Abstract
Thyroid hormone (TH) levels decline after a myocardial infarction. Treatment with TH has been shown to improve left ventricular (LV) function in myocardial infarction (MI) and other cardiovascular diseases, but the mechanisms are not clear. We have previously shown that TH can prevent myocyte apoptosis via Akt signaling in cultured neonatal rat cardiomyocytes. In this study, the effects of triiodo-L-thyronine (T3) on LV function and myocyte apoptosis after MI was examined in rats. After surgery, MI rats were treated with T3 for 3 days. Compared with sham-operated rats, MI rats showed significantly increased LV chamber dimension during systole and decreased LV function. T3 treatment increased LV ±dP/dt but did not change LV chamber dimensions. MI rats also showed significantly increased myocyte apoptosis in the border area as assessed by DNA laddering and TUNEL assay. T3 treatment decreased the amount of DNA laddering, and reduced TUNEL positive myocytes in the border area, which was associated with phosphorylation of Akt at serine 473. These results suggest that T3 can protect myocytes against ischemia induced apoptosis, which may be mediated by Akt signaling.
Keywords: thyroid hormone, myocardial infarction, cardiomyocyte, apoptosis, Akt
1. INTRODUCTION
It is well known that myocyte apoptosis is an important event after acute myocardial infarction (MI ) and may be responsible for a significant portion of myocyte death during the acute ischemic stage, as well as progressive loss of surviving myocytes during the subacute and chronic stages[1, 2]. In addition to the infarcted area, profound ongoing myocyte apoptosis has been demonstrated in the border area after MI[1, 2, 3, 4]. Inhibition of myocyte apoptosis after MI may improve left ventricular (LV) remodeling and cardiac function[5].
Thyroid hormones (TH) are known to have multiple effects on the cardiovascular system. Both hyper- and hypothyroidism are associated with cardiac disorders. Correspondingly, severe heart diseases including MI are often accompanied by altered metabolism of TH, characterized by low serum T3, T4, FT3 and FT4 levels, high serum rT3 levels, and normal or low serum TSH levels. These changes have been demonstrated in both animal and clinical studies[6, 7, 8, 9, 10, 11]. It is not known if reduced TH activity after MI is beneficial or detrimental. Some studies indicated that supplementary treatment with TH can improve LV function after MI and may be beneficial for prognosis[10, 12].
We have previously shown that triiodo-L-thyronine (T3) treatment can protect serum starvation-induced neonatal cardiomyocytes apoptosis via Akt signaling[13]. We have also shown that L-thyroxine (T4) can activate Akt signaling in the heart[14]. Whether TH have the same potentially beneficial effect on adult myocardial cells in vivo is unknown. Therefore, we established a myocardial infarction model by coronary ligation in adult female Sprague-Dawley rats and treated the animals with 3,5,3′-triiodothyronine (T3) after surgery for 3 days. The purpose of the study was to confirm whether or not T3 treatment post-MI leads to reduced apoptosis in the border area.
2. MATERIALS AND METHODS
2.1. Experimental design
Myocardial infarction was produced by ligation of the left descending coronary artery of adult female Sprague-Dawley rats weighing between 220 and 290g using a technique described in detail elsewhere[15]. Immediately following this procedure, survivors were randomly assigned to the following groups: (1) MI+T3 group (n=12); (2) MI group (n=10); and (3) Sham-operated group (n=11). Shams were produced with a similar procedure except the suture was tied loosely around the coronary artery. T3 (14ng/g body weight, daily. Sigma, St. Louis, MO) was administrated to the MI+T3 animals by intraperitoneal injection (IP) shortly after surgery and continued for 3 days. A similar amount of sterilized water was given to the MI and sham-operated animals over the same time period. Animals were housed two per cage and kept on a 12h light/dark cycle with food and water provided ad libitum. At termination, cardiac function was assessed by echocardiography and LV catheterization for each animal in the study. All experiments and protocols were performed in accordance with the Guide for the Care and Use of Laboratory Animals (US Department of Health, Education, and Welfare, Department of Health and Human Services, NIH Publication 85-23), and approved by the University of South Dakota Animal Care and Use Committee.
2.2. Echocardiographic measurements
Echocardiography was performed in each animal before it was killed using a Visualsonics 660 imaging system (with a 20 MHz transducer) as described before[16]. Briefly, Rats were anesthetized using isoflurane (1.5%). Two dimensional echocardiograms were obtained from short-axis views of the left ventricle at the level of the papillary muscle tips. Two-dimensionally targeted M-mode echocardiograms were used to measure the LV dimensions in systole and diastole.
2.3. Cardiac hemodynamic measurements
LV hemodynamics were obtained by catheterization of the right carotid artery using a Millar Micro-tip catheter (Millar Instruments; Houston, TX) as described before[17]. Data were recorded using a Digi-Med Heart Performance Analyzer system (model HPA 410a; MicroMed; Louisville, KY). The following data were collected: heart rate (HR), LV peak systolic pressure (LVSP), LV end-diastolic pressure (LVEDP), and positive/negative change in pressure over time (dP/dt).
2.4. Tissue collection
The rats in each group were separated into two groups, one for Western blotting assay and DNA laddering, and the other for TUNEL study. After hemodynamic data were collected, hearts were arrested in diastole by an intravenous injection of saturated potassium chloride solution via the left jugular vein.
For the first group, the chest was opened, the hearts quickly removed and immediately placed in ice-cold PBS. Hearts were cannulated with an 18 gauge gavage needle through the aorta, perfused with ice cold PBS, and subsequently trimmed, blotted, and weighed. The LV plus septum and the right ventricle (RV) were dissected and weighed. LVs were then cut into 3 pieces transversely, perpendicularly to the LV long axis. The middle slice, which was cut 1mm below the suture, was divided into infarcted, border and septal parts, frozen in liquid nitrogen and stored in −80ºC until used.
For the second group, after the aorta was cannulated and perfused with ice cold PBS to clear red blood cells from the coronary circulation, hearts were fixed by coronary perfusion with 4% paraformaldehyde at a pressure of 80mmHg for 15 min. Subsequently, LV plus septum and RV weights were recorded. Again, LVs were cut into 3 pieces transversely, perpendicularly to the LV long axis. The middle slice was fixed in 4% paraformaldehyde for no more than 24h, transferred to PBS, embedded in O.C.T. compound (Sakura Finetek; Torrance, CA), and cryosectioned at 5 μm thickness for histology and assessment of myocytes apoptosis.
2.5. ELISA
Blood samples were collected from opened chests after hearts were removed and separated into serum aliquots by centrifugation and stored at −70ºC until assayed. Serum T3, T4, and TSH levels were measured using ELISA kits according to the manufacturers’ specification. The T3 kit was obtained from Bio-Quant (human kit, San Diego, CA), the T4 and TSH kits were from Diagnostic Systems Laboratories, Inc (human kit, Webster, TX).
2.6. Hematoxylin and eosin staining
Paraformaldehyde-fixed transverse cryosectioned tissue slides were stained with hematoxylin and eosin, and viewed under a microscope with a color video camera to identify infarcted and non-infarcted areas and the border between these areas. Infarct size was estimated by measuring the percentage of endocardial and epicardial circumferences replaced by infarcted tissue using the following formula: Infarct size (%) = [(Infarcted tissue outer length + Infarcted tissue inner length)/(Left ventricular transversal epicardial circumference + Left ventricular transversal endocardial circumference)] × 100%[18, 19]. MI animals with infarct size less than 20% or larger than 50% were excluded from this study.
2.7. TUNEL labeling
Terminal deoxynucleotidyl transferase-mediated nick-end labeling (TUNEL) assays were performed to detect apoptotic cardiomyocytes using the APO-BrdU TUNEL Assay Kit (A-23210, Invitrogen, Carlsbad, CA) according to the manufacturer’s procedure. The 4% paraformaldehyde perfusion fixed cryosectioned tissue slices described above were used. Samples were treated with Proteinase K for 20 min and 3% H2O2 for 3 min, incubated with terminal deoxynucleotidyl transferase (TdT) in reaction buffer containing Br-dUTP for 60 min at 37°C. Positive controls were treated with DNAse I. Negative controls were incubated in reaction buffer not containing TdT. The cardiomyocytes were counter-stained with DAPI and actin conjugated phalloidin (77418, Sigma, St. Louis, MO). TUNEL positive nuclei were counted using a Nikon fluorescence microscope (TE200-5) and expressed as percentage of the total number of cardiomyocyte nuclei (DAPI stained). Only the initial 500 μm of LV free wall adjacent to each side of the infarcted area were included in this sampling and considered to correspond to the region bordering the infarcted myocardium (Fig. 1). Eight 267×200 μm2 fields (20× objective; approximately 2000 cells per heart) were selected randomly from the border and remote (septal) regions respectively from each sample and examined for TUNEL positive cells.
Figure 1.
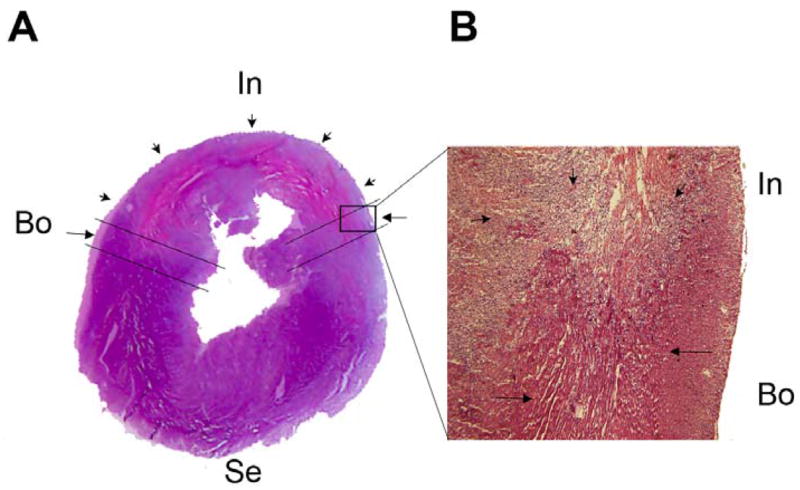
Transverse myocardial section, hematoxylin and eosin staining. (A) Left ventricle and septum. (B) Enlarged image (×10) of the boxed area in A. The infarcted area (In, short arrows), border area (Bo, long arrows), and septum (Se) are illustrated as indicated.
2.8. DNA laddering
DNA from the border and septal areas of LV tissues was extracted respectively. A semi-quantitative PCR-based DNA laddering kit from Maxim Biotech, Inc. (San Francisco, CA) was used to assess the degree of apoptosis. Semi-quantitative PCR amplification of glyceraldehyde-3 phosphate-dehydrogenase (GAPDH) was used to determine if equal amounts of genomic DNA were used for the DNA laddering assay.
2.9. Western blotting
Frozen tissue samples from border and septal regions of LV were sonicated to completely homogenize in ice and RIPA buffer with protease cocktail inhibitor (EMD Biosciences Inc., San Diego, CA), 1 mM PMSF, and 1 mM sodium orthovanadate added. Cell lysates were centrifuged at 14,000 rpm for 10 min. The supernatant was collected, aliquoted and stored at −80 °C until time of use. Protein concentrations of cell lysates were determined by a BCA protein assay. Samples were then mixed with Laemelli buffer containing 5% β-mercaptoethanol and evenly loaded onto SDS-PAGE gels. Protein was transferred to PVDF membranes and detected by antibodies (Cell Signaling Technology, Beverly, MA) specific to total Akt (1:1000), phospho Akt (ser473; 1:300) and Akt2 (1:300). Resultant bands were detected through chemiluminescence (Pierce, Rockford, IL) and quantified using a Versadoc Imaging System model 3000 (Biorad Laboratories, Inc., Hercules, CA).
2.10. Statistical analysis
All data are expressed as means (SD) and were compared using a paired Student’s t-test, two-tailed Student’s t-test, or one-way ANOVA. In addition, a Student-Newman-Keuls post-hoc test was used to examine significant differences between groups. A value of P < 0.05 was considered statistically significant.
3. RESULTS
3.1. Effects of T3 on Heart weight and Body weight
Average body weights, heart weights and heart weight-to-body weight ratios are shown in Table 1. Compared with the sham-operated group, all MI groups including T3-treated animals had significant cardiac hypertrophy characterized by increased heart weight, heart weight-to-body weight ratios, LV weight, and right ventricular (RV) weight. There were no significant differences in heart weight, LV weight, or RV weight between the MI groups. There were no significant differences in body mass between any animal groups before surgery. There was a significant decrease in body mass for all groups three days after surgery but body weight was not different between either MI group vs Shams. There were no significant differences in infarct size between the two MI groups.
Table 1.
Changes in body weight, heart weight, and infarct size.
| Body Wt1 (gm) | Body Wt2 (gm) | Heart Wt (mg) | Hw/BWt2 (mg/gm) | LV Wt (mg) | RV Wt (mg) | Infarct size (%) | |
|---|---|---|---|---|---|---|---|
| Sham (n=11) | 249(13) | 233(10)§§ | 837(81) | 3.6(0.4) | 558(87) | 180(40) | |
| MI (n=10) | 265(12) | 243(11)§§ | 1036(117) † | 4.3(0.5)‡ | 685(64)‡ | 217(47) | 36(4) |
| MI+T3 (n=12) | 251(20) | 223(23)**§§ | 1047(113)‡ | 4.7(0.5)*‡ | 669(58)‡ | 220(50)† | 34(8) |
Data presented as means (SD).
Body Wt1= body weight before surgery; Body wt2 = body weight at terminal study. Heart Wt = heart weight; Hw/BWt2 = heart wt-to-body wt2 ratio; LV Wt = left ventricular weight; RV Wt = right ventricular weight;
P ≤ 0.05,
P ≤ 0.01 vs. MI rats;
P ≤ 0.05,
P ≤ 0.01 vs. Sham operated rats; ANOVA with Student-Newman-Keuls’ Multiple Comparison Test;
P ≤ 0.01 vs. Body Wt1; Paired Student’s t-test.
3.2. Changes of serum T3, T4 and TSH levels after T3 treatment
Serum T3, T4 and TSH levels are shown in Table 2. After MI, there was a significant decrease in serum T3 and T4 levels, and a tendency for increased serum TSH levels as compared with the sham-operated group. Treatment with T3 significantly increased serum T3 levels, decreased serum T4 levels, and tended to decrease TSH levels as compared with MI and sham-operated groups.
Table 2.
Serum T3, T4, and TSH levels
| T3 | T4 | TSH | |
|---|---|---|---|
| Sham | 0.41(0.22) | 6.5(0.9) | 83(23) |
| MI | 0.28(0.10)‡ | 5.4(0.8)† | 94(8) |
| MI+T3 | 0.77(0.31)**† | 1.0(0.5)**‡ | 83(16) |
Data presented as means (SD), μg/dl.
P ≤ 0.01 vs. MI rats;
P ≤ 0.05,
P ≤ 0.01 vs. Sham operated rats; ANOVA with Student-Newman-Keuls’ Multiple Comparison Test.
3.3. Echocardiographic changes after T3 treatment
Table 3 shows echocardiographic data. Compared with sham-operated animals, LV chamber dimension during systole for MI rats was significantly increased; LV chamber dimensions during diastole tended to increase. There were no significantly changes in LV wall thickness during systole and diastole in MI rats. Treatment with T3 did not show any changes in the above indices.
Table 3.
Echocardiographic data
| IVSd | IVSs | LVPWd | LVPWs | LVIDd | LVIDs | |
|---|---|---|---|---|---|---|
| Sham (n=8) | 1.7(0.2) | 2.6(0.4) | 1.7(0.5) | 2.5(0.3) | 6.8(0.5) | 4.2(0.5) |
| MI (n=7) | 1.9(0.3) | 2.4(0.7) | 1.8(0.5) | 2.5(0.7) | 7.1(0.5) | 5.2(0.9)† |
| MI+T3 (n=9) | 1.8(0.2) | 2.2(0.6) | 1.9(0.4) | 2.5(0.2) | 7.1(0.5) | 5.5(0.5)‡ |
Data presented as means (SD).
IVSd and IVSs = interventricular septal thickness in end diastole and systole, respectively, mm; PWTd and PWTs = diastolic and systolic posterior wall thickness, respectively, mm; LVIDd and LVIDs = left ventricular diastolic and systolic internal diameter, respectively, mm;
P ≤ 0.05,
P ≤ 0.01 vs. Sham operated rats; ANOVA with Student-Newman-Keuls’ Multiple Comparison Test.
3.4. Effects of T3 on Hemodynamics
Hemodynamic data are summarized in Table 4. In MI animals, LV systolic pressure and −dP/dt were significantly decreased, LV end-diastolic pressure was significantly increased, and there was a tendency for +dP/dt to decrease as compared with the sham-operated animals. Compared with MI animals, T3 treatment of MI led to increased ±dP/dt, resulting in normalization of these values when compared to the sham-operated group. Heart rate tended to increase in MI group but did not reach significance as compared with Sham-operated animals. T3 treatment resulted in significantly increased heart rate but no change in LVEDP as compared with MI group.
Table 4.
Hemodynamic data
| Heart rate (beats/min) | LVSP (mmHg) | LVEDP (mmHg) | +dP/dt (mmHg/sec) | −dP/dt (mmHg/sec) | |
|---|---|---|---|---|---|
| Sham (n=9) | 353(24) | 132(16) | 7.0(2.3) | 8660(1300) | 7330(920) |
| MI (n=10) | 376(32) | 112(19)† | 11.7(4.0)‡ | 7650(1420) | 5870(1190)† |
| MI+T3 (n=12) | 420(48)*‡ | 124(14) | 12.0(3.1)‡ | 9270(1420)* | 7290(1300)** |
Data presented as means (SD).
LVSP and LVEDP = left ventricular end-systolic and end-diastolic pressure, respectively; +dP/dt and −dP/dt = maximal rate of pressure development and decline, respectively;
P ≤ 0.05,
P ≤ 0.01 vs. MI rats;
P ≤ 0.05,
P ≤ 0.01 vs. Sham operated rats; ANOVA with Student-Newman-Keuls’ Multiple Comparison Test.
3.5. T3 Prevented Ischemia Induced Myocyte Apoptosis
Figure 1A and 1B show low and high power photomicrographs of a transverse slice of LV from a rat with MI. Reduced H&E staining intensity of myocytes in the infarcted area is readily apparent. Selection of border areas for apoptosis sampling is indicated. Myocytes with normal staining intensity immediately adjacent to the infarct were selected. Three days after MI, there was a significant increase in apoptosis in the border area as shown by DNA laddering (Figure 2A) and TUNEL assay (Figure 2B). The ratio of TUNEL positive nuclei was 37 (14)%. No evident myocyte apoptosis was detected by either DNA laddering or TUNEL labeling in the septal area. T3 treatment dramatically reduced the amount of DNA laddering and the number of TUNEL positive cells to 18 (5)% in the border area, suggesting that T3 protects cardiomyocytes from ischemia induced apoptosis.
Figure 2.
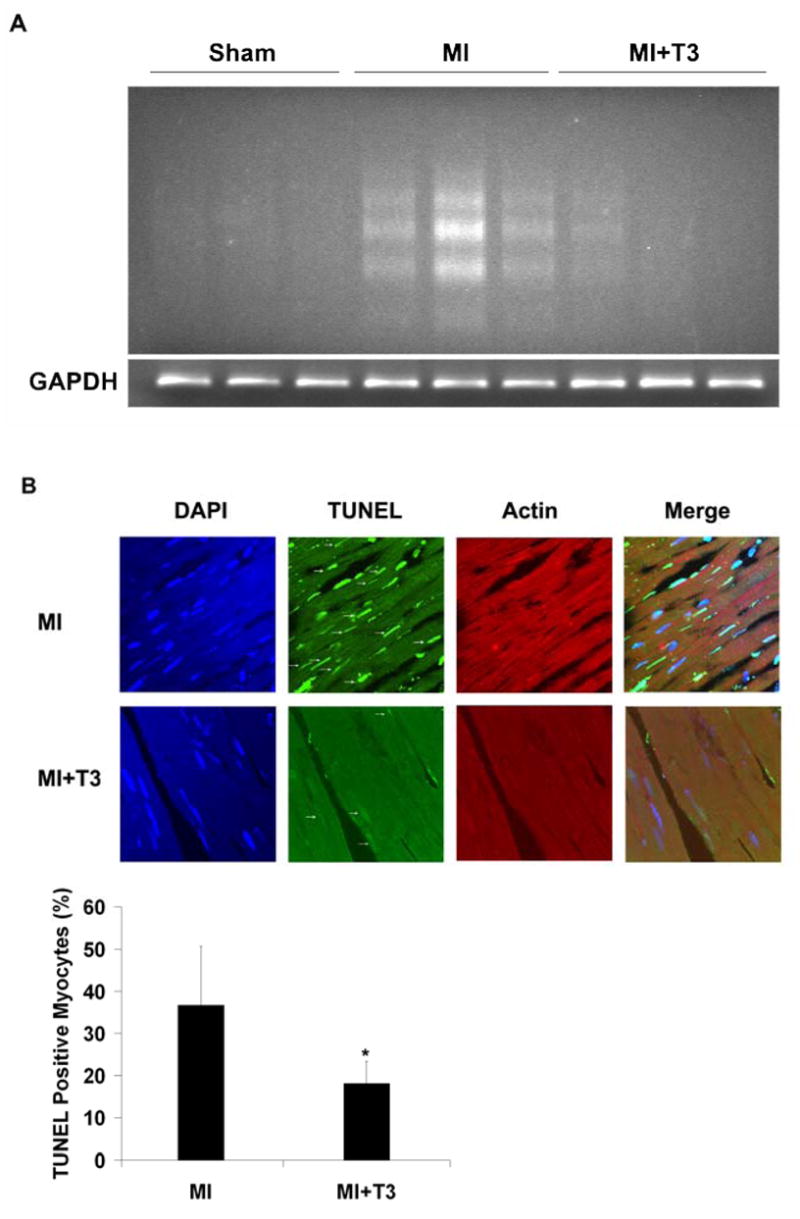
Myocyte apoptosis in border area was attenuated by T3 treatment. (A) T3 reduced DNA fragmentation significantly in cardiomyocytes in border area. Semi-quantitative PCR amplification of GAPDH gene showed that equal amount of genomic DNA were used for the DNA laddering assay. (B) T3 markedly reduced TUNEL positive cardiomyocytes (arrows). Results are mean (SD) with n=4 rats per group. *P ≤ 0.05 vs. MI group; two-tailed Student’s t-test.
3.6. T3 Activated Akt Signaling
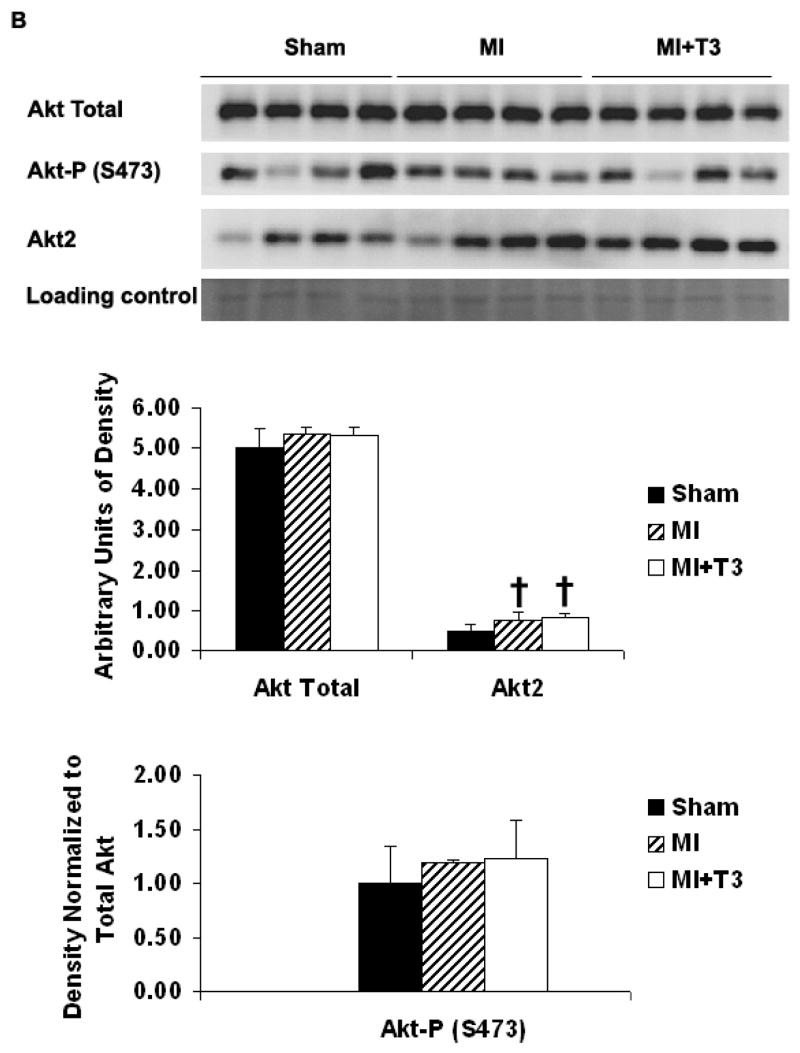
Compared with sham operated animals, total Akt expression was not changed in either border or septal areas after MI as examined by Western blots. There was a tendency for increased phosphorylation of Akt at serine 473 in the border area in the MI group (Figure 3A). T3 treatment did not change total Akt and Akt2 expression in either the border or septal areas (Figure 3A, 3B). However, T3 significantly induced phosphorylation of Akt at serine 473 in the border area but not in the septum (Figure 3A, 3B). There was a slight but significant increase in Akt2 expression in the septal area but not in the border area of the MI groups (Figure 3A, 3B).
Figure 3.
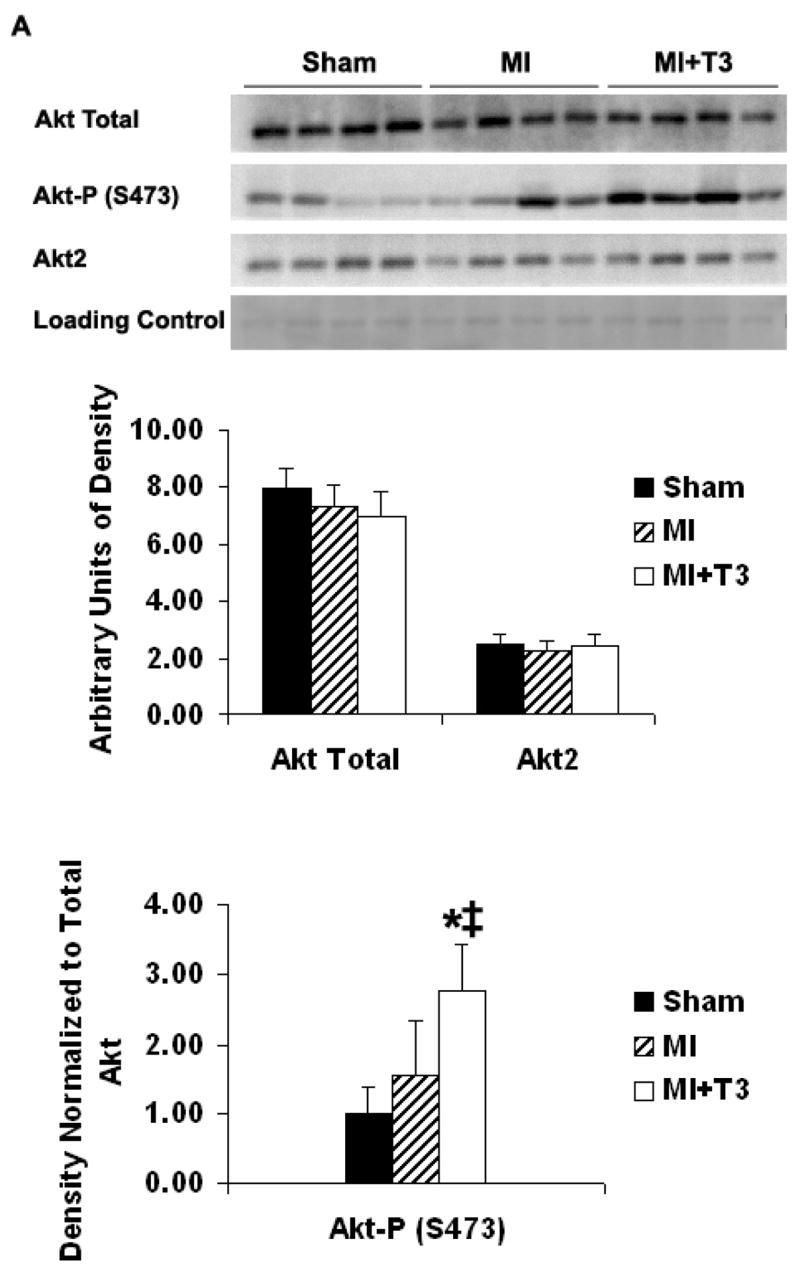
Figure 3A. Border area. Representative Western blot and densitometry for total Akt, phospho-Akt (S473) and Akt2 expressions. Results are mean (SD) with n=4 rats per group. *P ≤ 0.05 vs. MI group. ‡P ≤ 0.01 vs. Sham operated group; ANOVA with Student-Newman-Keuls’ Multiple Comparison Test.
Figure 3B. Septal area. Representative Western blot and densitometry for total Akt, phospho-Akt (S473) and Akt2 expression. Results are mean (SD) with n=4 rats per group. †P ≤ 0.05 vs. Sham operated group; ANOVA with Student-Newman-Keuls’ Multiple Comparison Test.
DISCUSSION
This study shows for the first time that T3 can inhibit cardiomyocyte apoptosis and activate Akt signaling in the border area after acute myocardial infarction. T3 treatment increased LV ±dP/dt and systolic pressure but had no effect on end diastolic pressure. MI surgery caused an increase in heart rate and a reduction in body weight with slightly larger changes in those parameters from T3 treatment.
This study shows a significant decrease in serum T3 and T4 levels and a tendency for increased serum TSH levels after MI, which is consistent with previous reports[6, 7, 9, 10]. T3 treatment significantly increased serum T3 level, decreased serum T4 levels, and tended to decrease serum TSH level, suggesting inhibition of the pituitary-thyroid axis by exogenous thyroid hormone. It should be noted here that the experiment was specifically designed to investigate the potential of T3 to alter apoptosis in vivo using the rat MI model. While labeling with various markers of apoptosis was clearly reduced by T3 in the MI border zone, we can not rule out the possibility that T3 might also increase the clearance of apoptotic myocytes.
In this experiment, the border area was defined histologically as the tissue lying within ~500 μm from the infarcted area. This area is considered ischemic and has many cellular and molecular events on going after MI. A high apoptotic rate of myocytes was found in this area in our study. Using a similar sampling protocol, Olivetti et al found an apoptotic rate up to 26% in human hearts post-MI[4]. Previously studies have shown that ischemia can induce myocyte apoptosis at a rate of 33% or as high as 62% in rat heart[20, 21]. It must be pointed out that some investigators such as Cheng et al[3] have studied the border area defined as the initial 2mm of LV free wall adjacent to each side of the septum, which was relatively far away from the infarcted area, and reported a very low myocyte apoptotic rate. In our experiment, we did not detect significant TUNEL positive signal in such areas. Thus, differences between our study and that of Cheng appear to be related to sampling.
Thyroid hormones play an important role in embryonic development and body growth, and have many beneficial effects on the cardiovascular system, including physiological hypertrophy, enhanced contractility, and vasodilatation. Recently, Trivieri et al have shown that cardiac specific elevation of T3 levels by increased cardiac type 2 deiodinase activity can enhance myocardial contractility and prevent impaired contractility induced by pressure overload[22]. Rooij et al reported that T3 induced increases in cardiac contractility via stimulation of αMHC expression and repression of βMHC expression are controlled by a specific MicroRNA (miR-208)[23]. With regard to the current study, it should be stressed that T3 induced increased in ±dP/dt in MI animals may be partially due to increased heart rate, and it is difficult to know how much of the anti-apoptotic effect of T3 contributes to the changes in LV ±dP/dt.
In addition to severe heart diseases, deficiency of T3 has also been reported in patients after cardiopulmonary bypass surgery[24, 25], and in heart transplantation donors and recipients, and may contribute to donor heart dysfunction after heart transplantation[26]. Treatment with TH has been shown to improve LV function in several heart diseases including MI[10]. In cardiopulmonary bypass surgery, T3 supplementation after surgery can improve low cardiac output status, reduce inotropic support, and increase recovery rate[27]. TH treatment can resuscitate donor hearts with poor function, allow rapid inotropic reduction, and may consequently, increase organ availability [28, 29, 30]. In recipients, immediate cardiac allograft dysfunction following transplantation can be relieved by T3 treatment and the amount and duration of inotropic support can also be decreased [27, 31]. Although the mechanisms of these TH benefits are not clear, the anti-apoptotic effect of T3 found in this study may be involved. The long term effect of TH on post-MI remodeling remains unknown. Some studies have shown that TH have anti-apoptotic effects on rat cerebellar granule neurons[32], promyeloleukemic HL-60 cells[33] and porcine granulose cells[34].
Both genomic and non-genomic mechanisms have been proposed to mediate the effects of TH in the heart. Pantos et al have shown an increase in TH receptor α1 and a decrease in β1 receptor expression 13 weeks after MI in rat hearts, indicating an altered genomic mechanism[35]. However, the mechanism through which TH exerts its anti-apoptotic effect shortly after MI may involve non-genomic signal transduction pathways. The PI 3-kinase/Akt signaling pathway has been shown to play a key role in regulating cardiomyocyte growth and survival. Activation of this pathway can inhibit cardiomyocyte apoptosis and promote cardiac hypertrophy[36, 37]. The Akt family of serine/threonine protein kinases is comprised of three members, Akt1, Akt2, and Akt3. Both Akt1 and Akt2 have been implicated in the inhibition of myocyte apoptosis[38, 39]. We have demonstrated previously that TH can activate Akt signaling in the heart[14] and prevent myocyte apoptosis via Akt signaling in cultured neonatal rat cardiomyocytes[13]. The present study shows that T3 treatment increased phospho-Akt levels in the border area, consistent with decreased myocyte apoptosis in the same area. Together with our previously findings, this suggests an Akt signaling mediated mechanism for the anti-apoptotic effect of T3. We noticed a regional difference in phospho-Akt expression in T3 treated MI rats. The cause is not clear at this time but may be related to altered response of myocytes in the post-MI border area being subjected to greater stress levels. In this study, we found a slight increase in Akt2 expression in the septal area but not in the border area after MI, which might be stress-induced. The significance of this change is uncertain, but our results suggest that the anti-apoptotic effect of T3 is not likely Akt2 mediated.
Several studies have shown reduced thyroid hormone levels after myocardial infarction, but whether this is beneficial or detrimental remains unanswered. Supplementation of TH as a treatment for MI has never been recommended. This preclinical study was not intended to answer the above questions. The current study was initiated largely in response to our recent data demonstrating that T3 can prevent serum starvation-induced apoptosis in neonatal rat cardiomyocytes[13]. In those initial experiments, a relatively high dose of T3 was intentionally selected to ensure that potential anti-apoptotic effects would be detected in vitro. The results from the current study indicate that decreased thyroid hormones might be detrimental and T3 supplementation may provide some benefits. Nonetheless, the long term effects of T3 remain uncertain and could be dangerous. It is not yet clear if the T3 effect noted here can be produced without also inducing an increase in heart rate or incidence of arrhythmias. The thyroid hormone analog DITPA, currently being investigated in clinical trials, is able to induce cardiac functional changes with reduced risk of tachycardia. Some studies have shown that DITPA can stimulate post MI arteriolar growth, modify LV remodeling and improve LV function[40, 41]. It would be of interest to know if DITPA has similar anti-apoptotic effects on cardiac myocytes.
Clearly, more work is needed before clinical trials can be considered. Important issues that should be addressed in future experiments include determination of: (1) minimal T3 dose required for the anti-apoptotic effect; (2) whether T4 (clinically used form of TH) or safer alternatives such as TH analogs will produce the same effect; (3) optimum treatment duration; and (4) potential long term beneficial changes in salvaged myocardium, improved cardiac function, and survival. Nonetheless, the results of our experiment are encouraging and support further study.
Acknowledgments
We thank Dr. James A. Kuzman for his assistance in this study. These studies were supported by grant P20 RR017662 from the National Institutes of Health (National Center for Research Resources), and the South Dakota 2010 Initiative Research Center Grant. Sanford Research/USD is a partnership between the Sanford School of Medicine at the University of South Dakota and Sanford Health (formerly Sioux Valley Hospital and Health Systems) in Sioux Falls.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sam F, Sawyer DB, Chang DL, Eberli FR, Ngoy S, Jain M, et al. Progressive left ventricular remodeling and apoptosis late after myocardial infarction in mouse heart. Am J Physiol Heart Circ Physiol. 2000;279:H422–428. doi: 10.1152/ajpheart.2000.279.1.H422. [DOI] [PubMed] [Google Scholar]
- 2.Palojoki E, Saraste A, Eriksson A, Pulkki K, Kallajoki M, Voipio-Pulkki LM, et al. Cardiomyocyte apoptosis and ventricular remodeling after myocardial infarction in rats. Am J Physiol Heart Circ Physiol. 2001;280:H2726–2731. doi: 10.1152/ajpheart.2001.280.6.H2726. [DOI] [PubMed] [Google Scholar]
- 3.Cheng W, Kajstura J, Nitahara JA, Li B, Reiss K, Liu Y, et al. Programmed myocyte cell death affects the viable myocardium after infarction in rats. Exp Cell Res. 1996;226:316–327. doi: 10.1006/excr.1996.0232. [DOI] [PubMed] [Google Scholar]
- 4.Olivetti G, Quaini F, Sala R, Lagrasta C, Corradi D, Bonacina E, et al. Acute myocardial infarction in humans is associated with activation of programmed myocyte cell death in the surviving portion of the heart. J Mol Cell Cardiol. 1996;28:2005–2016. doi: 10.1006/jmcc.1996.0193. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac hypertrophy. J Clin Invest. 1997;100:1991–1999. doi: 10.1172/JCI119730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Marinis L, Mancini A, Masala R, Torlontano M, Sandric S, Barbarino A. Evaluation of pituitary-thyroid axis response to acute myocardial infarction. J Endocrinol Invest. 1985;8:507–511. doi: 10.1007/BF03348548. [DOI] [PubMed] [Google Scholar]
- 7.Veres I, Pechan J, Murin J, Ondrejka P. Changes in levels of thyroid hormones and TSH in acute myocardial infarction. Bratisl Lek Listy. 1992;93:167–174. [PubMed] [Google Scholar]
- 8.Takada K, Shimai S, Takano T, Hayakawa H. The prevalence and significance of abnormal thyroid hormone metabolism in acute myocardial infarction. Nippon Ika Daigaku Zasshi. 1994;61:220–231. doi: 10.1272/jnms1923.61.220. [DOI] [PubMed] [Google Scholar]
- 9.Eber B, Schumacher M, Langsteger W, Zweiker R, Fruhwald FM, Pokan R, et al. Changes in thyroid hormone parameters after acute myocardial infarction. Cardiology. 1995;86:152–156. doi: 10.1159/000176862. [DOI] [PubMed] [Google Scholar]
- 10.Ojamaa K, Kenessey A, Shenoy R, Klein I. Thyroid hormone metabolism and cardiac gene expression after acute myocardial infarction in the rat. Am J Physiol Endocrinol Metab. 2000;279:E1319–1324. doi: 10.1152/ajpendo.2000.279.6.E1319. [DOI] [PubMed] [Google Scholar]
- 11.Friberg L, Werner S, Eggertsen G, Ahnve S. Rapid down-regulation of thyroid hormones in acute myocardial infarction: is it cardioprotective in patients with angina? Arch Intern Med. 2002;162:1388–1394. doi: 10.1001/archinte.162.12.1388. [DOI] [PubMed] [Google Scholar]
- 12.Gay R, Gustafson TA, Goldman S, Morkin E. Effects of L-thyroxine in rats with chronic heart failure after myocardial infarction. Am J Physiol. 1987;253:H341–346. doi: 10.1152/ajpheart.1987.253.2.H341. [DOI] [PubMed] [Google Scholar]
- 13.Kuzman JA, Gerdes AM, Kobayashi S, Liang Q. Thyroid hormone activates Akt and prevents serum starvation-induced cell death in neonatal rat cardiomyocytes. J Mol Cell Cardiol. 2005;39:841–844. doi: 10.1016/j.yjmcc.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 14.Kuzman JA, Vogelsang KA, Thomas TA, Gerdes AM. L-Thyroxine activates Akt signaling in the heart. J Mol Cell Cardiol. 2005;39:251–258. doi: 10.1016/j.yjmcc.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 15.Selye H, Bajusz E, Grasso S, Mendell P. Simple techniques for the surgical occlusion of coronary vessels in the rat. Angiology. 1960;11:398–407. doi: 10.1177/000331976001100505. [DOI] [PubMed] [Google Scholar]
- 16.Tamura T, Said S, Harris J, Lu W, Gerdes AM. Reverse remodeling of cardiac myocyte hypertrophy in hypertension and failure by targeting of the renin-angiotensin system. Circulation. 2000;102:253–259. doi: 10.1161/01.cir.102.2.253. [DOI] [PubMed] [Google Scholar]
- 17.Zimmer HG, Gerdes AM, Lortet S, Mall G. Changes in heart function and cardiac cell size in rats with chronic myocardial infarction. J Mol Cell Cardiol. 1990;22:1231–1243. doi: 10.1016/0022-2828(90)90060-f. [DOI] [PubMed] [Google Scholar]
- 18.Pfeffer JM, Pfeffer MA, Braunwald E. Influence of chronic captopril therapy on the infarcted left ventricle of the rat. Circ Res. 1985;57:84–95. doi: 10.1161/01.res.57.1.84. [DOI] [PubMed] [Google Scholar]
- 19.Litwin SE, Katz SE, Morgan JP, Douglas PS. Long-term captopril treatment improves diastolic filling more than systolic performance in rats with large myocardial infarction. J Am Coll Cardiol. 1996;28:773–781. doi: 10.1016/0735-1097(96)00215-x. [DOI] [PubMed] [Google Scholar]
- 20.Fliss H, Gattinger D. Apoptosis in ischemic and reperfused rat myocardium. Circ Res. 1996;79:949–956. doi: 10.1161/01.res.79.5.949. [DOI] [PubMed] [Google Scholar]
- 21.Pozzi S, Malferrari G, Biunno I, Samaja M. Low-flow ischemia and hypoxia stimulate apoptosis in perfused hearts independently of reperfusion. Cell Physiol Biochem. 2002;12:39–46. doi: 10.1159/000047825. [DOI] [PubMed] [Google Scholar]
- 22.Trivieri MG, Oudit GY, Sah R, Kerfant BG, Sun H, Gramolini AO, et al. Cardiac-specific elevations in thyroid hormone enhance contractility and prevent pressure overload-induced cardiac dysfunction. Proc Natl Acad Sci U S A. 2006;103:6043–6048. doi: 10.1073/pnas.0601072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Rooij E, Sutherland LB, Qi X, Richardson JA, Hill J, Olson EN. Control of stress-dependent cardiac growth and gene expression by a microRNA. Science. 2007;316:575–579. doi: 10.1126/science.1139089. [DOI] [PubMed] [Google Scholar]
- 24.Robuschi G, Medici D, Fesani F, Barboso G, Montermini M, d’Amato L, et al. Cardiopulmonary bypass: a low T4 and T3 syndrome with blunted thyrotropin (TSH) response to thyrotropin-releasing hormone (TRH) Horm Res. 1986;23:151–158. doi: 10.1159/000180311. [DOI] [PubMed] [Google Scholar]
- 25.Bettendorf M, Schmidt KG, Tiefenbacher U, Grulich-Henn J, Heinrich UE, Schonberg DK. Transient secondary hypothyroidism in children after cardiac surgery. Pediatr Res. 1997;41:375–379. doi: 10.1203/00006450-199703000-00012. [DOI] [PubMed] [Google Scholar]
- 26.Jeevanandam V. Triiodothyronine: spectrum of use in heart transplantation. Thyroid. 1997;7:139–145. doi: 10.1089/thy.1997.7.139. [DOI] [PubMed] [Google Scholar]
- 27.Carrel T, Eckstein F, Englberger L, Mury R, Mohacsi P. Thyronin treatment in adult and pediatric heart surgery: clinical experience and review of the literature. Eur J Heart Fail. 2002;4:577–582. doi: 10.1016/s1388-9842(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 28.Jeevanandam V, Todd B, Hellman S, Eldridge C, McClurken J, Addonizio VP. Use of triiodothyronine replacement therapy to reverse donor myocardial dysfunction: creating a larger donor pool. Transplant Proc. 1993;25:3305–3306. [PubMed] [Google Scholar]
- 29.Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. Hormonal resuscitation yields more transplanted hearts, with improved early function. Transplantation. 2003;75:1336–1341. doi: 10.1097/01.TP.0000062839.58826.6D. [DOI] [PubMed] [Google Scholar]
- 30.Jeevanandam V, Todd B, Regillo T, Hellman S, Eldridge C, McClurken J. Reversal of donor myocardial dysfunction by triiodothyronine replacement therapy. J Heart Lung Transplant. 1994;13:681–687. 685–687. [PubMed] [Google Scholar]
- 31.Novitzky D, Cooper DK, Chaffin JS, Greer AE, DeBault LE, Zuhdi N. Improved cardiac allograft function following triiodothyronine therapy to both donor and recipient. Transplantation. 1990;49:311–316. doi: 10.1097/00007890-199002000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Muller Y, Rocchi E, Lazaro JB, Clos J. Thyroid hormone promotes BCL-2 expression and prevents apoptosis of early differentiating cerebellar granule neurons. Int J Dev Neurosci. 1995;13:871–885. doi: 10.1016/0736-5748(95)00057-7. [DOI] [PubMed] [Google Scholar]
- 33.Hara M, Suzuki S, Mori J, Yamashita K, Kumagai M, Sakuma T, et al. Thyroid hormone regulation of apoptosis induced by retinoic acid in promyeloleukemic HL-60 cells: studies with retinoic acid receptor-specific and retinoid x receptor-specific ligands. Thyroid. 2000;10:1023–1034. doi: 10.1089/thy.2000.10.1023. [DOI] [PubMed] [Google Scholar]
- 34.Asahara S, Sato A, Aljonaid AA, Maruo T. Thyroid hormone synergizes with follicle stimulating hormone to inhibit apoptosis in porcine granulosa cells selectively from small follicles. Kobe J Med Sci. 2003;49:107–116. [PubMed] [Google Scholar]
- 35.Pantos C, Mourouzis I, Xinaris C, Kokkinos AD, Markakis K, Dimopoulos A, et al. Time-dependent changes in the expression of thyroid hormone receptor alpha 1 in the myocardium after acute myocardial infarction: possible implications in cardiac remodelling. Eur J Endocrinol. 2007;156:415–424. doi: 10.1530/EJE-06-0707. [DOI] [PubMed] [Google Scholar]
- 36.Matsui T, Nagoshi T, Rosenzweig A. Akt and PI 3-kinase signaling in cardiomyocyte hypertrophy and survival. Cell Cycle. 2003;2:220–223. [PubMed] [Google Scholar]
- 37.Oudit GY, Sun H, Kerfant BG, Crackower MA, Penninger JM, Backx PH. The role of phosphoinositide-3 kinase and PTEN in cardiovascular physiology and disease. J Mol Cell Cardiol. 2004;37:449–471. doi: 10.1016/j.yjmcc.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 38.Chen WS, Xu PZ, Gottlob K, Chen ML, Sokol K, Shiyanova T, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15:2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.DeBosch B, Sambandam N, Weinheimer C, Courtois M, Muslin AJ. Akt2 regulates cardiac metabolism and cardiomyocyte survival. J Biol Chem. 2006;281:32841–32851. doi: 10.1074/jbc.M513087200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahaffey KW, Raya TE, Pennock GD, Morkin E, Goldman S. Left ventricular performance and remodeling in rabbits after myocardial infarction. Effects of a thyroid hormone analogue. Circulation. 1995;91:794–801. doi: 10.1161/01.cir.91.3.794. [DOI] [PubMed] [Google Scholar]
- 41.Zheng W, Weiss RM, Wang X, Zhou R, Arlen AM, Lei L, et al. DITPA stimulates arteriolar growth and modifies myocardial postinfarction remodeling. Am J Physiol Heart Circ Physiol. 2004;286:H1994–2000. doi: 10.1152/ajpheart.00991.2003. [DOI] [PubMed] [Google Scholar]