Abstract
βarrestins mediate the desensitization of the β2-adrenergic receptor (β2AR) and many other G protein-coupled receptors (GPCRs). Additionally, βarrestins initiate the endocytosis of these receptors via clathrin coated-pits and interact directly with clathrin. Consequently, it has been proposed that βarrestins serve as clathrin adaptors for the GPCR family by linking these receptors to clathrin lattices. AP-2, the heterotetrameric clathrin adaptor protein, has been demonstrated to mediate the internalization of many types of plasma membrane proteins other than GPCRs. AP-2 interacts with the clathrin heavy chain and cytoplasmic domains of receptors such as those for epidermal growth factor and transferrin. In the present study we demonstrate the formation of an agonist-induced multimeric complex containing a GPCR, βarrestin 2, and the β2-adaptin subunit of AP-2. β2-Adaptin binds βarrestin 2 in a yeast two-hybrid assay and coimmunoprecipitates with βarrestins and β2AR in an agonist-dependent manner in HEK-293 cells. Moreover, β2-adaptin translocates from the cytosol to the plasma membrane in response to the β2AR agonist isoproterenol and colocalizes with β2AR in clathrin-coated pits. Finally, expression of βarrestin 2 minigene constructs containing the β2-adaptin interacting region inhibits β2AR endocytosis. These findings point to a role for AP-2 in GPCR endocytosis, and they suggest that AP-2 functions as a clathrin adaptor for the endocytosis of diverse classes of membrane receptors.
The agonist-mediated internalization of G protein-coupled receptors (GPCRs) represents a mechanism by which desensitized receptors are recycled to the plasma membrane as competent resensitized receptors (1–3). Many GPCRs internalize through the clathrin-coated vesicle endocytic pathway (4–7). βarrestins, which interact with the phosphorylated form of agonist-activated GPCRs to desensitize their signaling, have been demonstrated to initiate clathrin-mediated endocytosis (8, 9). The β2-adrenergic receptor (β2AR), which belongs to the GPCR family, has been postulated to endocytose via clathrin-coated pits through a direct interaction between βarrestin and clathrin (10). Consequently, it has been proposed that βarrestins serve as clathrin adaptors for GPCR endocytosis (10–12).
Clathrin-coated vesicle-mediated endocytosis of integral membrane proteins such as receptor tyrosine kinases (RTKs) involves interaction of the receptor with the clathrin adaptor complex AP-2 (13), an intrinsic component of the endocytic machinery (14). The heterotetrameric AP-2 protein complex is involved in the formation of clathrin-coated pits and functions as an adaptor by linking receptors directly to the clathrin lattice (11, 14, 15). The α subunit of AP-2 binds clathrin and has been implicated in interacting with dynamin (16, 17), a GTPase involved in the budding of clathrin-coated vesicles. This subunit also interacts with Eps15, a protein that both binds and acts as a substrate for the epidermal growth factor receptor tyrosine kinase (18). The β2 chain of AP-2 interacts with clathrin (19) and is able to promote clathrin lattice assembly (15). The μ2 chain recognizes the tyrosine-based internalization signals within the cytosolic domains of some receptors (20), whereas the function of the σ2 chain remains unclear.
Even though βarrestins bind clathrin in vitro with high affinity (21) they are not constitutively associated with clathrin-coated vesicles (22), nor do they promote clathrin-coat assembly like the AP-2 adaptor complex (15, 23). βarrestins are cytosolic proteins that translocate to activated GPCRs prior to initiating receptor internalization and resensitization (22). The ability of βarrestins to serve as clathrin adaptors would suggest that at least two distinct mechanisms might have arisen to direct receptor internalization through clathrin-coated pits. In this paper, we report that an agonist-mediated association occurs between the β2-adaptin subunit of AP-2 and βarrestin during clathrin-mediated GPCR endocytosis. This association suggests that AP-2 acts as a clathrin adaptor for GPCR endocytosis, thus indicating that different classes of receptors endocytose through common clathrin-interacting intermediates.
METHODS
Cell Transfection and β2AR Sequestration.
The βarrestin 2 clathrin-binding-deficient mutant in pcDNA 3.1 (+) zeo (βarr2AAEA) and βarrestin 2 C-terminal minigene constructs with (βarr2 310–410) or without (βarr2 310–410AAEA) the clathrin-binding site were generated by PCR, and the integrity of each construct was verified by dideoxynucleotide sequencing. The hemagglutinin epitope (HA)-tagged β2AR construct is described elsewhere (24). HEK-293 or COS-7 cells were transiently transfected with cDNA by using a modified calcium-phosphate method (5), and βarrestin expression was assessed by Western blotting using a rabbit polyclonal antibody (25). For determination of sequestration, transfected cells expressing HA-tagged β2AR alone or coexpressing HA-tagged β2AR with βarr2, βarr2AAEA, βarr2 310–410, or βarr2 310–410AAEA were exposed to 10 μM isoproterenol for 30 min at 37°C followed by washing in ice-cold phosphate-buffered saline (PBS). Cells were then incubated on ice with a mouse monoclonal anti-HA (1:500, Boehringer Mannheim) for 40–60 min followed by incubation with FITC-conjugated goat anti-mouse IgG antibody (1:250, Sigma). Sequestration was assessed by flow cytometry (24). Statistical significance was determined by a paired two-tailed t test.
Yeast Two-Hybrid Assays.
A GAL4 BD-βarrestin 2 fusion protein was constructed by excising the full-length βarrestin 2 cDNA from a pCMV5 vector with the enzymes NcoI and SalI and was cloned into pAS2–1 (CLONTECH) by using the same restrictions sites. The GAL4 AD-α, -β2, -μ2, and σ2 constructs were kindly provided by M. S. Robinson (Univ. of Cambridge). The fusion GAL4 AD-clathrin N-terminal domain was generated by excising a 2.4-kbp fragment of the rat clathrin heavy chain cDNA (kindly provided by T. Kirchhausen, Harvard Medical School) with the enzymes NcoI and SalI, and cloned into pACT2 digested with NcoI and XhoI. GAL4 BD fragments of βarrestin 2 gene with BamHI and SalI restriction sites were generated by PCR and cloned into pAS2–1 by using the same sites. Fusion genes were transformed into the yeast Y187 or PJ69–4A strain by lithium acetate transformation as described in the instructions for the Matchmaker two-hybrid kit (CLONTECH). Transformants were allowed to grow at 30°C for 2–4 days and were assayed for β-galactosidase activity. Yeast colonies were transferred directly onto sterile filter paper for the colony-lift filter assay or grown overnight in selective medium for the liquid culture assays. Filters were assayed for β-galactosidase activity in Z-buffer containing 5-bromo-4-chloro-3-indolyl d-galactoside. β-Galactosidase activities of yeast transformants in liquid culture were assayed with a chemiluminescent β-galactosidase assay kit (CLONTECH). Constructs positive for β-galactosidase activity were also tested for their adenine prototrophy in yeast strain PJ69–4A.
Immunoprecipitation and Immunodetection of AP-2.
PCR was used to introduce a Flag sequence (DYKDDDDK) at the C-terminus of βarrestin. The functional activity of the Flag-tagged βarrestin 2 was measured by assessing the ability of the Flag-tagged βarrestin 2 to promote β2AR sequestration in COS-7 cells (5, 8, 22). This construct was found to be as potent as the wild-type βarrestin 2 for rescuing β2AR sequestration. HEK-293 cells expressing HA-tagged β2AR, Flag-tagged βarrestin 1 and 2, or HA-tagged μ opioid receptor (μOR) were serum-starved overnight in minimal essential medium (MEM). Cells were treated at 37°C for different periods of time with 10 μM isoproterenol or 0.5 μM etorphine. Medium was removed, and cells were solubilized in digitonin buffer [20 mM triethanolamine⋅HCl, pH 8.0/1% (wt/vol) digitonin/20% (vol/vol) glycerol/300 mM NaCl/1 mM EDTA containing 0.1 mM phenylmethylsulfonyl fluoride, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 μg/ml pepstatin A]. Lysates were cleared by centrifugation at 100,000 × g for 15 min at 4°C, and 15 μg of mouse monoclonal anti-HA or M2 anti-Flag (Sigma) was added to each supernatant. Aliquots were taken for determinations of the total amount of adaptin in each sample. The supernatants were incubated for 1 h at 4°C with a 50% slurry mixture of protein A/G Sepharose beads (Pharmacia Biotech). The beads were recovered by centrifugation and washed three times with digitonin buffer, and bound proteins were solubilized in SDS-sample buffer [8% SDS/25 mM Tris⋅HCl, pH 6.5/10% (vol/vol) glycerol, 5% (vol/vol) 2-mercaptoethanol/0.003% bromophenol blue]. Equal amounts of proteins were loaded into wells, resolved by electrophoresis on 10% acrylamide gels, transferred onto nitrocellulose membranes, and subjected to Western blotting analysis using a mouse monoclonal anti-β2-adaptin antibody (1:1000, Transduction Laboratories).
Immunofluorescence Microscopy.
A construct of enhanced green fluorescent protein (in this paper abbreviated GFP) fused to the N terminus of β2-adaptin was made by excising β2-adaptin cDNA from pGAD24 with the restriction enzymes SmaI and BglII and ligating the resulting fragment into the SmaI and BamHI sites of pEGFP-C1 (CLONTECH). This generated a 21-aa spacer (SGLRSRAQASNSAVDGTAGPG) between the GFP C terminus and the initial methionine of β2-adaptin (GFP/β2-adaptin). HEK-293 cells transfected with HA-tagged β2AR and GFP/β2-adaptin were plated onto ethanol-sterilized glass coverslips (2.5 × 105 cells per well) in a 6-well plate in complete MEM at least 24 h before observation. The cells were incubated in MEM containing 20 mM Hepes with or without 10 μM isoproterenol for 5 min at 37°C, washed, and immediately fixed with 4% paraformaldehyde in PBS at room temperature for 20 min. Receptors were labeled in PBS containing 1% bovine serum albumin (BSA) and a mouse monoclonal anti-HA conjugated with rhodamine (1:500; Boehringer Mannheim). Clathrin was labeled by using a permeabilization solution of PBS containing 0.1% Triton X-100 and 1% BSA at pH 7.4. Cells were incubated with a crude extract of mouse anti-clathrin X22 (1:70; American Type Culture Collection) for 1 h at room temperature, washed three times, and incubated with goat anti-mouse IgG conjugated with Texas red (1:200; Molecular Probes), washed, and mounted on glass slides. GFP/β2-adaptin fluorescence was observed at 488 nm with a fluorescein filter set. Texas red and rhodamine were observed with a rhodamine filter set as previously described (26).
RESULTS AND DISCUSSION
βarrestins have been shown in vitro to bind clathrin (10), and the residues LIEF/L found in the βarrestin 1 and 2 C termini have been shown to mediate this interaction (21). Alanine substitution of three of these residues profoundly reduces βarrestin binding to clathrin without affecting the ability of βarrestin to bind phosphorylated receptor (21). However, the role of the βarrestin/clathrin interaction in cells is less well appreciated. To test whether the βarrestin/clathrin interaction is necessary and sufficient for receptor endocytosis, we examined β2AR endocytosis in COS-7 cells in the presence of a βarrestin 2 clathrin-binding mutant (βarrestin 2AAEA: L374 → A, I375 → A, F377 → A). These cells express lower levels of endogenous βarrestins than many other cells (25), enabling an assessment of the role of βarrestin on β2AR endocytosis. The overexpression of βarrestin 2AAEA enhanced β2AR internalization to the same extent as wild-type βarrestin 2, and to a level normally observed for β2AR sequestration in HEK-293 cells (Fig. 1) (5, 25). These results are inconsistent with the expected reduction in sequestration that should occur if the LIEF-mediated clathrin binding to βarrestin were the only required interaction for receptor endocytosis.
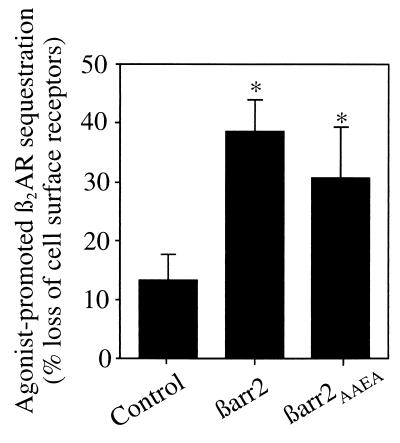
Figure 1.
Effect of βarrestins on β2AR sequestration in COS-7 cells. The agonist-mediated sequestration of β2AR was measured in the presence of endogenous βarrestin only (Control), overexpressed βarrestin 2 (βarr2), or overexpressed βarrestin 2AAEA (βarr2AAEA). Equal amounts of βarr2 and βarr2AAEA were expressed (data not shown). Sequestration is defined as the fraction of total cell surface receptors that are not accessible to antibodies after exposure to agonist, and is expressed as percent loss of cell surface receptors. Results demonstrate that βarr2AAEA and wild-type βarr2 expression similarly increase sequestration above control levels (∗, P < 0.01). The data represent the mean ± SD of three or four independent experiments.
The failure of the βarrestin 2 clathrin-binding mutant to inhibit β2AR sequestration suggests that other proteins may be required to initiate β2AR endocytosis. One potential candidate is the AP-2 adaptor complex because of its involvement in clathrin-mediated endocytosis of other classes of membrane proteins. Therefore, we initially examined whether AP-2 interacts with βarrestin by using a yeast two-hybrid assay, because it provides a sensitive method for revealing protein–protein interactions that may otherwise be difficult to detect biochemically. Different subunits of AP-2 were coexpressed with βarrestin 2 in yeast, and β-galactosidase activity resulting from their interaction was assessed qualitatively by a colony-lift filter assay and quantitatively with a chemiluminescent liquid assay (Fig. 2). The results revealed that βarrestin 2 selectively binds to the β2-adaptin subunit (Fig. 2a). The interaction between βarrestin 2 and β2-adaptin resulted in a 70-fold increase in β-galactosidase activity, whereas the other three subunits of adaptin produced no significant increases over basal activity. βarrestin 1 interacted less well with β2-adaptin as revealed by a 4- to 5-fold increase in β-galactosidase activity (data not shown).
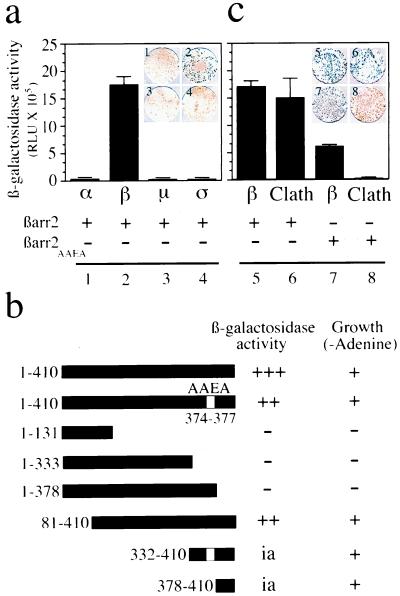
Figure 2.
Interaction between βarrestin 2 and individual subunits of AP-2 or clathrin as assessed by β-galactosidase activity in yeast. Results of colony-lift filter assays, colony growth assays on adenine-deficient medium, and liquid culture β-galactosidase assays are shown. (a) The binding interactions between βarrestin 2 (βarr2) and each of the four subunits of AP-2 were analyzed in yeast cells transformed with plasmids encoding GAL4 BD-βarr2 and one of the various GAL4 AD-adaptin subunits (α, β2, μ2, and σ2). (b) Results of the expression of the lacZ reporter gene and adenine growth in yeast cells transformed with GAL4 BD-βarr2, -βarr2AAEA, or different fragments of βarr2 with the GAL4 AD-β2-adaptin subunits. Only transformants expressing interacting proteins were able to grow in the absence of adenine, eliminating the contribution of transformants with intrinsic activity (ia). Positive results for β-galactosidase activity or growth on adenine-deficient plates are indicated by (+), and no interaction is indicated by (−). (c) The interaction of βarr2 or βarrestin2 clathrin-binding deficient mutant (βarr2AAEA) with GAL4 AD-clathrin (Clath) or GAL4 AD-β2-adaptin (β) was examined. A more intense blue coloration on the filter indicates a stronger binding interaction between the two proteins. Results, expressed in relative light units (RLU), are the mean ± SD of triplicate determinations. All results are representative of four to six independent experiments.
We next identified the region of βarrestin involved in the binding of β2-adaptin by using GAL4 BD fusion proteins of different domains of βarrestin 2 (Fig. 2b). We found that the minimal β2-adaptin binding region for transactivation of yeast reporter genes resides in residues 378–410 of the C-terminal domain of βarrestin 2. Interestingly, these residues are immediately downstream of the clathrin-binding site. This location suggests that the LIEF clathrin-binding motif is not necessary for the interaction with β2-adaptin. Using the same yeast-based assay, we could demonstrate that βarrestin 2 binding sites for clathrin and β2-adaptin are different (Fig. 2c). Whereas βarr2AAEA produced a 25-fold increase in β-galactosidase activity when assayed with β2-adaptin, no interaction was detected with clathrin. βarrestin 2 interacted equally well with both clathrin and β2-adaptin (Fig. 2c). Taken together, these data confirm that removal of the LIEF motif eliminates the interaction of βarrestin2 with clathrin. The interaction of βarrestin 2 with clathrin appears to be confined to a single region of βarrestin 2, unlike what has been suggested recently (27). Moreover, the ability of βarrestin 2 to interact with the β2-adaptin component of the endocytic machinery even in the absence of a clathrin interaction provides a potential basis to explain how the βarrestin 2AAEA mutant could retain its ability to enhance β2AR sequestration.
We next examined whether a functional complex consisting of βarrestin 2, β2AR, and β2-adaptin could be detected in HEK-293 cells by using immunoprecipitation of either epitope-tagged β2AR or βarrestins. To maximize the number of membrane-bound receptor complexes, experiments were performed in the presence of overexpressed dynamin-K44A, a dominant-negative mutant that inhibits clathrin-mediated endocytosis (28). Under these conditions the dissociation of clathrin-coated vesicles from the plasma membrane is blocked and agonist-activated β2ARs accumulate in coated pits (5). Immunoprecipitation of β2AR from isoproterenol-treated HEK-293 cells revealed a robust time-dependent increase in the association between immunoreactive β2-adaptin and the receptor, which peaked at 2 min and decreased after 5 min (Fig. 3a). The transient nature of this interaction was also observed in the absence of dynamin-K44A, but the intensity of the signal was weaker (data not shown). There were no significant differences in β2-adaptin immunoprecipitation signals between untransfected cells (mock) and unstimulated cells expressing either β2AR or βarrestin 2 (Fig. 3). β2-Adaptin could also be recovered by immunoprecipitation of Flag-tagged βarrestin 1 and 2 (Fig. 3b) or the endogenous βarrestins (data not shown), further supporting the idea that β2-adaptin interacts with βarrestins in cells. Interestingly, as opposed to the yeast two-hybrid data, βarrestin 1 and 2 interacted comparably with β2-adaptin in HEK-293 cells. The ability of β2-adaptin to interact with a receptor/βarrestin 2 complex is not restricted to the β2AR. Indeed, β2-adaptin is also recovered in immunoprecipitates of agonist-activated μOR, which like the β2AR, internalizes via clathrin-coated pits (Fig. 3c) (6).
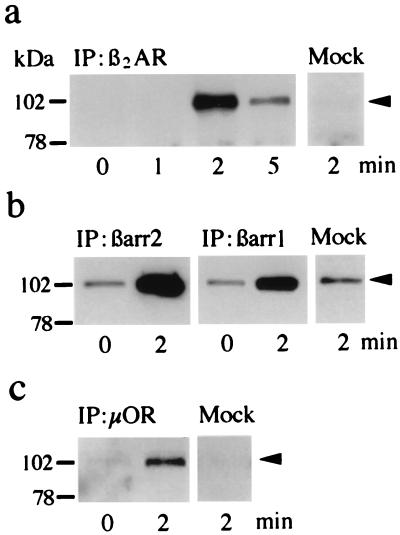
Figure 3.
β2-Adaptin immunoprecipitation in HEK-293 cells. Cells were transfected with receptor and/or βarrestin 1 (βarr1) or βarrestin 2 (βarr2) cDNA and treated as described in the text. Transfected cells were exposed to agonist for the indicated times, and the cell lysates were immunoprecipitated. (a–c) Amounts of β2-adaptin (arrowheads) that were immunoprecipitated with HA-tagged β2AR and a monoclonal antibody against the HA epitope (a), Flag-tagged βarr1 or βarr2 and a monoclonal antibody against the Flag epitope (b), or HA-tagged μOR and the anti-HA antibody (c). Immunoprecipitation results with agonist-treated mock-transfected cells are shown to the right of each panel for comparison. Results are representative of three to eight experiments.
These biochemical data suggest that β2-adaptin plays a dynamic role in the early stages of GPCR endocytosis. We investigated this possibility by using a fusion protein between GFP and β2-adaptin (GFP/β2-adaptin). HEK-293 cells were transfected with β2-adaptin or GFP/β2-adaptin, and the expression of each protein was verified by immunoblotting cell extracts with monoclonal antibodies against β2-adaptin or GFP (Fig. 4a). Since the AP-2 complex is a major structural component of clathrin-coated pits (11, 14, 15), we expected that a biologically active GFP/β2-adaptin would colocalize with clathrin. Overexpressed GFP/β2-adaptin did colocalize with clathrin in punctate regions of the plasma membrane but could also be found diffusely distributed in the cytoplasm as shown by epifluorescence microscopy (Fig. 4b). As expected, GFP/β2-adaptin colocalized with activated β2ARs in the early stages of endocytosis because β2ARs internalize via clathrin-coated pits (5). When cells were treated with isoproterenol, an agonist-mediated colocalization of GFP/β2-adaptin with β2AR became apparent as revealed by the enhanced size and intensity of punctate regions of the plasma membrane (Fig. 4b vs. 4c). Taken together, these results suggest that a redistribution of the receptor and/or the β2-adaptin might take place. To investigate this potential agonist-mediated recruitment of GFP/β2-adaptin by activated β2AR, confocal microscopy was employed (Fig. 4d). In the presence of isoproterenol, the amount of plasma membrane GFP/β2-adaptin was found to increase at the expense of the cytosolic fraction, colocalizing with plasma membrane β2AR (Fig. 4d) within the same time frame that β2-adaptin immunoprecipitated with β2AR. This increase is reflected by the enhancement of the β2-adaptin fluorescence at the plasma membrane (Fig. 4d Middle) and the increased coincidence of the GFP/β2-adaptin and β2AR signals in presence of isoproterenol (Fig. 4d Bottom). These results indicate that a dynamic agonist-mediated association occurs between β2-adaptin and the β2AR/βarrestin complex.
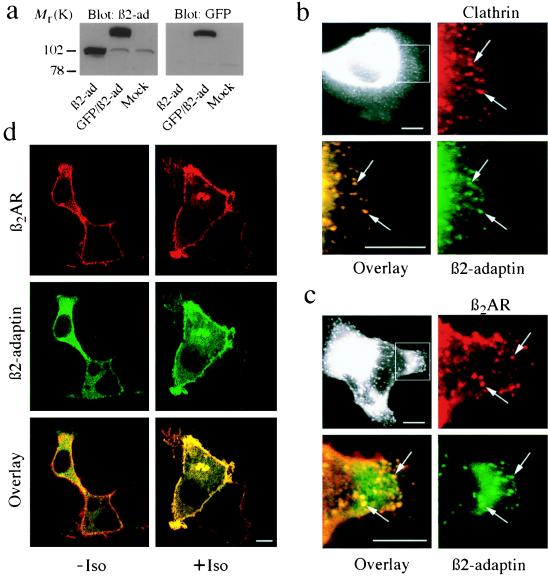
Figure 4.
Expression and distribution of GFP/β2-adaptin in HEK-293 cells. (a) Immunoblots of extracts from HEK-293 cells overexpressing β2-adaptin, GFP/β2adaptin, or endogenous β2-adaptin (mock). Proteins were detected with an antibody to β2-adaptin (Left) or GFP (Right). (b) Epifluorescence microscopy of HEK-293 cells shows transfected GFP/β2-adaptin colocalization with clathrin-coated pits. The cell area outlined in Upper Left has been enlarged in the other three panels. Upper Right shows clathrin immunostaining (red), Lower Right shows GFP/β2-adaptin fluorescence (green), and Lower Left shows the overlap (yellow or arrows) obtained from the superimposed clathrin and GFP/β2-adaptin images. (c) Demonstration of colocalization of GFP/β2-adaptin (green, Lower Right) to plasma membrane-bound β2AR (red, Upper Right) after isoproterenol treatment. The cell area outlined in Upper Left has been enlarged in the other three panels. The arrows indicate punctate areas of the plasma membrane where β2AR and β2-adaptin colocalize after isoproterenol treatment. These punctate areas correspond to clathrin-coated pits (5). (d) Confocal images demonstrating the isoproterenol-induced translocation of GFP/β2-adaptin to the plasma membrane. (Top) Plasma membrane distribution of β2AR (red) with (Right) or without (Left) isoproterenol. (Middle) Distribution of GFP/β2-adaptin (green) with or without agonist stimulation. Note the significant increase in plasma membrane localization of GFP/β2-adaptin in the presence of agonist at the expense of the cytosolic signal. In the absence of agonist (Bottom Left), the β2AR signal (red) appears at the plasma membrane, whereas that for the GFP/β2-adaptin (green) is mostly cytosolic. In the presence of agonist (Bottom Right) the fluorescent signals become predominantly coincident at the plasma membrane (yellow). Epifluorescence microscopy was done as previously described (26). Confocal images were obtained on a Zeiss LSM-410 laser scanning confocal microscope. (All scale bars = 10 μm.)
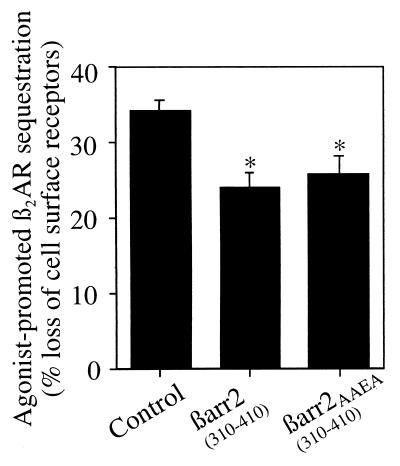
βarrestin C-terminal minigene constructs have been shown to inhibit β2AR internalization (27, 29). To investigate whether the interaction of AP-2 with βarrestin 2 was necessary for β2AR endocytosis, we overexpressed minigene constructs of βarrestin 2 containing the β2-adaptin interacting region with (βarr2 310–410) or without (βarr2 310–410AAEA) the clathrin-binding site. Both constructs were found to inhibit the agonist-induced β2AR sequestration up to 30% (Fig. 5). The moderate level of inhibition observed with these constructs is likely attributable to their low efficiency of expression (≈20%) compared with the expression of a similar construct of wild-type βarrestin 2 (data not shown). These results are consistent with our previous observations (Fig. 1) and suggest that the βarrestin minigenes function by competing for β2-adaptin binding to the endogenous βarrestins.
Figure 5.
Effect of minigene βarrestin on β2AR sequestration in HEK-293 cells. The agonist-mediated sequestration of β2AR was measured in the presence of endogenous βarrestin only (Control) and βarrestin 2 C-terminal minigene with (βarr2 310–410) or without (βarr2 310–410AAEA) the clathrin-binding site. Similar amounts of βarr2 310–410 and βarr2 310–410AAEA were expressed (data not shown). Results demonstrate that βarr2 310–410 and βarr2 310–410AAEA expression similarly decrease sequestration below control levels (∗, P < 0.01). The data represent the mean ± SD of four independent experiments.
We have shown that βarrestins specifically interact with β2-adaptin, that β2-adaptin translocates to the plasma membrane in an agonist-dependent manner, and that a stable membrane association occurs between AP-2 and the receptor/βarrestin 2 complex during β2AR (or μOR) endocytosis. In addition, overexpression of a βarrestin C-terminal minigene construct containing the β2-adaptin-binding region but lacking the LIEF clathrin-binding site is able to inhibit β2AR endocytosis, whereas the full-length βarrestin clathrin-binding mutant retains its ability to induce sequestration. These results suggest that AP-2 acts as a clathrin adaptor for GPCRs. Our findings support the postulated role of AP-2 as a common adaptor for clathrin/receptor binding and clathrin coat assembly, and they suggest that a role of βarrestin 2 in this process is to serve as a docking protein between GPCRs and AP-2. This novel role for βarrestin 2 may be analogous to the proposed function of Eps15 and/or epsin as AP-2 docking proteins for RTK endocytosis (18, 30–32). Our results thus challenge the paradigm that βarrestins assume the role of AP-2 in the GPCR endocytic process (11, 12).
The processing of plasma membrane proteins via clathrin-coated pits depends on the coordinated actions of several endocytic accessory proteins, the strength of their interactions, and their phosphorylation status (11, 15, 33, 34). Certain protein interactions may be required in the early stages of endocytosis, as appears to be the case for those between βarrestins and AP-2. Other associations such as those between βarrestins and clathrin may regulate later events in the process of endocytosis. Thus it might not be surprising that abrogation of the βarrestin 2/clathrin interaction does not prevent receptor internalization. For RTKs, multiple interactions of the receptor and associated proteins with components of the endocytic machinery have been demonstrated (13, 18, 30, 31). Similarly, additional interactions between GPCRs and AP-2 and/or other endocytic components may also occur and contribute to the endocytic process.
Results from these and other studies suggest that interactions between GPCRs and βarrestin 1 or 2 might differ. In contrast to βarrestin 2, βarrestin 1 was found to interact only weakly with β2-adaptin in the in vitro yeast two-hybrid assay. However, in HEK-293 cells βarrestin 1 appears just as effective as βarrestin 2 in forming a complex with the β2-adaptin. Interestingly, these differences may be related to the observation that the endocytic function of βarrestin 1 seems to be regulated by its phosphorylation (35). It is tempting to speculate that, like other endocytic accessory proteins (34), βarrestin 1 might be regulated by phosphorylation to interact with AP-2. Moreover, in different cells with several GPCRs, βarrestin 1 is generally less efficient than βarrestin 2 in translocating to activated receptor and promoting sequestration (unpublished data). These observations raise the possibility that these two proteins, although highly homologous, may not have totally redundant endocytic functions.
On the basis of our results, we propose a model in which an activated membrane GPCR is first desensitized by the recruitment of βarrestin from the cytosol. This complex then serves as a membrane anchor for the docking of β2-adaptin, and subsequently, for nucleation or association of other subunits of AP-2 and clathrin. Different subunits of AP-2 apparently have the ability to bind selectively to distinct classes of receptors or docking proteins (11, 13, 15, 36). This ultimately may explain why multiple types of receptors can simultaneously internalize via clathrin-coated pits.
Acknowledgments
We thank Dr. M. S. Robinson for materials and helpful discussion. We thank Drs. T. Kirchhausen for the clathrin cDNA and W. E. Miller and R. J. Lefkowitz for the Flag-tagged βarrestin 1 and helpful comments. S.A.L. is a recipient of a fellowship award from the Heart and Stroke Foundation of Canada. S.S.G.F. is a recipient of a McDonald Scholarship award from the Heart and Stroke Foundation of Canada. This work was supported in part by National Institutes of Health Grants NS 19576 (to M.G.C.) and HL 03422 (to L.S.B.). M.G.C. is an Investigator of the Howard Hughes Medical Institute and a recipient of an unrestricted Neuroscience Award from Bristol–Myers Squibb.
ABBREVIATIONS
- GPCR
G protein-coupled receptor
- β2AR
β2-adrenergic receptor
- RTK
receptor tyrosine kinase
- βarr1
βarrestin 1
- βarr2
βarrestin 2
- HA
hemagglutinin epitope
- μOR
μ opioid receptor
- GFP
enhanced green fluorescent protein
References
- 1.Yu S S, Lefkowitz R J, Hausdorff W P. J Biol Chem. 1993;268:337–341. [PubMed] [Google Scholar]
- 2.Pippig S, Andexinger S, Lohse M J. Mol Pharmacol. 1995;47:666–676. [PubMed] [Google Scholar]
- 3.Ferguson S S, Barak L S, Zhang J, Caron M G. Can J Physiol Pharmacol. 1996;74:1095–1110. doi: 10.1139/cjpp-74-10-1095. [DOI] [PubMed] [Google Scholar]
- 4.von Zastrow M, Kobilka B K. J Biol Chem. 1992;267:3530–3538. [PubMed] [Google Scholar]
- 5.Zhang J, Ferguson S S G, Barak L S, Menard L, Caron M G. J Biol Chem. 1996;271:18302–18305. doi: 10.1074/jbc.271.31.18302. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J, Ferguson S S, Barak L S, Bodduluri S R, Laporte S A, Law P Y, Caron M G. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tolbert L M, Lameh J. J Biol Chem. 1996;271:17335–17342. doi: 10.1074/jbc.271.29.17335. [DOI] [PubMed] [Google Scholar]
- 8.Ferguson S S, Downey W E, 3rd, Colapietro A M, Barak L S, Menard L, Caron M G. Science. 1996;271:363–366. doi: 10.1126/science.271.5247.363. [DOI] [PubMed] [Google Scholar]
- 9.Zhang J, Barak L S, Winkler K E, Caron M G, Ferguson S S. J Biol Chem. 1997;272:27005–27014. doi: 10.1074/jbc.272.43.27005. [DOI] [PubMed] [Google Scholar]
- 10.Goodman O B, Jr, Krupnick J G, Santini F, Gurevich V V, Penn R B, Gagnon A W, Keen J H, Benovic J L. Nature (London) 1996;383:447–450. doi: 10.1038/383447a0. [DOI] [PubMed] [Google Scholar]
- 11.Kirchhausen T, Bonifacino J S, Riezman H. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- 12.Krupnick J G, Benovic J L. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 13.Sorkin A, Carpenter G. Science. 1993;261:612–615. doi: 10.1126/science.8342026. [DOI] [PubMed] [Google Scholar]
- 14.Robinson M S. Curr Opin Cell Biol. 1994;6:538–544. doi: 10.1016/0955-0674(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 15.Schmid S L. Annu Rev Biochem. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- 16.Goodman O B, Jr, Keen J H. J Biol Chem. 1995;270:23768–23773. doi: 10.1074/jbc.270.40.23768. [DOI] [PubMed] [Google Scholar]
- 17.Wang L H, Sudhof T C, Anderson R G. J Biol Chem. 1995;270:10079–10083. doi: 10.1074/jbc.270.17.10079. [DOI] [PubMed] [Google Scholar]
- 18.Benmerah A, Begue B, Dautry-Varsat A, Cerf-Bensussan N. J Biol Chem. 1996;271:12111–12116. doi: 10.1074/jbc.271.20.12111. [DOI] [PubMed] [Google Scholar]
- 19.Shih W, Gallusser A, Kirchhausen T. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- 20.Ohno H, Stewart J, Fournier M C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 21.Krupnick J G, Goodman O B, Jr, Keen J H, Benovic J L. J Biol Chem. 1997;272:15011–15016. doi: 10.1074/jbc.272.23.15011. [DOI] [PubMed] [Google Scholar]
- 22.Barak L S, Ferguson S S, Zhang J, Caron M G. J Biol Chem. 1997;272:27497–27500. doi: 10.1074/jbc.272.44.27497. [DOI] [PubMed] [Google Scholar]
- 23.Goodman O B, Jr, Krupnick J G, Gurevich V V, Benovic J L, Keen J H. J Biol Chem. 1997;272:15017–15022. doi: 10.1074/jbc.272.23.15017. [DOI] [PubMed] [Google Scholar]
- 24.Barak L S, Tiberi M, Freedman N J, Kwatra M M, Lefkowitz R J, Caron M G. J Biol Chem. 1994;269:2790–2795. [PubMed] [Google Scholar]
- 25.Menard L, Ferguson S S, Zhang J, Lin F T, Lefkowitz R J, Caron M G, Barak L S. Mol Pharmacol. 1997;51:800–808. [PubMed] [Google Scholar]
- 26.Barak L S, Ferguson S S, Zhang J, Martenson C, Meyer T, Caron M G. Mol Pharmacol. 1997;51:177–184. doi: 10.1124/mol.51.2.177. [DOI] [PubMed] [Google Scholar]
- 27.Orsini M J, Benovic J L. J Biol Chem. 1998;273:34616–34622. doi: 10.1074/jbc.273.51.34616. [DOI] [PubMed] [Google Scholar]
- 28.van der Bliek A M, Redelmeier T E, Damke H, Tisdale E J, Meyerowitz E M, Schmid S L. J Cell Biol. 1993;122:553–563. doi: 10.1083/jcb.122.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krupnick J G, Santini F, Gagnon A W, Keen J H, Benovic J L. J Biol Chem. 1997;272:32507–32512. doi: 10.1074/jbc.272.51.32507. [DOI] [PubMed] [Google Scholar]
- 30.Benmerah A, Lamaze C, Begue B, Schmid S L, Dautry-Varsat A, Cerf-Bensussan N. J Cell Biol. 1998;140:1055–1062. doi: 10.1083/jcb.140.5.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coda L, Salcini A E, Confalonieri S, Pelicci G, Sorkina T, Sorkin A, Pelicci P G, Di Fiore P P. J Biol Chem. 1998;273:3003–3012. doi: 10.1074/jbc.273.5.3003. [DOI] [PubMed] [Google Scholar]
- 32.Chen H, Fre S, Slepnev V I, Capua M R, Takei K, Butler M H, Di Fiore P P, De Camilli P. Nature (London) 1998;394:793–797. doi: 10.1038/29555. [DOI] [PubMed] [Google Scholar]
- 33.Cremona O, De Camilli P. Curr Opin Neurobiol. 1997;7:323–330. doi: 10.1016/s0959-4388(97)80059-1. [DOI] [PubMed] [Google Scholar]
- 34.Slepnev V I, Ochoa G C, Butler M H, Grabs D, De Camilli P. Science. 1998;281:821–824. doi: 10.1126/science.281.5378.821. [DOI] [PubMed] [Google Scholar]
- 35.Lin F T, Krueger K M, Kendall H E, Daaka Y, Fredericks Z L, Pitcher J A, Lefkowitz R J. J Biol Chem. 1997;272:31051–31057. doi: 10.1074/jbc.272.49.31051. [DOI] [PubMed] [Google Scholar]
- 36.Boge M, Wyss S, Bonifacino J S, Thali M. J Biol Chem. 1998;273:15773–15778. doi: 10.1074/jbc.273.25.15773. [DOI] [PubMed] [Google Scholar]