Abstract
Aims/hypothesis
This 52-week multinational, randomised, open-label, parallel-group, non-inferiority trial compared clinical outcomes following supplementation of oral glucose-lowering drugs with basal insulin analogues detemir and glargine in type 2 diabetic patients.
Methods
Insulin-naive adults (n = 582, HbA1c 7.5–10.0%, BMI ≤ 40.0 kg/m2) were randomised 1:1 to receive insulin detemir or glargine once daily (evening) actively titrated to target fasting plasma glucose (FPG) ≤ 6.0 mmol/l. An additional morning insulin detemir dose was permitted if pre-dinner plasma glucose (PG) was >7.0 mmol/l after achieving FPG < 7.0 mmol/l. Due to labelling restrictions, no second glargine dose was allowed.
Results
Baseline HbA1c decreased from 8.6 to 7.2 and 7.1% (NS) with detemir and glargine, respectively. FPG improved from 10.8 to 7.1 and 7.0 mmol/l (NS), respectively. With detemir, 45% of participants completed the study on once daily dosing and 55% on twice daily dosing, with no difference in HbA1c. Overall, 52% of participants achieved HbA1c ≤ 7.0%: 33% (detemir) and 35% (glargine) without hypoglycaemia. Within-participant variability for self-monitored FPG and pre-dinner PG did not differ by insulin treatment, nor did the relative risk of overall or nocturnal hypoglycaemia. Modest reductions in weight gain were seen with detemir vs glargine in completers (3.0 vs 3.9 kg, p = 0.01) and in the intention-to-treat population (2.7 vs 3.5 kg, p = 0.03), primarily related to completers on once-daily detemir. Mean daily detemir dose was higher (0.78 U/kg [0.52 with once daily dosing, 1.00 U/kg with twice daily dosing]) than glargine (0.44 IU/kg). Injection site reactions were more frequent with detemir (4.5 vs 1.4%).
Conclusions/interpretation
Supplementation of oral agents with detemir or glargine achieves clinically important improvements in glycaemic control with low risk of hypoglycaemia. Non-inferiority was demonstrated for detemir using higher insulin doses (mainly patients on twice daily dosing); weight gain was somewhat reduced with once daily insulin detemir.
ClinicalTrials.gov ID no.: NCT00283751.
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-007-0911-x) contains supplementary material, which is available to authorised users.
Keywords: Body weight, Fasting plasma glucose, Glucose variability, Glucose control, Hypoglycaemia, Insulin detemir, Insulin glargine, Insulin supplementation, Oral glucose-lowering agents, Type 2 diabetes
Introduction
People with type 2 diabetes inadequately controlled by oral glucose-lowering drugs can achieve clinically relevant improvements in HbA1c with the addition of insulin therapy [1–3]. However, patients and healthcare providers are often reluctant to initiate insulin due to concerns over injections, fear of hypoglycaemia and additional weight gain, and also because insulin treatment is perceived as complex and an added burden to diabetes management [4, 5]. Moreover, once insulin is initiated, recommended targets for glycaemic control (HbA1c < 6.5–7.0%) are often not met [5–7].
In recent years, the basal insulin analogues glargine and detemir have been introduced. These were developed to improve upon the limitations of NPH insulin (NPH) and other conventional basal insulins, which have an inadequate duration of action, a marked peak glucose-lowering effect and variability in response from one injection to another [8]. These analogues might help to overcome some of the barriers to insulin initiation and optimisation, including concerns over hypoglycaemia and weight gain.
Several recent studies have assessed basal insulin as an add-on therapy to oral glucose-lowering drugs, comparing either insulin glargine or insulin detemir with NPH, and using titration algorithms based on glucose monitoring [1, 9–13]. These studies have demonstrated that simple regimens involving a once or twice daily injection of a basal insulin analogue can achieve clinically important improvements in glycaemic control similar to those achievable with NPH, but with less risk of hypoglycaemia. Insulin detemir has consistently shown less body weight gain than NPH when used in this way, as well as when used in basal plus mealtime insulin therapy [14, 15], whereas a weight advantage has been reported in only a few of the comparative trials of glargine vs NPH, as for example in the recent LANMET study [11].
Insulin glargine is licensed only for once daily use as a basal insulin for people with diabetes. Insulin detemir, in contrast, is available for once or twice daily use. Glucose clamp comparisons between these insulins have given contradictory information on whether their duration of effect is comparable [16, 17]. The only direct comparison in patients with type 2 diabetes suggests very similar pharmacodynamic profiles at clinically relevant doses [16], but methodological issues remain controversial. The objective of the current study was to compare treatment with insulin detemir and insulin glargine in line with their licensed indications as add-on therapy to oral glucose-lowering agents in insulin-naive patients with type 2 diabetes.
Methods
Study protocol This 52-week, parallel-group trial was conducted in 2003 and 2004 at 80 sites in Europe and the USA and included 582 insulin-naive people with type 2 diabetes, who were randomised (1:1) and treated with insulin detemir (Levemir; Novo Nordisk, Bagsværd, Denmark) or insulin glargine (Lantus; sanofi-aventis, Paris, France) as add-on therapy to oral glucose-lowering drugs. The trial was conducted in accordance with the Declaration of Helsinki and principles for Good Clinical Practice and was approved by ethics committees/review boards in all countries. All participants gave written informed consent.Concealed randomisation was carried out by an automatic telephone response system and was stratified according to oral glucose-lowering drug mono- or combination-therapy at entry. An open-label design was required to allow twice daily administration of insulin detemir if needed, according to the dosing algorithm targets for pre-dinner plasma glucose (PG) concentrations (Table 1). Glargine was only administered once daily at bedtime as per study protocol and in accordance with its licence [18]. To reduce potential bias, HbA1c results were only disclosed to investigators at randomisation and at trial end.
Table 1.
Algorithm used for insulin dose titration
| Algorithm | Adjustment of insulin dose (U) | |
|---|---|---|
| If positive response to previous dose adjustment | If no response to previous dose adjustmenta | |
| Evening insulin dose adjustment | ||
| Average pre-breakfast self-monitored PG | ||
| >10.0 mmol/l | +12 | +12 |
| 9.1–10.0 mmol/l | +8 | +10 |
| 8.1–9.0 mmol/l | +6 | +8 |
| 7.1–8.0 mmol/l | +4 | +6 |
| 6.1–7.0 mmol/l | +2 | +2 |
| If one self-monitored PG measurement | ||
| 3.1–4.0 mmol/l | −2 | –2 |
| <3.1 mmol/l | −4 | –4 |
| Morning insulin dose adjustmentb | ||
| Average pre-dinner self-monitored PG | ||
| >10.0 mmol/l | +8 | +8 |
| 9.1–10.0 mmol/l | +6 | +8 |
| 8.1–9.0 mmol/l | +4 | +6 |
| 7.1–8.0 mmol/l | +2 | +4 |
| 6.1–7.0 mmol/l | +2 | +2 |
| If one self-monitored PG measurement | ||
| 3.1–4.0 mmol/l | –2 | –2 |
| <3.1 mmol/l | –4 | –4 |
a Non-responses: the average self-monitored PG level is increased and/or within the same range as at the last contact
bSome insulin detemir-treated participants only
+, insulin dose titrated up; –, insulin dose titrated down
Participants Insulin-naive men and women with type 2 diabetes and the following characteristics were recruited: ≥18 years old, ≥12 months disease duration, BMI ≤ 40.0 kg/m2 and HbA1c 7.5–10.0%. For inclusion, they had to be receiving one or two oral agents (metformin, insulin secretagogues, α-glucosidase inhibitors) ≥4 months on at least one-half the maximum recommended dose, according to local guidelines. Exclusion criteria included treatment with thiazolidinediones (due to labelling restrictions in Europe), use of more than two oral agents within 6 months, hypoglycaemic unawareness or other medical conditions likely to interfere with trial conduct. Withdrawal criteria included pregnancy, HbA1c > 11.0% after the first 12 weeks of treatment and initiation of medication interfering with glucose metabolism.
Study medications Oral glucose-lowering therapy, diet and physical activity were recommended to remain stable during the study; no meal-time insulin was allowed. Basal insulin was initiated once daily in the evening at a dose of 12 U and titrated according to a structured treatment algorithm. In line with its licence, people allocated to insulin detemir were allowed to receive an additional morning insulin dose if pre-dinner PG was >7.0 mmol/l, but only if pre-breakfast PG was <7.0 mmol/l or nocturnal hypoglycaemia (major episode or PG ≤ 4.0 mmol/l) precluded achievement of the fasting plasma glucose (FPG) target. Insulin detemir was administered with a pen-injector (FlexPen, Novo Nordisk) 1 h before to 1 h after dinner, and if needed within 30 min of breakfast. Glargine was given once daily at bedtime, via a pen-injector in the EU (OptiPen Pro 1, sanofi-aventis) and with syringes in the USA. The evening dose of insulin detemir was administered somewhat earlier than that of insulin glargine, to allow for the possibility that recipients might be switched to a twice daily schedule, in which case a more even distribution of the dose across 24 h would be achieved.
Titration of basal insulin Participants attended 16 scheduled visits and nine telephone contacts over 1 year. Evening insulin doses were titrated throughout the trial to a FPG target ≤6.0 mmol/l in the absence of hypoglycaemia. An identical pre-dinner glucose target was applied for people administering a morning dose of insulin detemir (Table 1). Participants measured capillary PG using glucose meters (Medisense Xtra; Abbott, Wiesbaden, Germany). Dose adjustments were to be based on the average of three self-measurements before breakfast (and before dinner if on twice daily insulin detemir). During the first 12 weeks, participants had weekly investigator contact. A titration committee monitored the algorithm for insulin dose optimisation and reviewed prescribed insulin doses periodically. This committee was not informed of the treatment insulin being used, but this would have been evident for participants on twice daily insulin detemir.
Outcome measures The primary endpoint was baseline-adjusted HbA1c at end of treatment. Secondary variables included clinic FPG, within-participant variation in PG, ten-point self-measured PG profiles, proportion of participants achieving HbA1c ≤ 7.0% with and without hypoglycaemia, change in body weight, incidence of hypoglycaemia, adverse events and standard safety parameters.
Analyses and assessments HbA1c was analysed by HPLC (Bio Rad, Munich, Germany [EU analyses] and Bio Rad, Hercules, CA, USA [US analyses], DCCT-harmonised), with a reference range 4.3–6.1%. Clinic FPG was measured centrally by a hexokinase method (Gluco-quant; Roche, Mannheim, Germany). Body weight was measured using calibrated scales. Participants recorded ten-point PG profiles during the last week of treatment. Hypoglycaemia was classified as ‘major’ if assistance from another person was required, ‘minor’ if confirmed by PG < 3.1 mmol/l or ‘symptoms only’ if PG ≥ 3.1 mmol/l or no measurement was made.
Statistical analyses The sample size was based on non-inferiority of insulin detemir relative to insulin glargine for HbA1c after 52 weeks. To achieve a power of 95% with an expected SD for change in HbA1c of 1.1% and allowing a dropout rate of 15%, it was originally planned to randomise 466 patients. However, this was extended to 566 patients to allow for potential regional differences between the EU and US. The possibility of a treatment × region interaction was tested, but eliminated from the model as no significant interaction was found. Non-inferiority was accepted if the upper limit of the two-sided 95% CI for the difference in HbA1c (detemir–glargine) was less than 0.4%-units, a value decided in discussion with the Food and Drug Administration (FDA) for the entire insulin detemir phase III study programme.Unless otherwise specified, all analyses were based on the intention-to-treat (ITT) population, this being all randomised patients exposed to insulin detemir or insulin glargine. A significance level of 5% was used for all analyses. Participants were regarded as being on once- or twice-daily insulin detemir according to the regimen used at trial completion. Statistical analyses were not made for these subsets of patients, as this was non-randomised and protocol-determined; results are given for observational comparison only.Analyses of HbA1c, clinic FPG and change in weight after 52 weeks were by ANOVA with treatment, region and oral glucose-lowering therapy as fixed effects and baseline (randomisation) value as covariate. The last observation carried forward (LOCF) principle was specified for HbA1c and clinic FPG in non-completers treated ≥12 weeks (time of the first on-treatment measurements). Change in weight was estimated based on all participants completing the trial. Additional weight analyses were prepared for the ITT population, applying the same LOCF principle or using imputed data, by treatment-specific linear regression, from week 12 onwards to account for continuing weight change and limit the influence of differences in withdrawal rates and times between treatments. Fisher’s exact test was used to compare the numbers of patients on insulin detemir and insulin glargine with an HbA1c ≤ 7.0% without symptomatic hypoglycaemia confirmed by PG <4.0 mmol/l or any single value <3.1 mmol/l during the last 3 months of treatment.The ten-point self-monitored PG profiles were analysed for parallelism using repeated measures ANOVA depending on treatment, region, oral glucose-lowering therapy, time and treatment × time interaction as fixed effects. Within-participant variation in self-measured fasting and pre-dinner PG was determined from four measurements for each during the last week of treatment. Hypoglycaemic episodes during the 52 week treatment period were analysed as recurrent events in a Cox regression analysis using a gamma frailty model (Splus 2000; Insightful, Seattle, WA, USA). Nocturnal episodes (2300 to 0600 hours) were analysed separately. Adverse events were summarised using descriptive statistics. All other analyses were performed using SAS version 8.2 (SAS Institute, Cary, NC, USA).
Results
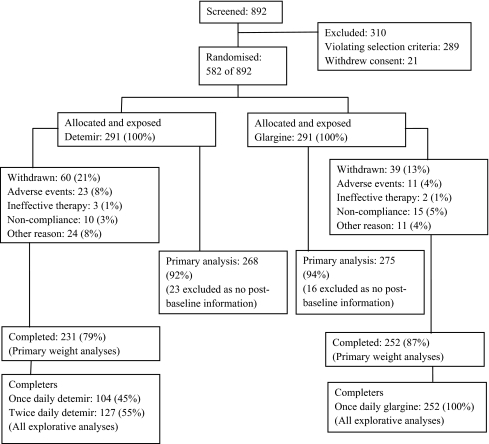
Participants Of 892 patients screened, 582 were randomised, while 289 did not fulfil the selection criteria and 21 withdrew consent. In each treatment arm, 291 patients were randomised and treated, of whom 231 (79%) and 252 (87%) completed the trial on insulin detemir and insulin glargine, respectively (Fig. 1). The higher withdrawal rate observed with insulin detemir was partly due to adverse events, ten of which were considered possibly or probably related to trial drug (Electronic supplementary material [ESM] Table 1). These included two cases of possible cutaneous allergy, five of injection site reactions and one each of injection site inflammation, hypoglycaemia and weight increase. Adverse event withdrawals with possible or probable relation to glargine included one case each of pruritus, myalgia, hyperglycaemia and hypoglycaemia. Details of all adverse events leading to withdrawal are provided in ESM Table 2. Other diabetes-related reasons for withdrawal with insulin detemir included HbA1c 12.0% (n = 1) and multiple hypoglycaemic episodes (n = 1). Other reasons for withdrawal included the closure of a trial site and various circumstantial events unrelated to the trial medications. Baseline characteristics (Table 2) were comparable between the two treatment groups. Participants completing the trial on once or twice daily insulin detemir had a baseline HbA1c of 8.60 and 8.66%, respectively.
Fig. 1.
Patient disposition during the trial and consequent analysis sets
Table 2.
Clinical characteristics of type 2 diabetic participants
| Characteristics | Detemir | Glargine |
|---|---|---|
| Randomised/exposed/ITT, n (%) | 291 (100) | 291 (100) |
| Men/women, n | 166/125 | 171/120 |
| Ethnicity (n) | ||
| Black | 22 | 12 |
| White | 250 | 263 |
| Asian-Pacific islander | 7 | 7 |
| Other | 12 | 9 |
| Oral glucose-lowering drugs, n (%) | ||
| Monotherapy | 73 (25) | 70 (24) |
| Metformin | 32 (11) | 33 (11) |
| Insulin secretagogues | 41 (14) | 37 (13) |
| Combination therapy | 218 (75) | 221 (76) |
| Metformin + secretagogue | 212 | 215 |
| Metformin + alpha glucosidase inhibitor | 3 | 1 |
| Secretagogue + alpha glucosidase inhibitor | 3 | 4 |
| Secretagogue + secretagogue (SU + glinide) | – | 1 |
| Age (years) | 58.4 (10.2) | 59.4 (9.6) |
| Weight (kg)a | 87.4 (16.6) | 87.4 (17.4) |
| BMI (kg/m2) | 30.6 (4.8) | 30.5 (4.6) |
| Duration of diabetes (years) | 9.1 (6.1) | 9.1 (6.4) |
| HbA1c (%)a | 8.64 (0.78) | 8.62 (0.77) |
| C-peptide (nmol/l) | 0.87 (0.56) | 0.85 (0.55) |
Values are n (%) or mean (SD)
aBefore randomisation
SU, sulfonylurea
Insulin dosing Of participants treated with insulin detemir, 104 (45%) completed the trial on one daily injection, while 129 (55%) administered an additional morning dose. The majority of patients administering insulin detemir twice daily (n = 103) were transferred to this regimen within 12 weeks of treatment. At the time of transfer, about two-thirds of the patients adding a morning injection fulfilled the formal dose transfer guidelines, the other one-third were changed through investigator discretion. Participants using insulin detemir twice daily administered about 40% of their daily insulin dose before breakfast. After 52 weeks the mean daily dose of insulin detemir (n = 227) was 0.78 U/kg (0.52 U/kg on once daily [n = 102] and 1.00 U/kg on twice daily [n = 125]) and the mean daily dose of glargine (n = 248) was 0.44 IU/kg.
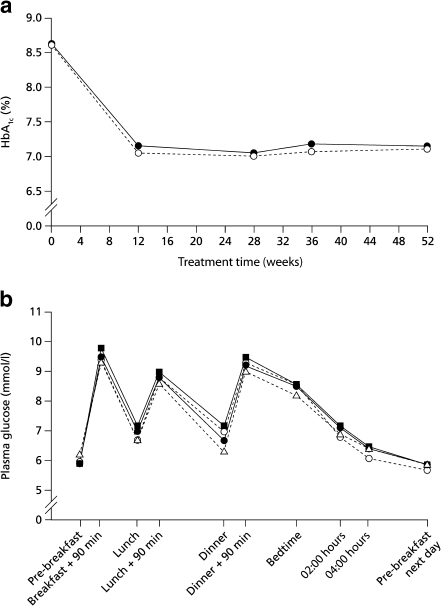
Glycaemic control HbA1c decreased by 1.5% with both insulins and was comparable after 52 weeks at 7.2% (n = 268) and 7.1% (n = 275) for detemir and glargine respectively (Table 3), with an estimated difference of 0.05 (−0.11, 0.21)%, thereby meeting the criteria for non-inferiority for insulin detemir vs glargine. HbA1c was similar in participants completing the study on once or twice daily insulin detemir (7.1 and 7.1%). Clinic FPG was 10.8 mmol/l at baseline in both arms and was comparable at end of treatment with insulin detemir and insulin glargine (7.1 and 7.0 mmol/l) (Table 3). The profile of change in HbA1c and FPG was very similar (Fig. 2a). Mean self-monitored FPG was comparable between treatments, being 6.1 mmol/l with insulin detemir and 6.0 mmol/l with insulin glargine; self-monitored PG pre-dinner (6.8 and 7.0 mmol/l) was also similar. Within-participant variation in self-monitored PG pre-breakfast and pre-dinner did not differ significantly between insulin detemir and insulin glargine (Table 3). The overall shape of the ten-point self-monitored PG profiles during the last week of treatment was parallel for insulin detemir (n = 218) and insulin glargine (n = 246) (NS) and appeared identical, regardless of treatment regimen (Fig. 2b).Of participants in both arms, 52% achieved HbA1c ≤ 7.0%, while 33% treated with insulin detemir and 35% treated with insulin glargine did so in the absence of hypoglycaemia. Fasting and pre-dinner PG targets of ≤6.0 mmol/l were achieved by 25 and 20% of participants treated with insulin detemir and insulin glargine (NS), respectively; the pre-breakfast target alone was achieved by 46 and 58% (p < 0.01) and the pre-dinner target by 38 and 30%, respectively (p < 0.05).
Table 3.
Glycaemic control in type 2 diabetic patients treated with insulin detemir or insulin glargine
| Detemir | Glargine | Difference (95% CI) (detemir/glargine) | p value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Completers on once daily | Completers on twice daily | All | All | |||||||
| Parameters | n | Mean | n | Mean | n | Mean | n | Mean | ||
| HbA1c % (SE)a | 104 | 7.12 (0.11) | 127 | 7.06 (0.10) | 268 | 7.16 (0.08) | 275 | 7.12 (0.08) | 0.05 (–0.11, 0.21) | – |
| Clinic FPG (mmol/l)a | 104 | 7.27 (0.31) | 127 | 6.73 (0.25) | 268 | 7.14 (0.21) | 272 | 6.98 (0.21) | 0.16 (–0.26, 0.58) | – |
| HbA1c ≤7.0%, n (%) | 103 | 52 (51) | 127 | 69 (54) | 248 | 129 (52) | 259 | 135 (52) | – | 1.00 |
| HbA1c ≤7.0% without hypoglycaemia, n (%) | 103 | 31 (30) | 127 | 48 (38) | 248 | 82 (33) | 259 | 90 (35) | – | 0.71 |
| Within-participant variation (mmol/l) | ||||||||||
| Pre-breakfast (mmol/l)b | 103 | 0.93 (15.0) | 125 | 1.15 (19.6) | 238 | 1.06 (17.5) | 257 | 1.03 (17.3) | – | 0.45 |
| Pre-dinner (mmol/l)b | 103 | 1.27 (19.8) | 125 | 1.84 (26.4) | 238 | 1.60 (23.6) | 258 | 1.55 (22.0) | – | 0.41 |
aMean (SE); n=number of patients for whom data are available
bWithin-participant SD (CV%)
Fig. 2.
Change in HbA1c with time (a). Black circles, insulin detemir; white circles, insulin glargine. b Mean ten-point self-monitored PG profiles during the last week of treatment. Triangles, insulin detemir (once daily); squares, insulin detemir (twice daily); black circles, insulin detemir (all patients); white circles, insulin glargine
Hypoglycaemia The risk of hypoglycaemia of any type was comparable between treatments (Table 4). The overall rate of hypoglycaemia was low at 5.8 vs 6.2 episodes per patient-year with insulin detemir versus insulin glargine (relative risk: 0.94 [96% CI 0.71, 1.25]), while the rate of nocturnal hypoglycaemia was only 1.3 episodes per patient-year with both insulins. Adjustment for HbA1c did not affect the outcome of the analyses. Major hypoglycaemic episodes were rare with both insulins, especially at night, and could not be statistically analysed.
Table 4.
Hypoglycaemic episodes in type 2 diabetic patients treated with insulin detemir or insulin glargine
| Events | Insulin detemir (n = 291) | Insulin glargine (n = 291) | Relative risk (detemir/glargine) (95% CI) | ||||
|---|---|---|---|---|---|---|---|
| Participants, n (%) | Episodes (n) | Rate (per patient-year) | Participants, n (%) | Episodes (n) | Rate (per patient-year) | ||
| All | 182 (63) | 1521 | 5.8 | 191 (66) | 1670 | 6.2 | 0.94 (0.71–1.25) |
| Nocturnal | 95 (33) | 352 | 1.3 | 93 (32) | 350 | 1.3 | 1.05 (0.69–1.58) |
| Major | 5 (2) | 9 | 0.0 | 8 (3) | 8 | 0.0 | – |
| Nocturnal | 3 (1) | 5 | 0.0 | 4 (1) | 4 | 0.0 | – |
| Minor | 135 (46) | 737 | 2.9 | 151 (52) | 786 | 2.9 | 1.05 (0.75–1.46) |
| Nocturnal | 73 (25) | 212 | 0.8 | 71 (24) | 192 | 0.7 | 1.17 (0.75–1.83) |
| Symptoms only | 137 (47) | 760 | 3.0 | 133 (46) | 866 | 3.2 | 0.88 (0.61–1.25) |
| Nocturnal | 48 (17) | 128 | 0.5 | 49 (17) | 151 | 0.6 | 0.88 (0.50–1.54) |
No statistical analyses were performed on the small numbers of major events.
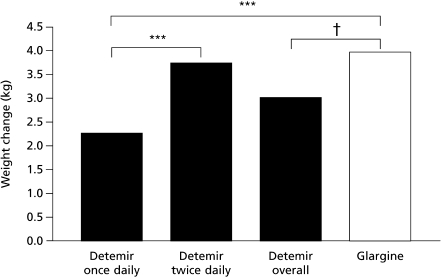
Body weight Weight gain in participants completing 52 weeks of treatment was lower with insulin detemir (n = 230) than with insulin glargine (n = 252) (3.0 [SE = 0.4] vs 3.9 [SE = 0.4] kg, p = 0.01). Change in weight analysed by LOCF (detemir n = 268, glargine n = 275) confirmed this lower weight gain (2.7 vs 3.5 kg, p = 0.03), as did analysis with imputation for non-completers (2.8 vs 3.5 kg, p = 0.04). Patients completing the study on once daily insulin detemir (n = 104) had a weight gain of 2.3 (SE = 0.5) kg, whereas those treated twice daily (n = 126) gained 3.7 (SE = 0.4) kg, similar to glargine (Fig. 3).
Fig. 3.
Mean weight change from baseline at week 52 in patients completing treatment on insulin detemir once or twice daily (and overall) and insulin glargine. ***p < 0.001; †p < 0.012
Adverse events and other safety measures Serious adverse events were less frequent with insulin detemir (42 patients with 47 events) than with glargine (53 patients with 73 events). However, only five serious events with insulin detemir (two hypoglycaemia, one each of hypothyroidism, injection-site reaction and a motor vehicle accident) and four with glargine (three hypoglycaemia, one hyperglycaemia) were considered by local investigators to be probably or possibly related to trial products. A participant treated with insulin detemir was found dead in bed (no autopsy report available), but had a history of myocardial infarction. A patient using glargine was hospitalised after the study for pulmonary fibrosis and died of cryptogenic fibrosing alveolitis. Adverse events recorded as serious tended to be of a wide-ranging disparate nature, with no clear pattern of between-treatment differences. Full details of all serious adverse events and events recorded as possibly or probably related to the study insulins are given as in the ESM Tables 1, 2, 3, and 4).The only differences in adverse events, judged as possibly or probably related to trial drugs, between treatments were: injection-site disorders (13 patients [4.5%] on insulin detemir compared with four [1.4%] on glargine), allergic reactions (three patients on detemir vs one on glargine) and skin disorders including pruritus and rash (six patients on insulin detemir vs one on glargine). There were no differences in standard safety parameters between treatments.
Discussion
The results of this first head-to-head comparison of insulin detemir and insulin glargine as add-on for treatment with oral glucose-lowering agents in people with type 2 diabetes using forced insulin titration suggest that clinically significant and similar improvements in glycaemic control can be achieved with both analogues, together with a similarly low risk of hypoglycaemia. Insulin doses were higher with insulin detemir overall, partly as a result of the doses used by those taking it twice daily. The withdrawal rate appeared to be higher with insulin detemir (21 vs 13%), which was partly accounted for by adverse events (8 vs 4%), this excess being primarily due to injection site reactions. Insulin detemir was associated with a modest relative reduction in weight gain, consistent with observations in previous comparisons of this analogue with NPH in type 2 diabetes [12, 13, 19, 20]. In the present study, the between-treatment difference in weight was primarily accounted for by those patients completing the study on once-daily insulin detemir. It is, however, possible that differences in eating pattern were contributory factors in both the insulin detemir dosing schedule and the different levels of weight gain observed between the two insulin detemir dosing schedules. The mechanism(s) responsible for relatively lower weight gain observed here and previously with insulin detemir remain the object of speculation [14, 15, 21, 22].
The present study was designed to compare the two basal insulin analogues as they had been used in previous studies and according to label. Thus, insulin glargine was given once daily at bedtime regardless of glycaemic profile, while for insulin detemir an option was provided for adding a second dose, primarily based on pre-dinner blood glucose level following a structured insulin titration protocol. The decision to use twice-daily insulin detemir could not be attributed to hypoglycaemia, as patients who were switched to a twice-daily regimen had on average 3.7 hypoglycaemic episodes per patient-year prior to transfer, compared with 4.7 episodes per patient-year in patients completing treatment on once-daily insulin detemir. The frequency of nocturnal hypoglycaemia was also lower in relevant patients before transfer (0.7 vs 0.9 episodes per patient-year).
Interestingly, the initiation of insulin with once-daily insulin detemir has recently been tested by Philis-Tsimikas et al. in type 2 diabetes patients on oral glucose-lowering agents, resulting in HbA1c reductions of 1.5% (morning injection) and 1.6% (evening injection) from baseline values of 9.1 and 8.9%, respectively [13]. These improvements in glycaemic control approach the magnitude of HbA1c reduction shown in the current study and in previous studies of once-daily insulin glargine [9–11], although the insulin detemir doses were higher at trial-end and the absolute HbA1c values achieved at trial-end were relatively high.
The design of the current study does not allow definitive conclusions for comparison of once and twice daily dosing with insulin detemir, although these post hoc observations do suggest that once daily administration can be an appropriate starting regimen for people using insulin detemir as add-on to oral glucose-lowering drug therapy. However, with dose optimisation, a significant proportion of patients may eventually need a twice-daily regimen, guided by structured glucose monitoring. Further studies are required to better define the differences between these two basal insulin analogues on the basis of a similar insulin administration regimen, once daily only, for both insulins. Alternatively, if the option of adding a second insulin dose is available for both insulins, then a properly designed study protocol should allow addition of this second insulin injection only when a normal or much lower FPG target is achieved. Indeed, the use of lower FPG targets (e.g. ≤5.5 mmol/l, as has been used previously) should also enable a higher proportion of patients to reach guideline HbA1c targets.
In conclusion, the use of insulin detemir or insulin glargine as add-on to oral glucose-lowering therapy resulted in comparable HbA1c improvements and a similarly low risk of hypoglycaemia. Further comparisons of detemir and glargine are required to fully understand how each may relatively benefit defined groups of patients starting on insulin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(PDF 12.4 kb)
Treatment emergent adverse events probably or possibly related to trial product by system organ class, ITT cohort (PDF 20.2 kb)
Adverse events leading to withdrawal (PDF 17.2 kb)
Treatment emergent serious adverse events by system organ class, ITT cohort (PDF 23.1 kb)
Treatment emergent serious adverse events preferred term, occurring in more than one subject, ITT (PDF 14.6 )
Acknowledgements
The investigators and participants are thanked for their participation. A full list of investigators can be seen in the ESM 1. The study was funded and monitored by Novo Nordisk. The authors and/or their institutions received support from Novo Nordisk (J. Larsen and C. Koenen are employees) and Aventis (now sanofi aventis) for consultation/research/teaching activities. The authors thank T. Rambrand, M. Edmunds and C. Jones for assistance with manuscript preparation. An abstract based on feasibility of titration data was presented at American Diabetes Association (ADA) meeting in 2004 (Rosenstock et al. Diabetes 53 (Suppl. 2): A145) and another abstract reporting the findings was presented at the ADA meeting in 2006 (Rosenstock et al. Diabetes 55(1): A132).
Duality of interest C. Koenen and J. Larsen are employees of Novo Nordisk. M. Davies has received grants in support of investigator and internal trials from Servier, Novartis, Novo Nordisk, Pfizer, sanofi-aventis and Lilly, and has acted as a consultant and/or speaker for Novartis, Novo Nordisk, sanofi-aventis, Lilly, Merck Sharp & Dohme, and Servier. P. Home has received consultancy fees and/or honoraria from Novo Nordisk and sanofi aventis. J. Rosenstock has served on advisory boards and received honoraria/consulting fees from Pfizer, sanofi-aventis, Novo Nordisk, Eli Lilly, GlaxoSmithKline, MannKind, Takeda, Centocor, Johnson & Johnson, Roche and Emisphere, and has also received grant support from Merck, Pfizer, sanofi aventis, Novo Nordisk, Eli Lilly, GlaxoSmithKline, Takeda, Novartis, AstraZeneca, Amylin, Roche, Sankyo and MannKind. G. Shernthaner has no relevant conflicts of interest.
Abbreviations
- FPG
fasting plasma glucose
- ITT
intention-to-treat
- LOCF
last observation carried forward
- NPH
NPH insulin
- PG
plasma glucose
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1007/s00125-007-0911-x) contains supplementary material, which is available to authorised users.
References
- 1.Riddle MC (2004) Timely initiation of basal insulin. Am J Med 116:3S–9S [DOI] [PubMed]
- 2.Wright A, Burden ACF, Paisey RB, Cull CA, Holman RR, for the UK Prospective Diabetes Study Group (2002) Sulfonylurea inadequacy: efficacy of addition of insulin over 6 years in patients with type 2 diabetes in the UK Prospective Diabetes Study (UKPDS 57). Diabetes Care 25:330–336 [DOI] [PubMed]
- 3.Marre M (2002) Before oral agents fail: the case for starting insulin early. Int J Obes 26(Suppl 3):S25–S30 [DOI] [PubMed]
- 4.Korytkowski M (2002) When oral agents fail: practical barriers to starting insulin. Int J Obes 26:S18–S24 [DOI] [PubMed]
- 5.Davies M (2004) The reality of glycaemic control in insulin treated diabetes: defining the clinical challenges. Int J Obes Relat Metab Disord 28(Suppl 2):S14–S22 [DOI] [PubMed]
- 6.Koro CE, Bowlin SJ, Bourgeois N, Fedder DO (2004) Glycaemic control from 1988 to 2000 among US adults diagnosed with type 2 diabetes. Diabetes Care 27:17–20 [DOI] [PubMed]
- 7.Turner RC, Cull CA, Frighi V, Holman RR, for the UK Prospective Diabetes Study (UKPDS) Group (1999) Glycaemic control with diet, sulfonylurea, metformin, or insulin in patients with type 2 diabetes mellitus: progressive requirement for multiple therapies (UKPDS 49). JAMA 281:2005–2012 [DOI] [PubMed]
- 8.Heise T, Nosek L, Ronn BB et al (2004) Lower within-subject variability of insulin detemir in comparison to NPH insulin and insulin glargine in people with type 1 diabetes. Diabetes 53:1614–1620 [DOI] [PubMed]
- 9.Riddle MC, Rosenstock J, Gerich J, on behalf of the Insulin Glargine 4002 Study Investigators (2003) The treat-to-target trial: randomized addition of glargine or human NPH insulin to oral therapy of type 2 diabetic patients. Diabetes Care 26:3080–3086 [DOI] [PubMed]
- 10.Eliaschewitz FG, Calvo C, Valbuena H et al, HOE 901/4013 LA Study Group (2006) Therapy in type 2 diabetes: insulin glargine vs NPH insulin both in combination with glimepiride. Arch Med Res 37:495–501 [DOI] [PubMed]
- 11.Yki-Järvinen H, Kauppinen-Mäkelin R, Tiikkainen M et al (2006) Insulin glargine or NPH combined with metformin in type 2 diabetes: the LANMET study. Diabetologia 49:442–451 [DOI] [PubMed]
- 12.Hermansen K, Davies M, Derezinski T, Martinez Ravn G, Clauson P, Home P (2006) A 26-week, randomized, parallel, treat-to-target trial comparing insulin detemir with NPH insulin as add-on therapy to oral glucose-lowering drugs in insulin-naive people with type 2 diabetes. Diabetes Care 29:1269–1274 [DOI] [PubMed]
- 13.Philis-Tsimikas A, Charpentier G, Clauson P, Martinez Ravn G, Roberts VL, Thorsteinsson B (2006) Comparison of once-daily insulin detemir with NPH insulin added to a regimen of oral antidiabetic drugs in poorly controlled type 2 diabetes. Clin Ther 28:1569–1581 [DOI] [PubMed]
- 14.Home P, Kurtzhals P (2006) Insulin detemir: from concept to clinical experience. Expert Opin Pharmacother 7:325–343 [DOI] [PubMed]
- 15.Hermansen K, Davies M (2007) Does insulin detemir have a role in reducing risk of insulin-associated weight gain. Diabetes Obes Metab 9:209–217 [DOI] [PubMed]
- 16.Klein O, Lynge J, Endahl L, Damholt B, Nosek L, Heise T (2007) Albumin-bound basal insulin analogues (insulin detemir and NN344): comparable time–action profiles but less variability than insulin glargine in type 2 diabetes. Diabetes Obes Metab 9:290–299 [DOI] [PubMed]
- 17.Porcellati F, Rossetti P, Busciantella Ricci N et al (2007) Comparison of pharmacokinetics and dynamics of the long-acting insulin analogs glargine and detemir at steady state in type 1 diabetes: A double-blind, randomized, crossover study. Diabetes Care 30:2447–2452 [DOI] [PubMed]
- 18.sanofi aventis (2005) Lantus label information, NDA 21-081/S-017. Available from http://www.fda.gov/cder/foi/label/2005/21081s017lbl.pdf, accessed 1 July 2007
- 19.Raslova K, Bogoev M, Raz I, Leth G, Gall MA, Hancu N (2004) Insulin detemir and insulin aspart: a promising basal-bolus regimen for type 2 diabetes. Diabetes Res Clin Pract 66:193–201 [DOI] [PubMed]
- 20.Haak T, Tiengo A, Draeger E, Suntum M, Waldhäusl W (2005) Lower within-subject variability of fasting blood glucose and reduced weight gain with insulin detemir compared to NPH insulin in patients with type 2 diabetes. Diabetes Obes Metab 7:56–65 [DOI] [PubMed]
- 21.Raslova K, Tamer SC, Clauson P, Karl D (2007) Insulin detemir results in less weight gain than NPH insulin when used in basal-bolus therapy for type 2 diabetes mellitus, and this advantage increases with baseline body mass index. Clin Drug Investig 27:279–85 [DOI] [PubMed]
- 22.Russell-Jones D, Khan R (2007) Insulin-associated weight gain in diabetes—causes, effects and coping strategies. Diabetes Obes Metab 9:799–812 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(PDF 12.4 kb)
Treatment emergent adverse events probably or possibly related to trial product by system organ class, ITT cohort (PDF 20.2 kb)
Adverse events leading to withdrawal (PDF 17.2 kb)
Treatment emergent serious adverse events by system organ class, ITT cohort (PDF 23.1 kb)
Treatment emergent serious adverse events preferred term, occurring in more than one subject, ITT (PDF 14.6 )