Abstract
This communication reports the biosynthesis of insulin in the bovine fetal pancreatic slices in vitro. Double-chain proinsulin and insulin were found as major components in the mitochondrial-granule fraction of bovine fetal pancreas. Tritiated leucine was incorporated into a single-chain proinsulin, a double-chain proinsulin, and insulin. Subcellular fractionation of the slices incubated with tritiated leucine showed that radioactive single-chain proinsulin was present in the deoxycholate-soluble microsomal fraction, deoxycholate-insoluble microsomal fraction, and mitochondrial-granule fraction. Labeled double-chain proinsulin and insulin were present in the deoxycholate-soluble microsomal and mitochondrial-granule fractions. These results are consistent with the hypothesis that insulin is synthesized as a single-chain polypeptide on the ribosomes, and that intracellular proteolysis in the subcellular membranous organelles and beta-granules converts the single-chain proinsulin to insulin via a double-chain intermediate.
Full text
PDF
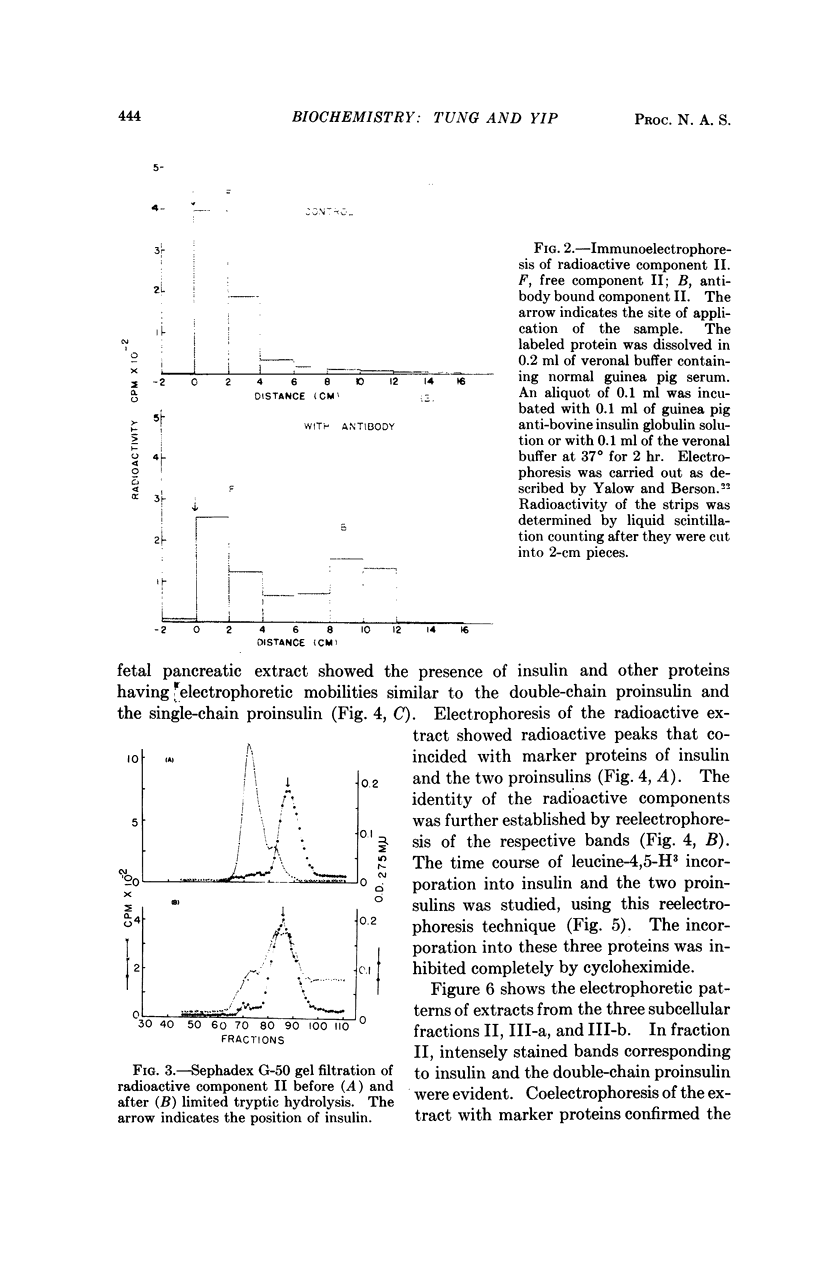
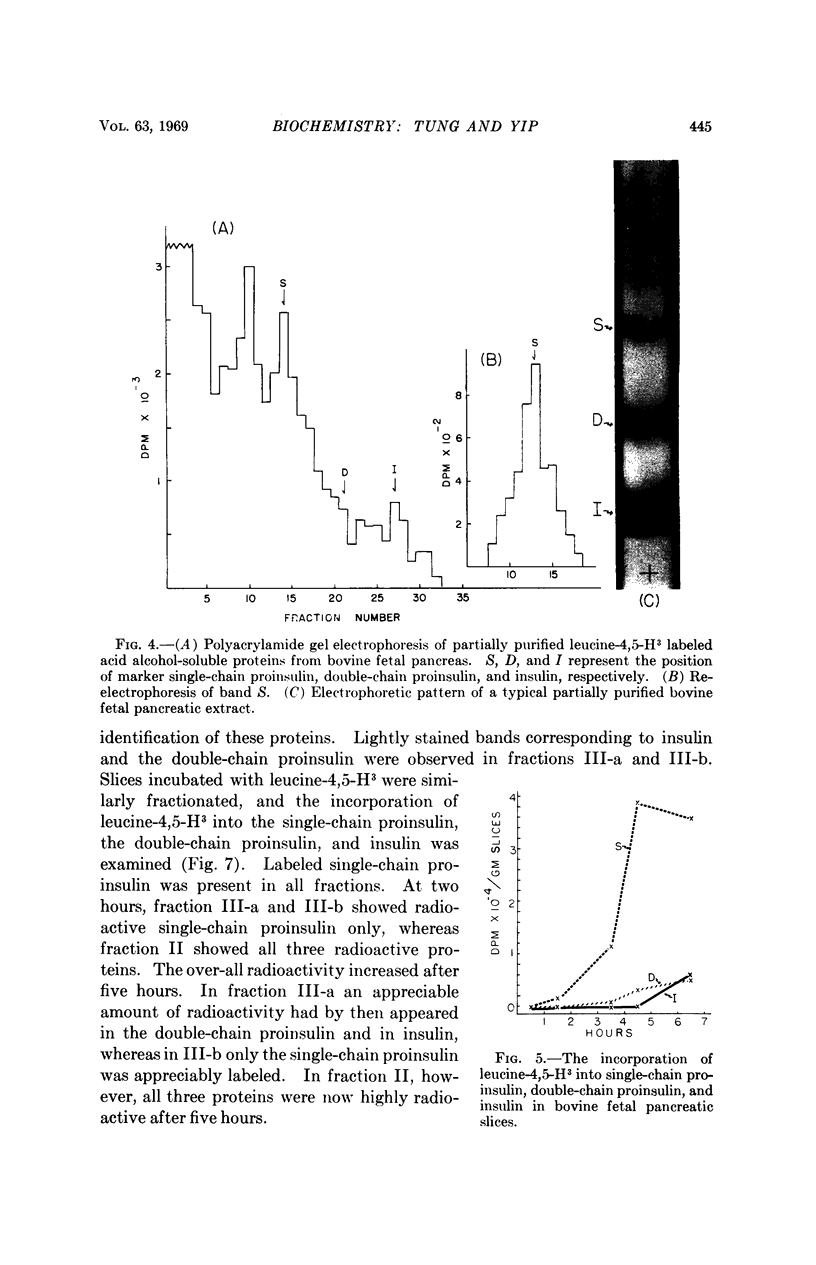

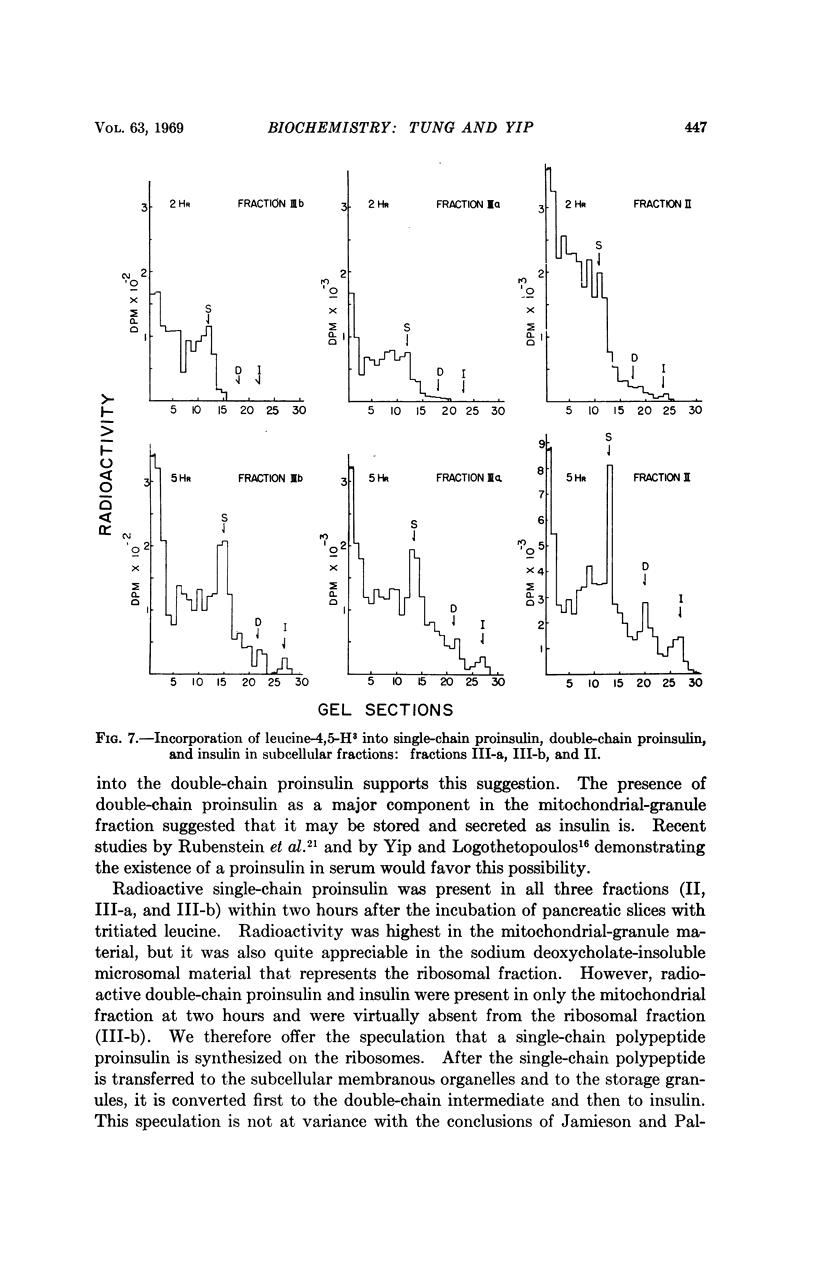
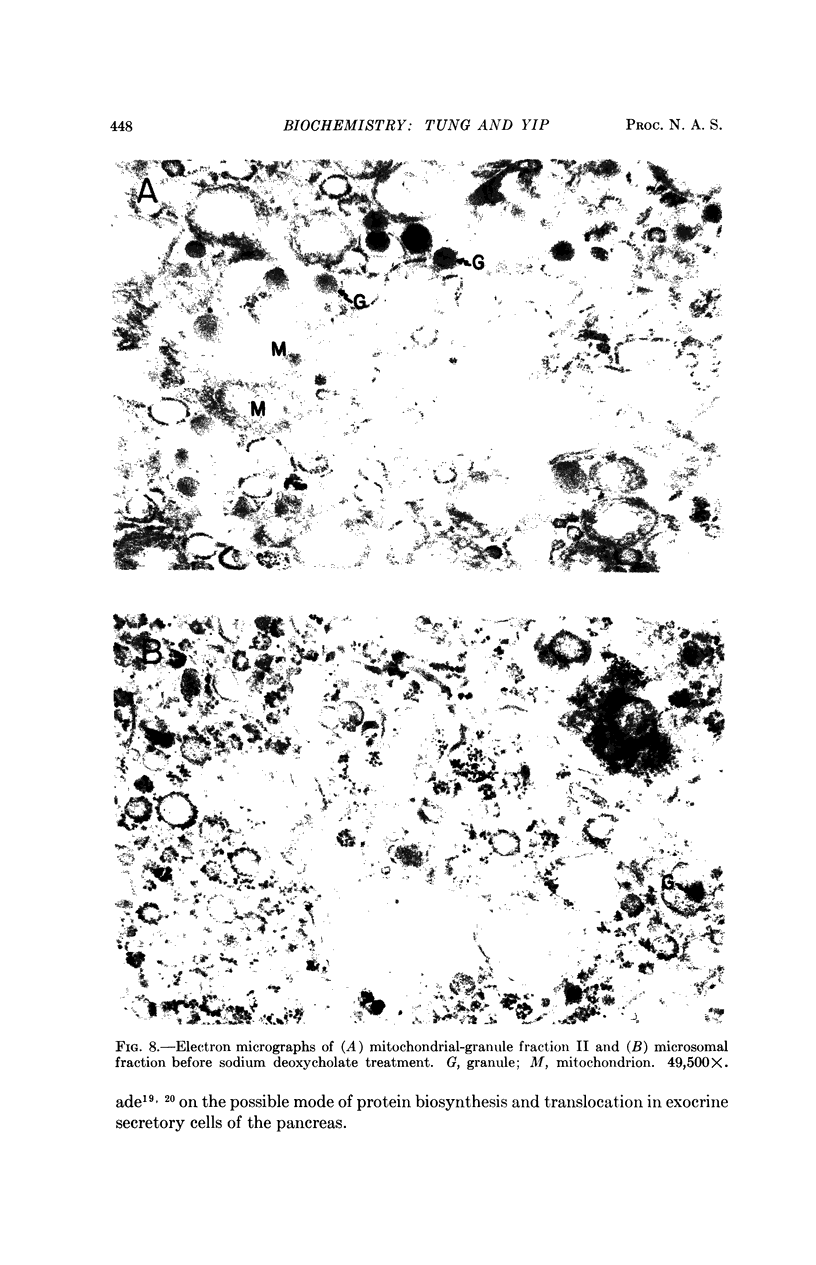

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chance R. E., Ellis R. M., Bromer W. W. Porcine proinsulin: characterization and amino acid sequence. Science. 1968 Jul 12;161(3837):165–167. doi: 10.1126/science.161.3837.165. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GRODSKY G. M., FORSHAM P. H. An immunochemical assay of total extractable insulin in man. J Clin Invest. 1960 Jul;39:1070–1079. doi: 10.1172/JCI104122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant P. T., Reid K. B. Biosynthesis of an insulin precursor by islet tissue of cod (Gadus callarias). Biochem J. 1968 Nov;110(2):281–288. doi: 10.1042/bj1100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B. S. Combustion analysis of radioactivity and densitometry of protein in polyacrylamide gels. Anal Biochem. 1968 Oct 24;25(1):172–180. doi: 10.1016/0003-2697(68)90090-0. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Rubenstein A. H., Cho S., Steiner D. F. Evidence for proinsulin in human urine and serum. Lancet. 1968 Jun 22;1(7556):1353–1355. doi: 10.1016/s0140-6736(68)92040-0. [DOI] [PubMed] [Google Scholar]
- Schmidt D. D., Arens A. Proinsulin vom Rind Isolierung, Eigneschaften und seine Aktivierung durch Trypsin. Hoppe Seylers Z Physiol Chem. 1968 Sep;349(9):1157–1168. [PubMed] [Google Scholar]
- Shaw W. N., Chance R. E. Effect of porcine proinsulin in vitro on adipose tissue and diaphragm of the normal rat. Diabetes. 1968 Dec;17(12):737–745. doi: 10.2337/diab.17.12.737. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Cunningham D., Spigelman L., Aten B. Insulin biosynthesis: evidence for a precursor. Science. 1967 Aug 11;157(3789):697–700. doi: 10.1126/science.157.3789.697. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Hallund O., Rubenstein A., Cho S., Bayliss C. Isolation and properties of proinsulin, intermediate forms, and other minor components from crystalline bovine insulin. Diabetes. 1968 Dec;17(12):725–736. doi: 10.2337/diab.17.12.725. [DOI] [PubMed] [Google Scholar]
- Steiner D. F., Oyer P. E. The biosynthesis of insulin and a probable precursor of insulin by a human islet cell adenoma. Proc Natl Acad Sci U S A. 1967 Feb;57(2):473–480. doi: 10.1073/pnas.57.2.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung A. K., Yip C. C. The biosynthesis of insulin and "proinsulin" in foetal bovine pancreas. Diabetologia. 1968 Jan;4(1):68–70. doi: 10.1007/BF01241035. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Lin B. J. Amino acid composition of bovine 'proinsulin'. Biochem Biophys Res Commun. 1967 Nov 17;29(3):382–387. doi: 10.1016/0006-291x(67)90467-6. [DOI] [PubMed] [Google Scholar]
- Yip C. C., Logothetopoulos J. A specific anti-proinsulin serum and the presence of proinsulin in calf serum. Proc Natl Acad Sci U S A. 1969 Feb;62(2):415–419. doi: 10.1073/pnas.62.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
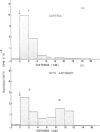
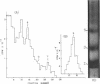
- Yip C. C. Terminal amino group determination, peptide mapping, biological and immunological activity of an insulin-like protein and the amino acid compositions of its two S-sulfo-polypeptide chains. Arch Biochem Biophys. 1968 Sep 20;127(1):741–748. doi: 10.1016/0003-9861(68)90284-1. [DOI] [PubMed] [Google Scholar]