Abstract
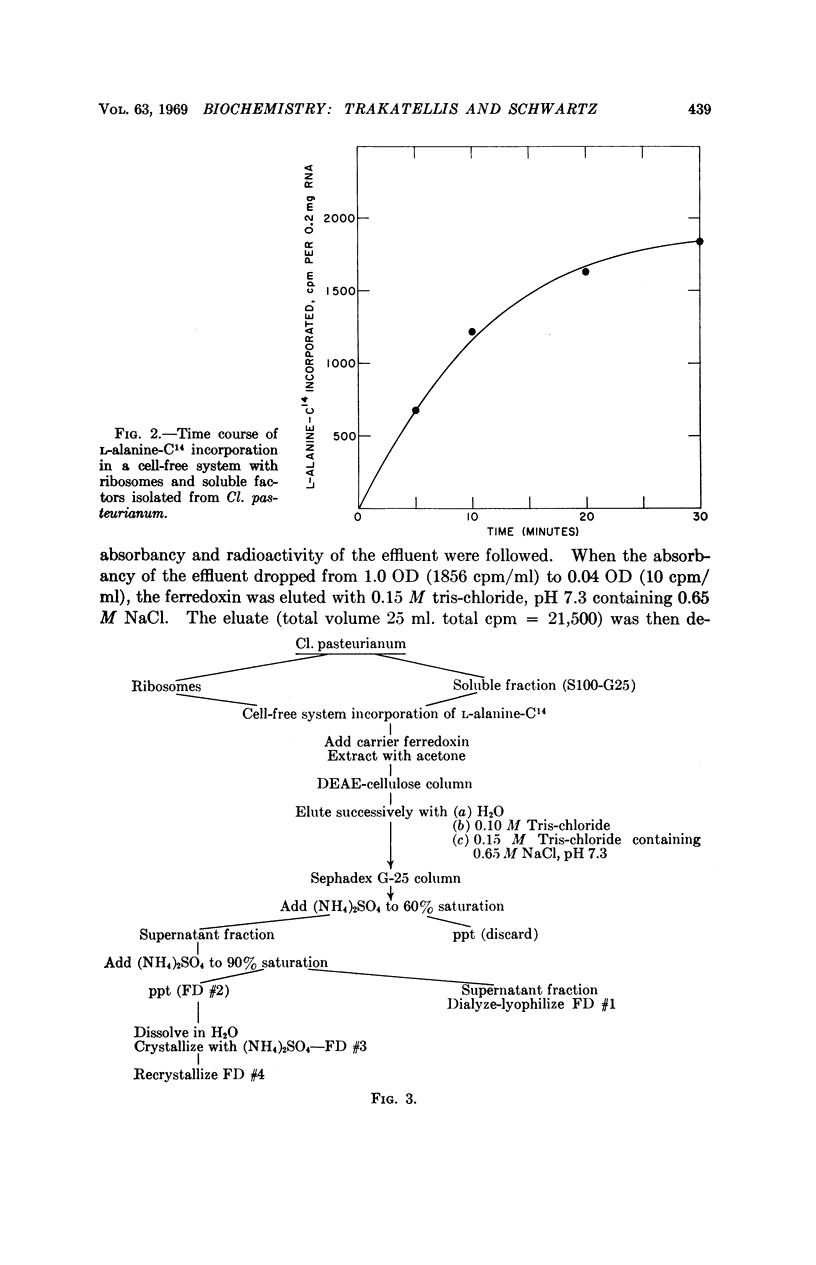
The investigation reported in this communication was concerned with the biosynthesis of ferredoxin in a cell-free system. A cell-free system, with the ability to incorporate amino acids into peptides and proteins, was developed from Cl. pasteurianum, and its requirements were established. After the incorporation of L-alanine-C14, ferredoxin-C14 was isolated in crystalline form with the aid of added carrier ferredoxin. Amino acid analyses and absorption spectra strongly indicated that the isolated ferredoxin-C14, crystallized three times, was essentially free from impurities. Furthermore, experiments with Fe59 strongly indicated that the iron may combine with the apoprotein after the latter is completely synthesized and released from the polysomes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUCHANAN B. B., LOVENBERG W., RABINOWITZ J. C. A comparison of clostridial ferredoxins. Proc Natl Acad Sci U S A. 1963 Mar 15;49:345–353. doi: 10.1073/pnas.49.3.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVENBERG W., BUCHANAN B. B., RABINOWITZ J. C. STUDIES ON THE CHEMICAL NATURE OF CLOSTRIDIAL FERREDOXIN. J Biol Chem. 1963 Dec;238:3899–3913. [PubMed] [Google Scholar]
- Lovenberg W., Sobel B. E. Rubredoxin: a new electron transfer protein from Clostridium pasteurianum. Proc Natl Acad Sci U S A. 1965 Jul;54(1):193–199. doi: 10.1073/pnas.54.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- TRAKATELLIS A. C., AXELROD A. E., MONTJAR M. ACTINOMYCIN D AND MESSENGER-RNA TURNOVER. Nature. 1964 Sep 12;203:1134–1136. doi: 10.1038/2031134a0. [DOI] [PubMed] [Google Scholar]
- TRAKATELLIS A. C., AXELROD A. E., MONTJAR M. STUDIES ON LIVER MESSENGER RIBONUCLEIC ACID. J Biol Chem. 1964 Dec;239:4237–4244. [PubMed] [Google Scholar]
- Tanaka M., Nakashima T., Benson A., Mower H. F., Yasunobu K. T. The amino acid sequence of Clostridium pasteurianum ferredoxin. Biochem Biophys Res Commun. 1964 Jul 27;16(5):422–427. doi: 10.1016/0006-291x(64)90369-9. [DOI] [PubMed] [Google Scholar]
- Trakatellis A. C. Effect of sparsomycin on protein synthesis in the mouse liver. Proc Natl Acad Sci U S A. 1968 Mar;59(3):854–860. doi: 10.1073/pnas.59.3.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VALENTINE R. C., BRILL W. J., SAGERS R. D. FERREDOXIN LINKED DPN REDUCTION BY PYRUVATE IN EXTRACTS OF CLOSTRIDIUM ACIDI-URICI. Biochem Biophys Res Commun. 1963 Aug 1;12:315–319. doi: 10.1016/0006-291x(63)90303-6. [DOI] [PubMed] [Google Scholar]
- VALENTINE R. C., JACKSON R. L., WOLFE R. S. Role of ferredoxin in hydrogen metabolism of Micrococcus lactilyticus. Biochem Biophys Res Commun. 1962 Jun 4;7:453–456. doi: 10.1016/0006-291x(62)90334-0. [DOI] [PubMed] [Google Scholar]




