Abstract
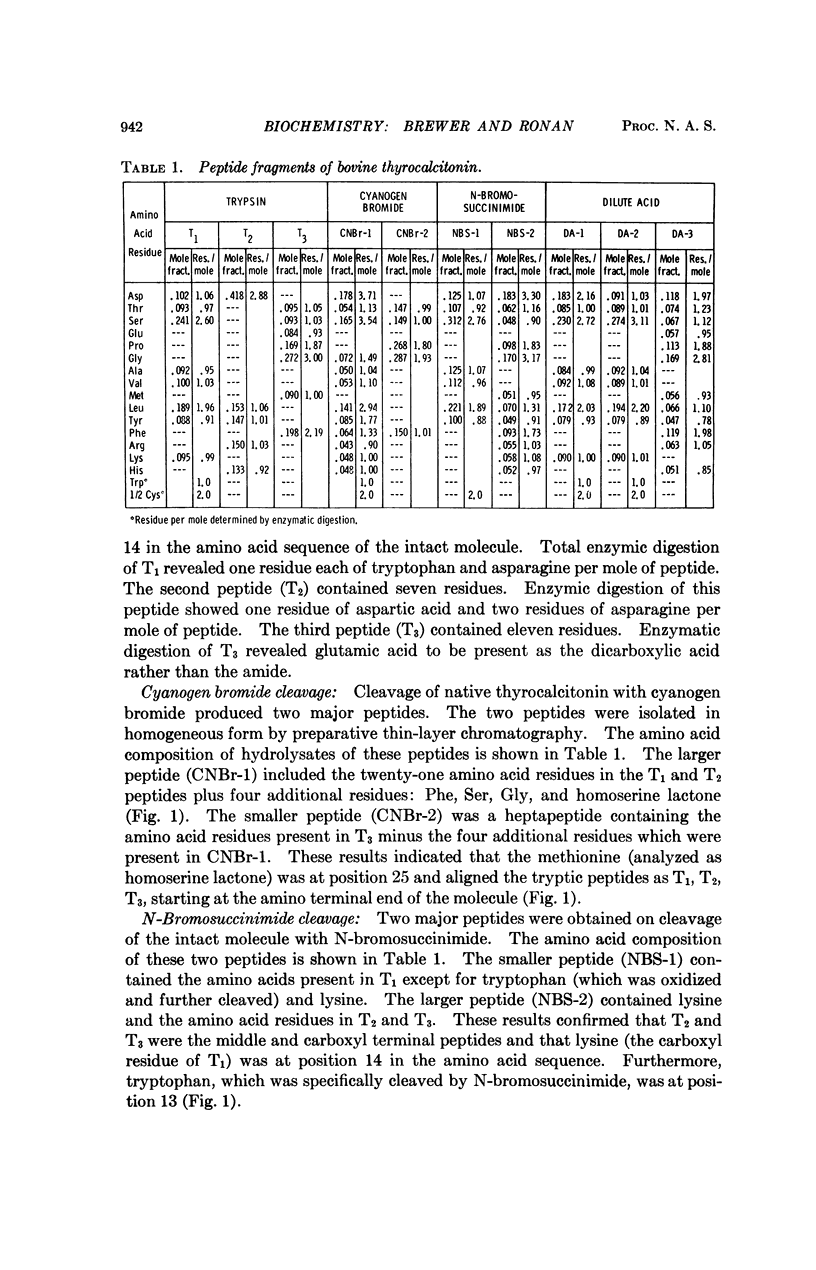
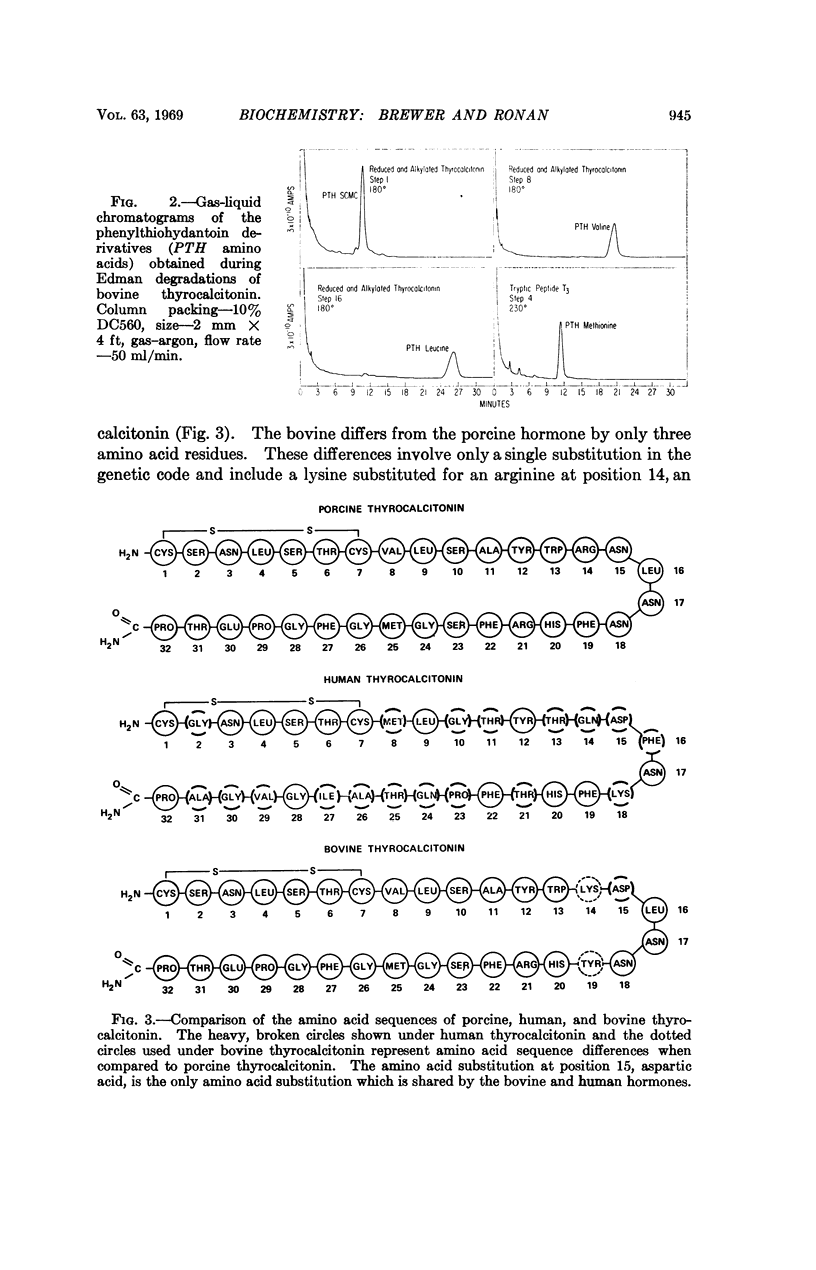
Bovine thyrocalcitonin has been isolated in homogeneous form, and its complete amino acid sequence determined. The bovine hormone is a singlechain 32 amino acid polypeptide. It contains a 1-7 amino terminal disulfide bridge, and the carboxyl terminal amino acid is prolinamide. The bovine thyrocalcitonin sequence differs from the porcine sequence by three amino acid residues and from the human sequence by 18 amino acid residues.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell P. H., Barg W. F., Jr, Colucci D. F., Davis M. C., Dziobkowski C., Englert M. E., Heyder E., Paul R., Snedeker E. H. Purification and structure of porcine calcitonin-1. J Am Chem Soc. 1968 May 8;90(10):2704–2706. doi: 10.1021/ja01012a050. [DOI] [PubMed] [Google Scholar]
- Benisek W. F., Cole R. D. Sodium/liquid ammonia reduction of proline-containing peptides. Biochem Biophys Res Commun. 1965 Sep 8;20(5):655–660. doi: 10.1016/0006-291x(65)90451-1. [DOI] [PubMed] [Google Scholar]
- Benson J. V., Jr, Jones R. T., Cormick J., Patterson J. A. Accelerated automatic chromatographic analysis of peptides on a spherical resin. Anal Biochem. 1966 Jul;16(1):91–106. doi: 10.1016/0003-2697(66)90084-4. [DOI] [PubMed] [Google Scholar]
- Blombäck B., Blombäck M., Edman P., Hessel B. Human fibrinopeptides. Isolation, characterization and structure. Biochim Biophys Acta. 1966 Feb 28;115(2):371–396. doi: 10.1016/0304-4165(66)90437-5. [DOI] [PubMed] [Google Scholar]
- Brewer H. B., Jr, Keutmann H. T., Potts J. T., Jr, Reisfeld R. A., Schlueter R., Munson P. L. Isolation and chemical properties of porcine thyrocalcitonin. J Biol Chem. 1968 Nov 10;243(21):5739–5747. [PubMed] [Google Scholar]
- Edman P., Begg G. A protein sequenator. Eur J Biochem. 1967 Mar;1(1):80–91. doi: 10.1007/978-3-662-25813-2_14. [DOI] [PubMed] [Google Scholar]
- Kahnt F. W., Riniker B., MacIntyre I., Neher R. Thyrocalcitonin. I. Isolierung und Charakterisierung wirksamer Peptide aus Schweineschilddrüsen. Helv Chim Acta. 1968;51(1):214–217. doi: 10.1002/hlca.19680510125. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Maier R., Byfield P. G., Gudmundsson T. V., MacIntyre I. Human calcitonin. Nature. 1968 Dec 7;220(5171):984–986. doi: 10.1038/220984a0. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Rittel W., Zuber H. Menschliches Calcitonin. 3. Struktur von Calcitonin M un. Helv Chim Acta. 1968;51(8):1900–1905. doi: 10.1002/hlca.19680510811. [DOI] [PubMed] [Google Scholar]
- Neher R., Riniker B., Zuber H., Rittel W., Kahnt F. W. Thyrocalcitonin. II. Struktur von alpha-Thyrocalcitonin. Helv Chim Acta. 1968 May 31;51(4):917–924. doi: 10.1002/hlca.660510430. [DOI] [PubMed] [Google Scholar]
- Potts J. T., Jr, Niall H. D., Keutmann H. T., Brewer H. B., Jr, Deftos L. J. The amino acid sequence of porcine thyrocalcitonin. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1321–1328. doi: 10.1073/pnas.59.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putter I., Kaczka E. A., Harman R. E., Rickes E. L., Kempf A. J., Chaiet L., Rothrock J. W., Wase A. W., Wolf F. J. The isolation and properties of thyrocalcitonin. J Am Chem Soc. 1967 Sep 27;89(20):5301–5302. [PubMed] [Google Scholar]
- Smyth D. S., Utsumi S. Structure at the hinge region in rabbit immunoglobulin-G. Nature. 1967 Oct 28;216(5113):332–335. doi: 10.1038/216332a0. [DOI] [PubMed] [Google Scholar]



