-Hel1–An-OH (n = 1–6) in water and 10 mol% trifluoroethanol at 25°C are shown in Fig. 2, along with the best fits of the helix–coil model to the data. The data are well fitted by the model when the standard propensity of alanine determined in alanine-based peptides (wA = 1.46 at 25°C) is used for residues i ≥ 4. Residues associated with hydrogen bonds to the template are given the reduced propensity of CwA, where the best-fit value of C is 0.41 (range: 0.39–0.44), yielding wi = 0.60 for 1 ≥ i ≥ 3. The best-fit parameters describing the template are vct= 0.27 (range: 0.21–0.32) and vctvse = 0.55 (range: 0.35–0.76). An excellent fit is also obtained in 10 mol% trifluoroethanol where the helix propensity of alanine is increased significantly (wA = 2.31 at 25°C). The values and confidence intervals of the best-fit parameters are C = 0.38 (range: 0.36–0.41), vct = 0.006 (range: 0.0–0.7), and vctvse = 1.9 (range: 1.4–2.0).
Figure 2.
Length dependence of t/c for the series Ac-Hel1–An-OH (n = 1–6) at 25°C in 2H2O (A) or 10 mol% trifluoroethanol/2H2O (B). Data are taken from ref. 4. Error bars represent a 3% uncertainty in the experimentally determined t/c ratios. Lines correspond to the best fit of the Lifson–Roig-based model to the data. The number of residues, Nr, differs from the number of attached amino acids, n, because the final amino acid is not flanked C-terminally by a peptide bond (see the Supplementary Material).
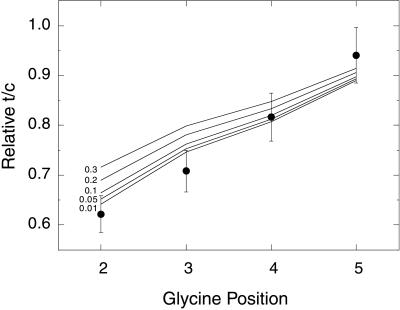
The parameters determined from fitting the data in Fig. 2 were then used to predict t/c ratios for other Ac-Hel1–peptide conjugates for which data have been reported in the literature. Fig. 3 shows relative t/c ratios measured for position isomers with composition Ac-Hel1–GA5-OH in which the position of the glycine residue is varied. The measured t/c ratios are reported relative to the t/c ratio of the all-alanine reference Ac-Hel1–A6-OH (2). The solid lines indicate the t/c values calculated for the glycine-containing Ac-Hel1–peptide conjugate relative to that calculated for the all-alanine reference. The different lines correspond to different values for the helix propensity of glycine. Other parameters in the calculations are set at the values determined from the analysis shown in Fig. 2A. The best agreement between experiment and theory is found for wG in the range 0.01–0.05.
Figure 3.
Relative t/c ratios for glycine position isomers. Data are taken from ref. 2 and are reported as the t/c ratio for each Ac-Hel1–GA5-OH position isomer divided by the t/c ratio of Ac-Hel1–A6-OH. Error bars indicate an uncertainty of 5.9% in relative t/c ratios, resulting from 3% uncertainty in the absolute t/c ratios. Lines correspond to the relative t/c ratios predicted by the Lifson–Roig-based model with template and nucleation parameters determined from fitting the Ac-Hel1–An-OH series in water and varying the helix propensity of glycine.
A similar comparison of observed and predicted t/c ratios for the peptide series Ac-Hel1–An-NH2 (n = 1–6) and Ac-Hel1–A5KAm-NH2 (m = 0–6) is shown in Fig. 4. Because the longer peptides contain only a single lysine at an invariant position, the predicted t/c ratios are fairly insensitive to the helix propensity of lysine. The solid line corresponds to the t/c values calculated by using a propensity for lysine of wK = 0.86, corresponding to the value determined by Rohl et al. (8), adjusted to 25°C as described above. Other parameters used are the best-fit values as determined for Fig. 2A. The predicted t/c values are in excellent agreement for the Ac-Hel1–An-NH2 (n = 1–6) series, as expected because this series differs from the series in Fig. 2A only by the addition of the C-terminal carboxamide moiety. The agreement between predicted and observed t/c values for the longer Ac-Hel1–A5KAm-NH2 series is significantly worse, and there is a roughly constant offset between the predicted and observed values. The predicted values do, however, reproduce the general curvature and magnitude of the observed t/c values as a function of chain length.
Figure 4.
Length dependence of t/c for the series Ac-Hel1–An-NH2 (n = 1–6; open circles) and Ac-Hel1–A5KAm-NH2 (m = 0–6; filled circles). Data are taken from ref. 5. Error bars represent a 3% uncertainty in the experimentally determined t/c ratios. The solid line corresponds to the t/c ratios predicted from the Lifson–Roig-based model by using the parameters determined from fitting the Ac-Hel1–An-OH series in water. The dotted line indicates the best fit to the Ac-Hel–A5KAm-NH2 series when the intrinsic template propensities are allowed to vary. The Inset shows the relative increase in the t/c ratio for Ac-Hel1–A5KAm-NH2 series members on addition of a single alanine residue (see Discussion).
Lysine shows complicated effects when placed in Ac-Hel1–peptide conjugates (refs. 5 and 6; see also Discussion), and, consequently, we considered the possibility that lysine may interact with the template. Such interactions would be reflected by a change in the parameters required to describe the conformational preferences of the template. The dotted line in Fig. 4 shows the best fit of the Lifson–Roig-based model to the Ac-Hel1–A5KAm-NH2 series. The values of the helix parameters are fixed to the values used in fitting the shorter peptide series (wK = 0.86; wA = 1.46; C = 0.41). The best-fit template parameters are vct = 1.2 (range: 0.7–1.6) and vctvse = 0.50 (range: 0.42–0.57). When the intrinsic propensities of the template are allowed to vary, the absolute magnitudes of t/c ratios of the Ac-Hel1–A5KAm-NH2 series are well predicted by the propagation propensities determined in standard peptide helices.
DISCUSSION
Helix Contents of Ac-Hel1–Peptide Conjugates Are Affected Strongly by Properties of the Peptide–Template Junction.
The helix propensity of an amino acid describes the equilibrium constant for adding that residue to a preexisting helix. In most helix–coil transition models, the minimal helix length is defined as three residues, the smallest unit capable of forming a single i, i + 4 hydrogen bond. For a homopolymer, the energetic cost of nucleation is attributed entirely to formation of this first hydrogen bond, and each additional hydrogen bond is assumed to form with identical helix propensity. The rationale behind this treatment is that, for each residue added to the minimal helix, one pair of dihedral angles must be fixed, and one helical hydrogen bond is formed. From an energetic perspective, however, formation of two or more helical hydrogen bonds may be required before all relevant van der Waals and dipole–dipole interactions are formed and the energetic contribution of each residue to helix formation becomes constant (for further discussion see ref. 22). Template-nucleated helices introduce further complications because the effect of the template on helix formation is not known. Consequently, it is unlikely that the energetic contributions to helix propagation in template-nucleated helices become constant after the formation of the first hydrogen bond, and the first residues added to the minimal helix are likely to have apparent helix propensities that differ from the true helix propensity.
When such junctional or end effects are ignored, and all residues are treated as having identical propensities, length-dependent errors are expected to occur and to become substantial for short peptides. In standard peptide helices, short helical segments are not populated significantly, and length-dependent helix propensities are not observed. In contrast, short helical segments are populated in template-nucleated helices, and the overall t/c ratios of Ac-Hel1–peptide conjugates are consequently highly sensitive to the helix propensities of residues at the template–peptide junction. In their analysis of the t/c ratios of Ac-Hel1–peptide conjugates, Kemp and coworkers (4) test for systematic variation in the value of the determined helix propensity of attached residues by determining average apparent helix propensities from t/c ratios of various subsets of peptides from the Ac-Hel1–An-OH series. Because average helix propensities are determined in the analysis of Kemp and coworkers (4), position-dependent effects are partially masked. Nevertheless, when t/c ratios from only the shortest peptides in the set are used (n = 1–4), a smaller average helix propensity is obtained than when only the longest peptides in the series are considered (n = 4–6) (see figure 4 in ref. 4). In addition, a significantly smaller average helix propensity is determined when the longest peptide in the series (n = 6) is excluded. These observations indicate that the average apparent helix propensity of alanine increases with peptide length, and they suggest that residues close to the template have lower propensities than those further from the template, consistent with the hypothesis that residues at the template–peptide junction have lower helix propensities than those distant from the template.
A more sensitive test of whether residues adjacent to the template have reduced helix propensities can be performed by examining the incremental increase in t/c ratio as a function of length. If all residues have identical propensities, then the t/c ratio is expected to increase in a monotonic fashion. The experimentally determined t/c ratios deviate, however, from such smooth curvature, showing a more complicated dependence on length. These deviations are small but are systematically reproduced both in water and trifluoroethanol. The Lifson–Roig-based model reproduces the complex shape of the length-dependence of the t/c ratio when the three residues associated with template–peptide hydrogen bonds are given reduced helix propensities (Fig. 2). Although this description of the physical nature of the nucleus is likely not exact, it significantly improves the agreement between theory and experiment and eliminates discrepancies between helix formation in Ac-Hel1–peptide conjugates and standard peptides. In addition to reproducing the t/c ratios of the Ac-Hel1–An-OH series, the parameters determined from fitting this series also predict the relative t/c ratios of the glycine position isomers (Fig. 3) and yield a glycine helix propensity of wG = 0.01–0.05, in excellent agreement with the helix propensity of glycine determined in alanine-based peptides (8, 23).
Lysine Interacts with the Ac-Hel1 Template.
Unlike glycine, lysine shows complex effects when incorporated into Ac-Hel1–peptide conjugates. When a single lysine residue is moved through an alanine-based Ac-Hel1–peptide conjugate, the t/c ratio first increases as the lysine is moved away from the template, passes through a maximum when lysine is located at position 5, and then decreases when lysine occurs in position 6 (5). This effect depends on the separation from the template rather than the local sequence context, suggesting that interactions between the lysine side chain and the template may contribute to the observed changes in t/c ratio. One possible explanation for the position-dependent effect is that lysine–template interactions occur in the helical state and increase the t/c ratio by increasing the extent of helix formation. On the basis of nuclear-Overhauser-effect evidence, Kemp and coworkers suggest that the lysine side chain interacts with carbonyl groups of residues spaced i, i − 3 and i, i − 4 (ref. 5; see also ref. 24). Lysine residues at positions 3 and 4, consequently, would interact with carbonyl groups on the Ac-Hel1 template, enhancing helix formation.
The parameters determined from fitting the Lifson–Roig-based model to the t/c ratios of the Ac-Hel1–A5KAm-NH2 series are consistent with an alternative model in which lysine interacts with the template in the nonhelical state and increases the t/c ratio of the template directly without stabilizing helical structure in the attached peptide. To predict quantitatively the t/c ratios of the lysine-containing template–peptide conjugates by using the standard helix propensities determined in alanine-based peptides, the intrinsic template propensities must be varied from those determined for the Ac-Hel1–An-NH2 series (Fig. 4). In particular, the fitted value of vct increases substantially when lysine is incorporated into Ac-Hel1–peptide conjugates, suggesting that lysine at position 6 selectively stabilizes the ts template conformation. In the absence of an independent measure of helix content, it is not known whether the changes in the t/c ratio observed when lysine is incorporated into Ac-Hel1–peptide conjugates reflect a change in helix content or only in the conformational state of the template. Consequently, the extent to which lysine stabilizes or destabilizes helix formation in template-nucleated helices cannot be determined. Further experimental work is required to determine at which positions lysine interacts with the template and whether such interactions stabilize helical or nonhelical conformations.
Alanine Is a Helix-Forming Residue.
Despite complications arising from lysine–template interactions, the t/c ratios of Ac-Hel1–A5KAm-NH2 series support the conclusion that alanine is a helix-forming residue. Although the exact magnitude of the predicted t/c ratio depends on the treatment of the template and template–peptide junction (see Fig. 4), the increase in t/c ratio resulting from the addition of a single residue should depend primarily on the helix propensity of the added residue. Regardless of the treatment of the helix nucleus, the helix propagation propensity of alanine determined in standard peptide helices accurately predicts the increase in t/c ratio with increasing chain length for the Ac-Hel1–A5KAm-NH2 series (Fig. 4 Inset). Differences between Ac-Hel1-nucleated and standard peptide helices are confined to the template–peptide junction. At large separations from the template, alanine residues have a substantially favorable propagation propensity that is indistinguishable from the value found in standard peptide helices.
If alanine were helix-neutral, lysine would have to be substantially helix-stabilizing to account for the observed helix contents of AK peptides. Kemp and coworkers (25) note that the helix contents of several peptides based on the (AAKAA) motif can be predicted almost equally well by attributing the helix-formation to alanine (wA = 1.7; wK = 1.0) or to lysine (wA = 1.07; wK = 3.7–5.0). In standard peptide helices, however, the propensity of alanine has been determined by using alanine-based sequences solubilized with differing mixtures of glutamine and lysine, allowing a unique set of propensities for A, K, and Q to be determined (8). In addition, data from standard peptides are generally consistent with the helix propensity of lysine being smaller than that of alanine, although measuring the exact helix propensity of lysine is complicated by potential side-chain interactions. Lysine is expected to interact electrostatically with both the partial charges of the helix backbone and with other charged side chains. Consequently, the apparent helix propensity of lysine will depend on context, position, and ionic concentration. Nevertheless, in a neutral AQ host, lysine-for-alanine substitutions are destabilizing at all positions examined over a wide range of salt concentrations (26). One recent study has attempted to measure the helix propensity of lysine in a polyalanine host devoid of any potential pairwise side-chain interactions (25). Analysis of the results in this study is complicated, however, by aggregation and by the use of ninhydrin and amino acid analysis for concentration determination, methods that are less precise and less accurate than tyrosine UV absorbance (13, 27).
Despite some uncertainty in the exact helix propensity of lysine, it is clear that alanine is a helix-forming residue. Substantial helix formation is observed for alanine-based peptides solubilized with a variety of polar residues, including lysine, glutamine, arginine, glutamate, aspartate, and histidine (8, 13–16). In addition, polyalanine sequences that contain charged residues only at the peptide termini also form helices (P. Luo and R.L.B., unpublished work and ref. 24). An ellipticity at 222 nm of −13,500 deg⋅cm2⋅dmol−1 at 0°C has been measured for the sequence Ac-KAAAAAAAAAKGGY-NH2, (P. Luo and R.L.B., unpublished work); this ellipticity corresponds to a helix content of ≈42% (8). With the propensities proposed by Kemp and coworkers (wA = 1.07; wK = 3.7–5.0; wG = 0.3; ref. 25), this peptide is predicted to be at most 11% helical. In direct contrast, propensities determined in alanine-based peptides (wA = 1.7; wK = 1.0; wG = 0.048; ref. 8) predict that this sequence should be ≈51% helical. Helix formation in this peptide cannot be explained by a high intrinsic propensity of lysine and must be attributed to the strong helix-forming tendency of alanine.
At least two effects contribute to the high helix propensity of alanine. The helix propensities of nonpolar amino acids are correlated linearly with side-chain entropy, indicating that alanine favors helix formation in part because no reduction in side-chain entropy is required on helix formation (28–30). In addition, water plays a role in determining the helix propensities of nonpolar amino acids (8). This effect can be explained in terms of solvent shielding: the small alanine side chain does not shield the backbone from solvent, allowing water to interact with the peptide carbonyl groups in a polyalanine helix (P. Luo and R.L.B., unpublished work). In contrast to alanine, charged and polar residues are generally helix-destabilizing. In a series of lysine homologs, a direct relation between the helix-destabilizing effect of a polar group and its proximity to the helix backbone is observed, suggesting that polar groups close to the peptide backbone interfere with helix formation (31).
The main conclusion from our analysis is that differences between alanine-based peptides and Ac-Hel1-nucleated helices can be explained by the properties of residues at the template–peptide junction. Helix contents of both peptide helices and template-nucleated helices can be predicted by the standard helix propensities if the residues at the template-peptide junction are treated separately from those distant from the template. The Ac-Hel1 template was developed to simplify the measurement of helix propensities by separating the contributions of helix nucleation and propagation, but the effect of the template on the helix structure is unknown. The energetic contributions to helix formation are probably not constant for the first few residues added to the template, and, because the template allows short helices to be significantly populated, the properties of the template–peptide junction strongly affect the macroscopic properties of Ac-Hel1–peptide conjugates. With increasing separation from the template, the helix propensities observed in standard peptides and in Ac-Hel1–peptide conjugates are indistinguishable, however, and are consistent with a substantial helix-forming tendency of alanine.
Supplementary Material
Acknowledgments
We thank N. R. Kallenbach for sharing a preprint copy of ref. 24. This work was supported by National Institutes of Health Grant GM19988 (to R.L.B.). C.A.R. is a fellow of the Cancer Research Fund of the Damon Runyon–Walter Winchell Foundation (Fellowship DRG-1405). Supplementary materials are available at the PNAS website.
Note Added in Proof
A paper has appeared recently in which an EF hand is used as a template for the growth of four additional helical residues (32). The authors point out that the quality of the template that nucleates the helix should be critical in determining the helix content of the added residues. In contrast to the results from Kemp’s laboratory discussed above, the helix content of residues added to the EF-hand template is quite high. In fact, the observed helix content is higher than the value predicted from the helix propensities measured in alanine-based peptides when the helix nucleus is treated as being completely formed. Circular dichroism is used to determine the fraction helix, and there is some ambiguity in converting ellipticity to helix content for a helical segment as short as four residues; this issue can be resolved by future studies of peptides with longer helical segments.
References
- 1.Schellman J A. C R Trav Lab Carlsberg. 1955;29:230–259. [PubMed] [Google Scholar]
- 2.Kemp D S, Boyd J G, Muendel C C. Nature (London) 1991;352:451–454. doi: 10.1038/352451a0. [DOI] [PubMed] [Google Scholar]
- 3.Kemp D S, Allen T J, Oslick S L. J Am Chem Soc. 1995;117:6641–6657. [Google Scholar]
- 4.Kemp D S, Oslick S L, Allen T J. J Am Chem Soc. 1996;118:4249–4255. [Google Scholar]
- 5.Groebke K, Renold P, Tsang K Y, Allen T J, McClure K F, Kemp D S. Proc Natl Acad Sci USA. 1996;93:4025–4029. doi: 10.1073/pnas.93.9.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Renold P, Tsang K Y, Shimizu L S, Kemp D S. J Am Chem Soc. 1996;118:12234–12235. [Google Scholar]
- 7.Park S H, Shalongo W, Stellwagen E. Biochemistry. 1993;32:7048–7053. doi: 10.1021/bi00078a033. [DOI] [PubMed] [Google Scholar]
- 8.Rohl C A, Chakrabartty A, Baldwin R L. Protein Sci. 1996;5:2623–2637. doi: 10.1002/pro.5560051225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Spek E J, Gong Y, Zhou H, Kallenbach N R. Protein Sci. 1996;6:1264–1272. doi: 10.1002/pro.5560060614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohl C A, Scholtz J M, York E J, Stewart J M, Baldwin R L. Biochemistry. 1992;31:1263–1269. doi: 10.1021/bi00120a001. [DOI] [PubMed] [Google Scholar]
- 11.Rohl C A, Baldwin R L. Biochemistry. 1994;33:7760–7767. doi: 10.1021/bi00191a003. [DOI] [PubMed] [Google Scholar]
- 12.Shalongo W, Dugad L, Stellwagen E. J Am Chem Soc. 1994;116:8288–8293. [Google Scholar]
- 13.Marqusee S, Baldwin R L. Proc Natl Acad Sci USA. 1987;84:8898–8902. doi: 10.1073/pnas.84.24.8898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scholtz J M, York E J, Stewart J M, Baldwin R L. J Am Chem Soc. 1991;113:5102–5104. [Google Scholar]
- 15.Huyghues-Despointes B M P, Scholtz J M, Baldwin R L. Protein Sci. 1993;2:80–85. doi: 10.1002/pro.5560020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huyghues-Despointes B M P, Baldwin R L. Biochemistry. 1997;36:1965–1970. doi: 10.1021/bi962546x. [DOI] [PubMed] [Google Scholar]
- 17.Lifson S, Roig A. J Chem Phys. 1961;34:1963–1974. [Google Scholar]
- 18.Qian H, Schellman J A. J Phys Chem. 1992;96:3987–3994. [Google Scholar]
- 19.Scholtz J M, Marqusee S, Baldwin R L, York E J, Stewart J M, Santoro M, Bolen D W. Proc Natl Acad Sci USA. 1991;88:2854–2858. doi: 10.1073/pnas.88.7.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholtz J M, Qian H, York E J, Stewart J M, Baldwin R L. Biopolymers. 1991;31:1463–1470. doi: 10.1002/bip.360311304. [DOI] [PubMed] [Google Scholar]
- 21.Johnson M L, Correia J J, Yphantis D A, Halvorson H R. Biophys J. 1981;36:575–588. doi: 10.1016/S0006-3495(81)84753-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muñoz V, Serrano L. Biopolymers. 1997;41:495–507. doi: 10.1002/(SICI)1097-0282(19970415)41:5<495::AID-BIP2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Chakrabartty A, Schellman J A, Baldwin R L. Nature (London) 1991;351:586–588. doi: 10.1038/351586a0. [DOI] [PubMed] [Google Scholar]
- 24.Spek, E. J., Olson, C. A. & Kallenbach, N. R. (1999) J. Am. Chem. Soc., in press.
- 25.Williams L, Kather K, Kemp D S. J Am Chem Soc. 1998;120:11033–11043. [Google Scholar]
- 26.Scholtz J M, Qian H, Robbins V H, Baldwin R L. Biochemistry. 1993;32:9668–9676. doi: 10.1021/bi00088a019. [DOI] [PubMed] [Google Scholar]
- 27.Chakrabartty A, Kortemme T, Padmanabhan S, Baldwin R L. Biochemistry. 1993;32:5560–5565. doi: 10.1021/bi00072a010. [DOI] [PubMed] [Google Scholar]
- 28.Creamer T P, Rose G D. Proteins. 1994;19:85–97. doi: 10.1002/prot.340190202. [DOI] [PubMed] [Google Scholar]
- 29.Blaber M, Zhang X J, Matthews B W. Science. 1993;260:1637–1640. doi: 10.1126/science.8503008. [DOI] [PubMed] [Google Scholar]
- 30.Lee K H, Xie D, Freire E, Amzel L M. Proteins. 1994;20:68–84. doi: 10.1002/prot.340200108. [DOI] [PubMed] [Google Scholar]
- 31.Padmanabhan S, York E J, Stewart J M, Baldwin R L. J Mol Biol. 1996;257:726–734. doi: 10.1006/jmbi.1996.0197. [DOI] [PubMed] [Google Scholar]
- 32.Siedlecka M, Goch G, Ejchart A, Sticht H, Bierzyǹski A. Proc Natl Acad Sci USA. 1999;96:903–908. doi: 10.1073/pnas.96.3.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.