Abstract
A large number of hormones, of diverse molecular structure, evoke characteristic responses in target cells via the intermediary 3′,5′-AMP, the specificity of hormone action upon cell type being achieved by selective stimulation of adenyl cyclase. In the fat cells of rat adipose tissue, adenyl cyclase is stimulated by a number of hormones of disparate molecular structure, posing the question whether this cell type posesses multiple cyclase systems with distinctive specificities for individual hormones, or a single cyclase with broad specificity to a variety of hormones.
Studies of the stimulatory effects of adenocorticotropin, glucagon, and epinephrine upon the adenyl cyclase of the rat fat cell “ghosts” (plasma membrane sacs) have shown that distinctive selectivity sites for each of these hormones can be differentiated. The β-adrenergic blocking agent Kö 592 abolished the stimulatory effect of epinephrine without influencing adenocorticotropin or glucagon; Ca was required for adenocorticotropin action, but not for glucagon or epinephrine. Dose-response curves show that the affinity of hormones to the cyclase system was in the order: glucagon > adenocorticotropin ≫ epinephrine; the magnitude of cyclase activation by maximal doses of hormones had a reversed order. Combinations of maximal doses of hormones failed to produce additive stimulation. The results show that in the membrane of the fat cell a single catalytic unit of adenyl cyclase is coupled to distinctive selectivity sites for three lipolytic hormones.
Full text
PDF

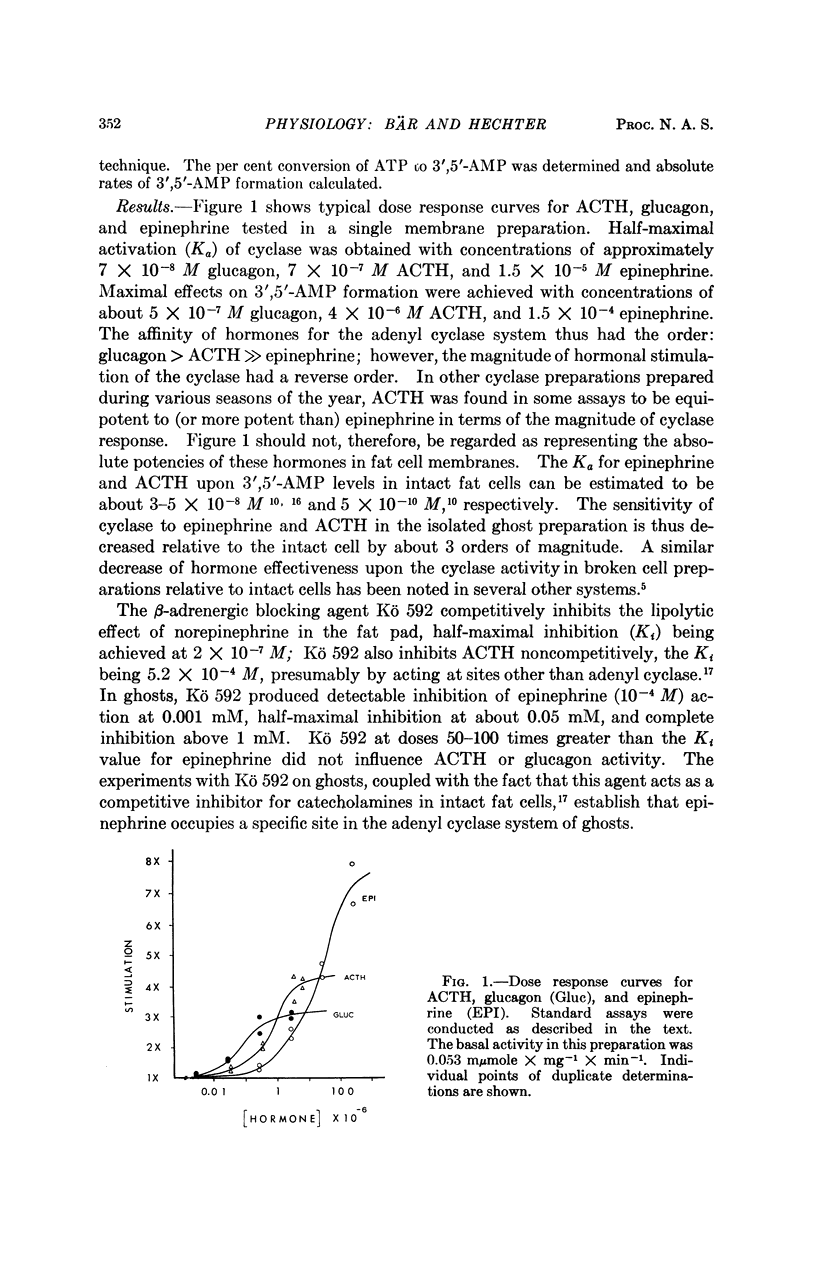
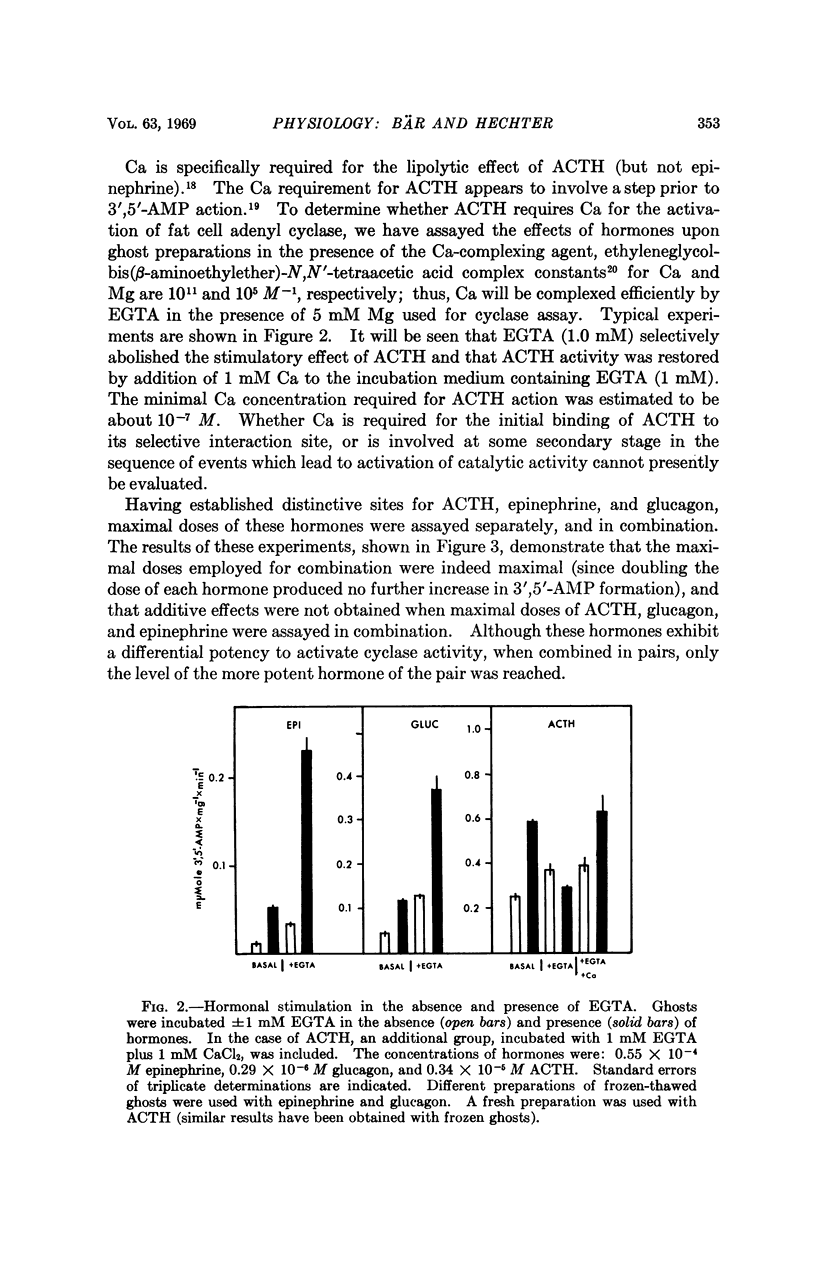
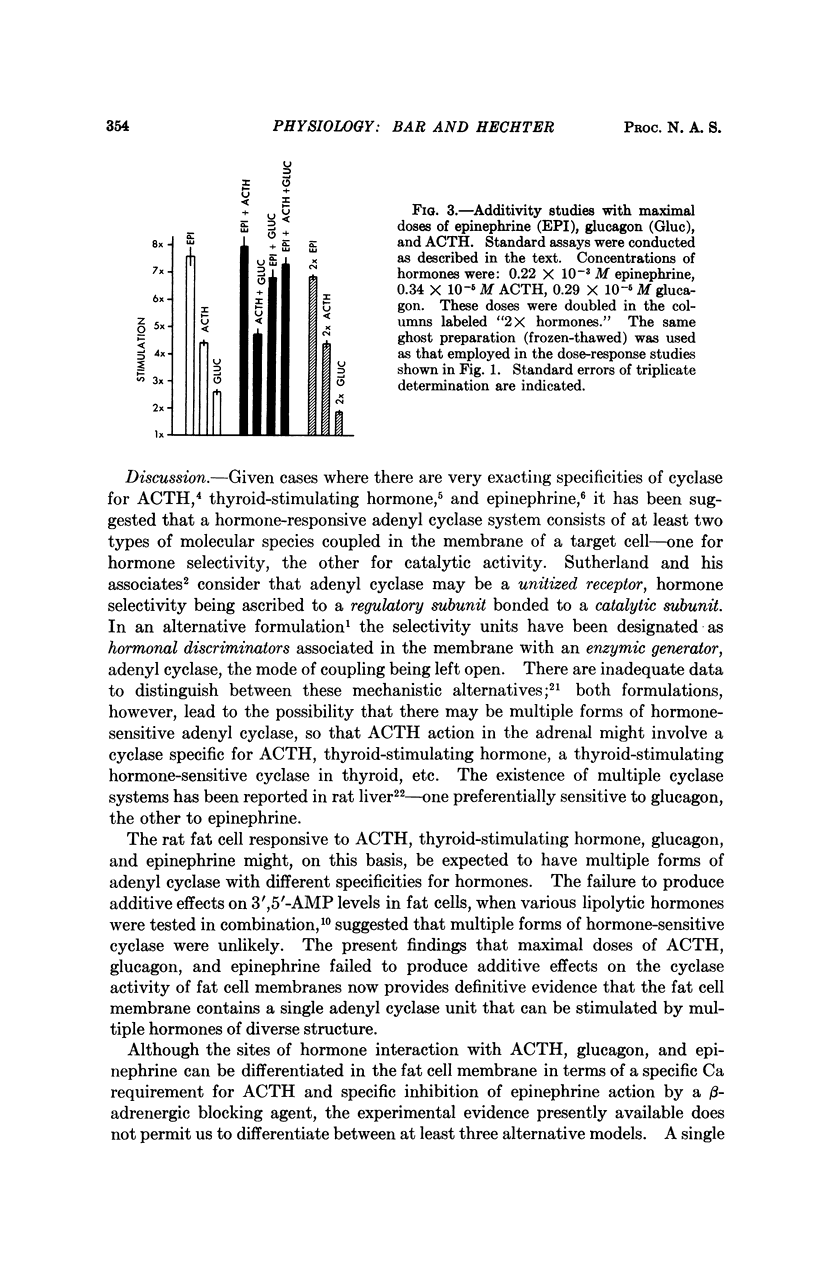


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bitensky M. W., Russell V., Robertson W. Evidence for separate epinephrine and glucagon responsive adenyl cyclase systems in rat liver. Biochem Biophys Res Commun. 1968 Jun 10;31(5):706–712. doi: 10.1016/0006-291x(68)90619-0. [DOI] [PubMed] [Google Scholar]
- Butcher R. W., Baird C. E., Sutherland E. W. Effects of lipolytic and antilipolytic substances on adenosine 3',5'-monophosphate levels in isolated fat cells. J Biol Chem. 1968 Apr 25;243(8):1705–1712. [PubMed] [Google Scholar]
- Chen R. F. Removal of fatty acids from serum albumin by charcoal treatment. J Biol Chem. 1967 Jan 25;242(2):173–181. [PubMed] [Google Scholar]
- Cunningham E. B. The enhancement of phosphoryl transfer by adenosine 3',5'-monophosphate in the presence of a membranous fraction from canine kidney. Biochim Biophys Acta. 1968 Oct 15;165(3):574–577. doi: 10.1016/0304-4165(68)90248-1. [DOI] [PubMed] [Google Scholar]
- DAVOREN P. R., SUTHERLAND E. W. THE EFFECT OF L-EPINEPHRINE AND OTHER AGENTS ON THE SYNTHESIS AND RELEASE OF ADENOSINE 3',5'-PHOSPHATE BY WHOLE PIGEON ERYTHROCYTES. J Biol Chem. 1963 Sep;238:3009–3015. [PubMed] [Google Scholar]
- Gilman A. G., Rall T. W. Factors influencing adenosine 3',5'-phosphate accumulation in bovine thyroid slices. J Biol Chem. 1968 Nov 25;243(22):5867–5871. [PubMed] [Google Scholar]
- LOPEZ E., WHITE J. E., ENGEL F. L. Contrasting requirements for the lipolytic action of corticotropin and epinephrine on adipose tissue in vitro. J Biol Chem. 1959 Sep;234:2254–2258. [PubMed] [Google Scholar]
- Langan T. A. Histone phosphorylation: stimulation by adenosine 3',5'-monophosphate. Science. 1968 Nov 1;162(3853):579–580. doi: 10.1126/science.162.3853.579. [DOI] [PubMed] [Google Scholar]
- MAKMAN M. H., SUTHERLAND E. W., Jr USE OF LIVER ADENYL CYCLASE FOR ASSAY OF GLUCAGON IN HUMAN GASTRO-INTESTINAL TRACT AND PANCREAS. Endocrinology. 1964 Jul;75:127–134. doi: 10.1210/endo-75-1-127. [DOI] [PubMed] [Google Scholar]
- Mosinger B., Vaughan M. Effects of electrolytes on epinephrine stimulated lipolysis in adipose tissue in vitro. Biochim Biophys Acta. 1967 Dec 5;144(3):556–568. doi: 10.1016/0005-2760(67)90045-8. [DOI] [PubMed] [Google Scholar]
- Oye I., Sutherland E. W. The effect of epinephrine and other agents on adenyl cyclase in the cell membrane of avian erythrocytes. Biochim Biophys Acta. 1966 Oct 31;127(2):347–354. doi: 10.1016/0304-4165(66)90389-8. [DOI] [PubMed] [Google Scholar]
- Pastan I., Katzen R. Activation of adenyl cyclase in thyroid homogenates by thyroid-stimulating hormone. Biochem Biophys Res Commun. 1967 Dec 29;29(6):792–798. doi: 10.1016/0006-291x(67)90289-6. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Adenyl cyclase as an adrenergic receptor. Ann N Y Acad Sci. 1967 Feb 10;139(3):703–723. doi: 10.1111/j.1749-6632.1967.tb41239.x. [DOI] [PubMed] [Google Scholar]
- Robison G. A., Butcher R. W., Sutherland E. W. Cyclic AMP. Annu Rev Biochem. 1968;37:149–174. doi: 10.1146/annurev.bi.37.070168.001053. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- Rosen O. M., Rosen S. M. The effect of catecholamines on the adenyl cyclase of frog and tadpole hemolysates. Biochem Biophys Res Commun. 1968 Apr 5;31(1):82–91. doi: 10.1016/0006-291x(68)90034-x. [DOI] [PubMed] [Google Scholar]
- Sutherland E. W., Robison G. A. The role of cyclic-3',5'-AMP in responses to catecholamines and other hormones. Pharmacol Rev. 1966 Mar;18(1):145–161. [PubMed] [Google Scholar]
- Taunton O. D., Roth J., Pastan I. ACTH stimulation of adenyl cyclase in adrenal homogenates. Biochem Biophys Res Commun. 1967 Oct 11;29(1):1–7. doi: 10.1016/0006-291x(67)90531-1. [DOI] [PubMed] [Google Scholar]
- Walsh D. A., Perkins J. P., Krebs E. G. An adenosine 3',5'-monophosphate-dependant protein kinase from rabbit skeletal muscle. J Biol Chem. 1968 Jul 10;243(13):3763–3765. [PubMed] [Google Scholar]
- Williams R. H., Walsh S. A., Hepp D. K., Ensinck J. W. Method for measuring hormonal effects on conversion of adenosine triphosphate to adenosine 3', 5'-monophosphate by isolated lipocytes. Metabolism. 1968 Aug;17(8):653–668. doi: 10.1016/0026-0495(68)90050-4. [DOI] [PubMed] [Google Scholar]



