Abstract
Multiple sclerosis affects more women than men. The reasons for this are unknown. Previously, we have shown significant differences in women versus men in inflammatory cytokine responses to the major protein component of myelin, proteolipid protein (PLP), which is thought to be a target in MS patients. Here, using the ELISPOT assay, we examined sex differences in single-cell secretion of Th1 and Th2 cytokines from freshly isolated PBMC between relapsing remitting (RR) MS patients and healthy individuals. Cells were stimulated with MS-associated antigens including proteolipid protein (PLP), myelin basic protein (MBP), myelin oligodendrocyte glycoprotein (MOG), and non-disease related antigens. Our data show a sex bias in the cytokine responses to multiple MS-relevant myelin antigens: Women with MS show IFNγ-skewed responses and men with MS show IL-5-skewed responses. These data extend our previous findings (Pelfrey et al., 2002): (1) by demonstrating gender skewing in cytokine responses to an expanded myelin antigen repertoire, which includes MBP, MOG and PLP; (2) by showing TNFα and IL-10 do not display comparable gender skewing compared to IFNγ and IL5; (3) by defining the patient population as early, untreated RR MS patients to avoid confounding factors, such as different disease stages/disability and immunomodulatory therapy; and (4) by showing HLA type does not appear to underlie the gender differences. These findings may explain increased susceptibility to MS in women and could contribute to the differences in disease severity between men and women.
Keywords: multiple sclerosis, inflammatory T helper-1 (Th1), anti-inflammatory T helper-2 (Th2), cytokines, sexual dimorphism, myelin
Introduction
Many autoimmune diseases are more frequent in women than men, including multiple sclerosis, rheumatoid arthritis, Grave's disease, systemic lupus erythematosus, myasthenia gravis, Sjogren's syndrome and Hashimotos thyroiditis. The reasons for a sex bias in MS and other autoimmune diseases are poorly understood but may include sex-related differences in immune responsiveness, sex steroid or hormonal effects, and sex-linked genetic factors (Whitacre et al., 1999).
MS is thought to be a T cell-mediated autoimmune disease with a T helper-1 (Th1)-type skewing of the immune response towards proinflammatory cytokines (e.g. IL-2, IFNγ, IL-12 and TNFα). IFNγ has been strongly linked to MS pathogenesis through several findings: increased production of IFNγ prior to clinical attacks (Beck et al., 1988; Lu et al., 1993); treatment of MS patients with rIFNγ induced exacerbations (Panitch et al., 1987); the CNS inflammatory process is characterized by increased IFNγ expression (Woodroofe and Cuzner, 1993). TNFα is another Th1 cytokine that is cytotoxic for oligodendrocytes in vitro and has been implicated in the pathology of multiple sclerosis and its animal model, experimental autoimmune encephalomyelitis (EAE) (Selmaj et al., 1991b; Selmaj et al., 1991a; Selmaj and Raine, 1988; Sharief and Hentges, 1991). On the other hand, Th2 cells, which are also associated with autoimmune disease, secrete anti-inflammatory cytokines (e.g. IL-4, IL-5, IL-10), which favor humoral-mediated responses (Lucey et al., 1996). IL-10 has been shown to suppress EAE (Bettelli et al., 1998; Cua et al., 1999; Mathisen et al., 1997) and may underlie the beneficial effects of beta-interferon treatment in RRMS (Rudick et al., 1998). Both IL-4 and IL-5 are key regulators in humoral-mediated and adaptive immunity by acting as co-stimulators for the growth/differentiation of B cells and stimulation of immunoglobulin secretion. Importantly, Th2 cytokines are associated with down-regulation of Th1 cytokines and may provide protection from Th1-mediated autoimmune disease (Bettelli et al., 1998; Cua et al., 1999; Racke et al., 1994).
In these studies, we evaluated the sex differences in cytokine responses that we observed previously (Pelfrey et al., 2002) with an expanded set of myelin antigens that have been implicated in MS, including MBP and MOG. We also evaluated whether the Th1/Th2 differences we observed with IFNγ and IL-5 extend to additional Th1 or Th2 cytokines that have known involvement in MS, including TNFα and IL-10. Since HLA genes are directly linked to susceptibility in MS, we analyzed whether HLA genotype had a direct interactive effect. We observed sex differences in cytokine responses to myelin in both men and women with MS. These findings may explain increased susceptibility to MS in women and could contribute to the differences in disease severity between men and women.
Materials and Methods
Study subjects
Sixty patients with mild RRMS (38F, 22M) and 94 healthy controls (53F, 41M) were recruited. All the MS patients were untreated for at least 3 months prior to the study. Careful selection of study subjects avoided the confounding factors of disease stage, immunomodulatory therapy, age and disease duration. For complete study subject characteristics, see Tables I and II. Patients were recruited from the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic. Informed consent was obtained from each study subject. The study was reviewed and approved by the Cleveland Clinic IRB.
Table 1.
MS patient characteristics
| MS Males |
MS Females |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| HLA Class II c |
HLA Class II |
||||||||
| Study ID |
DDa |
Ageb |
DRB1 |
DQB1 |
Study ID |
DD |
Age |
DRB1 |
DQB1 |
| 18 | 1.5 | 46 | -d | - | 4 | 0.4 | 53 | - | - |
| 29 | 4.4 | 48 | - | - | 5 | 4.3 | 40 | - | - |
| 50 | 4.0 | 50 | - | - | 13 | 0.4 | 39 | 1,15* | 5,6 |
| 74 | 0.2 | 30 | 12,15 | 3,6 | 15 | 1.5 | 50 | - | - |
| 87 | 0 | 47 | 3,7 | 3,2 | 17 | 9 | 43 | - | - |
| 103 | 2.9 | 48 | 7 | 3 | 19 | 0.3 | 36 | - | - |
| 106 | 0.4 | 46 | 4,15 | 3,6 | 20 | 1.3 | 37 | - | - |
| 117 | 3.1 | 34 | 1,15 | 5,6 | 31 | 0.3 | 37 | - | - |
| 122 | 3.5 | 29 | 9,11 | 3 | 37 | 7 | 46 | 8,12 | 3,4 |
| 133 | 0.08 | 40 | 7,15 | 2,6 | 45 | 1.8 | 28 | 1,13 | 5,6 |
| 139 | 0.1 | 33 | 8,15 | 4,6 | 46 | .3 | 42 | - | - |
| 140 | 0.07 | 35 | 4,15 | 3,6* | 48 | .3 | 31 | 15,14 | 5,6 |
| 141 | 0.1 | 42 | 3,8 | 2,3 | 52 | 1.1 | 29 | 10,13 | 5,3 |
| 143 | 0.1 | 29 | 1,15 | 5,6 | 60 | 3.3 | 40 | - | - |
| 145 | 0.3 | 45 | 1*,15 | 3,6 | 76 | 2.5 | 46 | 4,16 | 3,5 |
| 147 | 0.08 | 47 | 7,15 | 3,6 | 78 | 1.0 | 33 | 3,15 | 2,6 |
| 160 | 0 | 33 | - | - | 81 | 0.04 | 23 | 1,15 | 5,6 |
| 174 | 46 | - | - | 83 | 1.2 | 46 | 13,14 | 5,6* | |
| 178 | 0.1 | 19 | 1,4 | 5,3 | 99 | 0.1 | 34 | 1,15 | 5,6 |
| 179 | 2.6 | 60 | 11 | 3 | 105 | 0.9 | 50 | 4,14 | 3,5 |
| 184 | 0 | 57 | - | - | 107 | 0.1 | 41 | 7,15 | 2,6 |
| 185 | 0 | 40 | - | - | 110 | 12.9 | 39 | 13,15 | 3,6 |
| 112 | 0.01 | 44 | 3,15 | 2,6* | |||||
| 113 | 0.09 | 33 | 13,14 | 5,6* | |||||
| 119 | 0.2 | 43 | 10,15 | 5,6 | |||||
| 123 | 9.5 | 52 | 15* | 6 | |||||
| 126 | 1.5 | 24 | 11,13 | 3,6 | |||||
| 127 | 0.2 | 33 | 4,15 | 3,6 | |||||
| 130 | 2.7 | 45 | 4,15 | 3,6 | |||||
| 131 | 3.3 | 35 | 7,15 | 3,6 | |||||
| 134 | 0.1 | 30 | 3,8 | 4,6 | |||||
| 135 | 0.2 | 34 | 7,11 | 2,3 | |||||
| 138 | 0.6 | 42 | - | - | |||||
| 146 | 0.1 | 23 | 1*,11 | 3 | |||||
| 148 | 12.2 | 41 | 11,15 | 3,6 | |||||
| 149 | 0.5 | 33 | 8,15 | 3,6 | |||||
| 151 | 0.1 | 31 | 13 | 3,6* | |||||
| 153 | 5.4 | 30 | 7,11 | 2,3 | |||||
DD is disease duration in years.
Average ages: MS males, 41.1 ± 9.9 yrs, MS females, 37.8 ± 7.9 yrs.
HLA Class II lists both alleles separated by a comma (homozygous individuals have only one number). Four-digit molecular HLA typing has been simplified to 2 digits (e.g. *1501 is shortened to 15; *1301 is shortened to 13; *0401 is 4; *0101 is 1; *0301 is 3). Numbers followed by “*” are allelic variants: 1* is *0103 (not the same as *0101); 15* is 1503, an allelic variation that is rare in Caucasians; 6* is *0602 but ambiguities exist with 0619 and 0620. Representation of HLA types: DR15: MS M 53%; MS F 43%; DR4: MS M 20%; MS F 14%; DR3: MS M 13%; MS F 11%.
Dashes are data that were not tested or unavailable.
Table 2.
Healthy Control characteristics
| Control males |
Control females |
||||||
|---|---|---|---|---|---|---|---|
| HLA Class IIb |
HLA Class II |
||||||
| Study ID |
Agea |
DRB1 |
DQB1 |
Study ID |
Age |
DRB1 |
DQB1 |
| 12 | 45 | -c | - | 8 | 26 | - | - |
| 22 | 35 | - | - | 10 | 27 | 3,7 | 2 |
| 28 | 30 | - | - | 21 | 48 | 13,15 | 6,6 |
| 32 | 25 | - | - | 23 | 30 | - | - |
| 35 | 45 | 15,1 | 5,6 | 24 | 25 | - | - |
| 38 | 40 | - | - | 25 | 24 | - | - |
| 47 | 34 | 15,7 | 6,2 | 27 | 45 | - | - |
| 49 | 44 | - | - | 30 | 38 | 4,16 | 3,5 |
| 56 | 34 | 13,4 | 3,6 | 36 | 39 | 4,11 | 3 |
| 62 | 42 | - | - | 39 | 35 | 15 | 6 |
| 65 | 22 | - | - | 40 | 48 | 1,3 | 5,6 |
| 66 | 21 | - | - | 41 | 45 | 3,11 | 2,3 |
| 68 | 24 | 1,3 | 5,2 | 42 | 34 | 15 | 6 |
| 71 | 45 | 7,4 | 2,3 | 43 | 33 | 3,13 | 2,6 |
| 72 | 32 | 3,4 | 3,2 | 51 | 50 | 4 | 3 |
| 73 | 22 | - | - | 53 | 47 | 3 | 2 |
| 79 | 41 | 3,11 | 2,3 | 55 | 50 | 13,14 | 5,6 |
| 80 | 48 | 4,7 | 3,2 | 58 | 36 | - | - |
| 84 | 24 | 10,11 | 3,5 | 59 | 20 | 1,13 | 5,3 |
| 94 | 40 | 4,13 | 3,6* | 70 | 44 | - | - |
| 96 | 27 | 4,15* | 3,6* | 75 | 27 | - | - |
| 100 | 40 | 11,13 | 6*,6 | 77 | 55 | 3,15 | 2,6 |
| 101 | 41 | - | - | 82 | 48 | 1,3 | 5,2 |
| 102 | 52 | - | - | 86 | 36 | 11,15 | 3,6 |
| 108 | 39 | 3,15* | 4,6 | 88 | 30 | 3 | 2 |
| 111 | 25 | 14,15 | 5,6* | 89 | 38 | 15 | 6 |
| 114 | 38 | 4,7 | 3,2 | 90 | 51 | 3,4 | 3,2 |
| 120 | 39 | 4,15 | 3,6 | 91 | 35 | 4,13 | 3 |
| 125 | 41 | 11,14 | 3,5 | 92 | 28 | 3,4 | 2,3 |
| 129 | 29 | 11,15 | 3,6 | 93 | 38 | 11,16 | 5,6 |
| 132 | 27 | 3,4 | 2,4 | 95 | 21 | 15* | 6 |
| 150 | 39 | 15 | 6 | 98 | 43 | 11,15* | 6 |
| 152 | 36 | 1 | 5 | 104 | 48 | - | - |
| 159 | 24 | 11,13 | 3,6* | 109 | 43 | 11,13 | 5,3 |
| 161 | 41 | 11,13 | 3,6 | 115 | 31 | 3,15 | 2,6 |
| 162 | 24 | 11,15 | 3,6 | 116 | 41 | 4,11 | 3,3 |
| 163 | 39 | 13,15 | 3,6 | 118 | 49 | 11,15 | 3,6 |
| 164 | 42 | 4,12 | 3,3 | 124 | 51 | - | - |
| 165 | 29 | 4,15 | 3,6 | 128 | 46 | 1,9 | 5,2 |
| 172 | 54 | 7,13 | 2,6* | 136 | 33 | 11 | 6 |
| 173 | 28 | 13 | 6* | 137 | 55 | 7,15 | 2,6 |
| 144 | 42 | 11,3 | 2,3 | ||||
| 154 | 34 | 7,12 | 3,3 | ||||
| 155 | 36 | 7 | 2 | ||||
| 156 | 27 | 1,15 | 5,6 | ||||
| 157 | 36 | 3,15 | 2,6 | ||||
| 166 | 34 | 1,10 | 5 | ||||
| 167 | 30 | 7,8 | 2,4 | ||||
| 168 | 45 | 1,15 | 5,6 | ||||
| 169 | 26 | 4,15 | 3,6 | ||||
| 170 | 29 | 1,3 | 2,5 | ||||
| 171 | 49 | 15,15* | 6,6 | ||||
| 181 | 45 | 1 | 5 | ||||
Average ages: Control males (CM), 35.4 ± 8.8 yrs : Control females (CF), 38.2 ± 9.3 yrs.
HLA Class II lists both alleles separated by a comma (homozygous individuals have only one number). Four-digit molecular HLA typing has been simplified to 2 digits (e.g. *1501 is shortened to 15; *1301 is 13; *0401 is 4; *0101 is 1; *0301 is 3). Numbers followed by “*” are allelic variants: 1* is *0103 (not the same as *0101); 15* is 1503, an allelic variation that is rare in Caucasians; 6* is *0602 but ambiguities exist with 0619 and 0620. Representations of HLA types: DR15: CM 31%; CF 33%; DR4: CM 38%; CF 19%; DR3: CM 17%; CF 30%.
Dashes are data that were not tested or unavailable.
HLA typing
The HLA class II typing was performed by the Allogen Laboratories at the Cleveland Clinic Foundation, Cleveland, OH. Genomic DNA was extracted from peripheral blood and HLA class II DR/DQ typing was performed by sequence-specific oligonucleotide probing after polymerase chain reaction amplification of gene segments of interest (PCR-SSOP). In some cases DRB1* typing was performed by direct sequencing of PCR amplified gene segments using dye-labeled terminators (Applied Biosystems, Foster City, CA).
ELISPOT assay
The ELISPOT method was used as previously described (Pelfrey et al., 2000; Pelfrey and Moldovan, 2005) with the following modifications: the ELISPOT plates (7770−0052, Whatman, Clifton, NJ) and the cell number (300,000 cells/well). Each antigen was tested in duplicate wells. The individual background value (mean of media wells) was subtracted from the value obtained in the presence of each stimulating agent. A response was considered positive if the reactivity exceeded the cut-off value, calculated by subtracting individual subjects' background values.
Antibodies, reagents and antigens
The anti-human capture/detection monoclonal antibodies were obtained from the following sources: IFNγ (Pierce-Endogen); TNF-α (BD Pharmingen), IL-10 and IL-5 (eBiosciences). We purchased the following reagents: streptavidin-HRP (DAKO, Carpenteria, CA); 30% H2O2, AEC substrate and BSA (fraction V) (Sigma, St. Louis, MO); DMF (Acros); Tween-20 (polyoxyetylene 20-sorbitan monolaurate) (Fisher, Pittsburgh, PA); PBS, RPMI-1640 (Cleveland Clinic, Central Services Media Lab). Complete culture medium consisted of RPMI-1640 supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2mM L-glutamine, 25 mM HEPES, and 10% newborn bovine serum (Life Technologies, Gaithersburg, MD). Antigens consisted of: PHA-P (10 μg/ml) (Sigma); Streptokinase (1,250 IU/ml; final 1:5 dilution) (Astra USA, Inc.,Westborough, MA); Tetanus toxoid (1:100,1:1,000 dilutions) (Accurate Chemical, Westbury, NY); Candida albicans whole cell extract (1:50 dilution) (Hollister Stier, Spokane, WA); Anti-CD3 mAb (final, 5 μg/ml) (BD Pharmingen, San Diego, CA). Control antigens were chosen as previously described (Pelfrey CM and Moldovan, 2005; Pelfrey et al., 2000).
Myelin-derived proteins and peptides
Myelin peptides for these experiments followed the sequence of human PLP, MBP and MOG. Myelin peptides relevant to MS are as follows: MBP 87−106 (Martin et al., 1990; Zhang et al., 1994); PLP 40−60 (Markovic-Plese et al., 1995; Pelfrey et al., 1993; van der Veen et al., 1993); PLP 89−106 (Pelfrey et al., 1994; Trotter et al., 1991); PLP 103−120 (Correale et al., 1995; Ohashi et al., 1995; Trotter et al., 1991); PLP 139−158 (Chou et al., 1992; Correale et al., 1995; Zhang et al., 1994); PLP 172−191 (Markovic-Plese et al., 1995); PLP 195−206 (Tuohy et al., 1997); MOG 1−22 (Kerlero et al., 1997); MOG 34−56 (Kerlero et al., 1997); MOG 64−96 (Kerlero et al., 1997). We purchased human MBP (100 μg/ml) from Advanced Immuno Chemical, Inc. (Long Beach, CA). Human PLP (10 or 50 μg/ml) was prepared as previously described (Folch et al., 1957; Tuohy et al., 1989). The peptides derived from PLP (40−60, 89−106, 103−120, 139−158, 172−191, and 195−206), MOG (1−22, 34−56, and 64−96), Tetanus toxoid (830−844 and 947−967) and MBP 87−106 were synthesized by FMOC solid phase method with > 96% purity (Protein Core Facility, Cleveland Clinic). Synthetic peptides were reconstituted in DMSO or water at a stock concentration of 1mg/ml and stored in 20uL aliquots at −20°C until use. Peptides were thawed and diluted in media to a final concentration of 10μg/ml.
Statistical analysis
The measurements in this study are the result of ELISPOT assays of developed spots, where each spot represents a single cytokine-secreting cell. They are the average count of the number of spots present in duplicate culture wells after the background (media) counts have been subtracted. The distribution of count data is Poisson, not normal, therefore analyses of the number of cytokine-secreting cells was performed using the negative binomial regression comparing MS patients to healthy controls (disease effect), males to females (gender effect) and interaction between diagnosis (MS/Control) and gender (Male vs. Female). Age, HLA type, and disease duration were also included as covariates. When a significant interaction was observed, pair-wise comparisons were performed to determine which groups contributed significantly to the interaction. The test statistic for each of the variables was the chi-square. The variable was considered statistically significant if the P value associated with the chi-square statistic was < 0.05. Analyses were adjusted for multiple comparisons using the Bonferroni correction. All analysis was performed using SAS (9.1). Negative binomial regressions were run using SAS Genmod Procedures. Bootstrap methods were applied to the data used to compute sample values of the IFNγ/IL-5 ratios for control and MS males and females for each of the cytokines. The resultant sample ratios for male and female control and male and female MS were differenced. For each cytokine one thousand such differences were generated. The means and standard deviations of this sample population of differences were computed and tested against the observed difference of the male and female ratios.
Results
Cytokine responses reveal highly significant sex differences
To examine sex differences in the cytokine responses to myelin proteins, peptides and control antigens, we used the ELISPOT assay to compare all male responses versus all female responses for each cytokine. Table 3 demonstrates several highly significant sex differences in IFNγ and IL-5 responses. Women showed strong IFNγ skewing to PLP 40−60 (p = 0.0356) and to PLP 195−206 (p=0.0124). In 12/20 or 60% of all antigens tested, the women with MS show the highest mean IFNγ responses, although this reached significance with only 5 antigens (Table 3, right side, labeled MS F, in bold). Where men showed significantly higher IFNγ responses than women (MOG 34−56, PLP 89−106), the differences were attributable to the control men, and not the men with MS. The mitogens, anti-CD3 and PHA, did not induce IFNγ skewing by gender. For control antigens, only diphtheria toxoid and candida extract showed IFNγ skewing in all females (Table 3, All M vs. F).
Table 3.
Summary of IFNγ and IL-5 cytokine responses: Sex and disease differences
| Control Males |
Control Females |
MS Males |
MS Females |
P valuesb |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Stimulation |
Meana |
SEM |
Mean |
SEM |
Mean |
SEM |
Mean |
SEM |
All M vs. F |
MS F vs. MS M |
|
| IFNγ | Candida | 4.8 | 1.4 | 5.8 | 1.7 | 9.4 | 4.3 | 42.9 | 23.2 | 0.0452 | .0265 MS F |
| Diphtheria | 10.2 | 3.6 | 9.9 | 3.5 | 4.1 | 1.7 | 55.3 | 33.4 | 0.0004 F | <.0001 MS F | |
| Streptokinase | 23.7 | 6.5 | 13.8 | 3.5 | 17.0 | 5.4 | 25.3 | 6.1 | 0.6747 | 0.7994 | |
| Tetanus toxoid | 26.3 | 21.4 | 21.9 | 7.6 | 10.8 | 3.9 | 41.9 | 19.8 | 0.19 | 0.0164 MS F | |
| TT 830−844 | 0.8 | 0.2 | 1.2 | 0.7 | 1.5 | 0.6 | 3.2 | 1.3 | 0.2577 | 0.0778 | |
| TT 947−967 | 1.6 | 0.5 | 1.0 | 0.4 | 1.0 | 0.4 | 3.9 | 1.0 | 0.132 | 0.0108 MS F | |
| anti-CD3 | 555.4 | 57.1 | 612.8 | 48.9 | 557.3 | 70.9 | 605.1 | 52.6 | 0.1799 | .1764 | |
| PHA | 709.0 | 57.0 | 724.1 | 41.6 | 691.8 | 57.6 | 757.0 | 38.5 | 0.4343 | .6463 | |
| MBP | 12.4 | 6.9 | 30.0 | 22.3 | 49.7 | 40.7 | 7.1 | 1.9 | 0.1727 | 0.0159 MS M | |
| MBP 87−106 | 1.7 | 1.2 | 0.4 | 0.1 | 0.9 | 0.3 | 0.5 | 0.3 | 0.3989 | 0.4609 | |
| MOG 1−22 | 2.9 | 1.5 | 1.5 | 0.8 | 1.6 | 0.8 | 1.4 | 0.4 | 0.4359 | 0.6189 | |
| MOG 34−56 | 3.7 | 2.5 | 0.5 | 0.2 | 2.3 | 1.0 | 2.2 | 1.1 | 0.0067 M | 0.3531 | |
| MOG 64−96 | 2.9 | 1.8 | 0.8 | 0.3 | 1.8 | 0.6 | 3.1 | 1.1 | 0.6929 | 0.1167 | |
| PLP-10 | 0.7 | 0.2 | 0.8 | 0.3 | 4.5 | 3.4 | 5.1 | 1.7 | 0.1797 | 0.1461 | |
| PLP-50 | 5.5 | 3.1 | 1.1 | 0.4 | 2.8 | 1.2 | 3.4 | 1.3 | 0.3411 | 0.4807 | |
| PLP 40−60 | 0.7 | 0.2 | 0.7 | 0.4 | 1.0 | 0.3 | 7.1 | 5.2 | 0.0356 F | 0.0002 MS F | |
| PLP 89−106 | 10.6 | 7.1 | 0.3 | 0.1 | 2.6 | 1.6 | 1.3 | 0.6 | 0.002 M | 0.3962 | |
| PLP 103−120 | 0.7 | 0.2 | 2.7 | 1.8 | 6.5 | 3.6 | 4.4 | 1.9 | 0.0941 | 0.9992 | |
| PLP 139−158 | 0.6 | 0.2 | 0.7 | 0.3 | 3.4 | 1.4 | 1.0 | 0.5 | 0.4059 | 0.1009 | |
| PLP 172−191 | 0.6 | 0.2 | 0.7 | 0.2 | 1.3 | 0.5 | 2.8 | 1.1 | 0.2688 | 0.1973 | |
| PLP 195−206 | 0.8 | 0.3 | 3.8 | 3.0 | 2.0 | 0.6 | 4.4 | 2.1 | 0.0124 F | 0.4152 | |
| All M vs. F |
MS M vs. MS F |
||||||||||
| IL-5 | Candida | 0.8 | 0.3 | 2.0 | 0.8 | 8.8 | 4.7 | 3.0 | 1.6 | 0.6518 | 0.039 MS M |
| Diphtheria | 1.6 | 0.5 | 2.1 | 1.6 | 3.3 | 2.1 | 9.3 | 6.3 | 0.3022 | 0.349 | |
| Streptokinase | 2.8 | 0.8 | 2.6 | 0.7 | 7.3 | 3.0 | 3.8 | 1.9 | 0.1133 | 0.0642 | |
| Tetanus toxoid | 21.5 | 8.0 | 14.4 | 4.7 | 3.8 | 1.7 | 17.1 | 7.1 | 0.2086 | 0.0784 | |
| TT 830−844 | 0.8 | 0.4 | 0.2 | 0.1 | 0.6 | 0.3 | 0.3 | 0.1 | 0.1036 | 0.4001 | |
| TT 947−967 | 0.6 | 0.2 | 0.4 | 0.1 | 0.7 | 0.5 | 0.4 | 0.2 | 0.2954 | 0.0973 | |
| anti-CD3 | 77.7 | 15.5 | 92.6 | 16.7 | 107.7 | 30.7 | 85.2 | 13.1 | 0.903 | 0.92 | |
| PHA | 97.5 | 16.1 | 119.5 | 14.4 | 128.6 | 29.3 | 85.0 | 11.3 | 0.4301 | 0.1992 | |
| MBP | 0.9 | 0.3 | 3.5 | 3.0 | 4.2 | 2.0 | 0.5 | 0.2 | 0.0106 M | 0.038 MS M | |
| MBP 87−106 | 1.3 | 0.8 | 0.6 | 0.2 | 1.7 | 1.2 | 0.4 | 0.1 | 0.0094 M | 0.1856 | |
| MOG 1−22 | 1.2 | 0.5 | 0.3 | 0.1 | 2.3 | 1.3 | 0.9 | 0.5 | <.0001 M | 0.0106 MS M | |
| MOG 34−56 | 0.4 | 0.1 | 0.2 | 0.1 | 1.6 | 0.9 | 0.3 | 0.1 | 0.0069 M | 0.0023 MS M | |
| MOG 64−96 | 0.4 | 0.1 | 0.3 | 0.1 | 1.5 | 0.8 | 0.4 | 0.2 | 0.3172 | 0.3579 | |
| PLP-10c | 5.2 | 3.9 | 0.4 | 0.2 | 0.5 | 0.2 | 6.2 | 5.8 | 0.0203 M | 0.6358 | |
| PLP-50c | 6.1 | 4.8 | 0.4 | 0.2 | 2.4 | 1.5 | 6.7 | 5.2 | 0.0217 M | 0.9666 | |
| PLP 40−60 | 0.8 | 0.5 | 0.3 | 0.1 | 2.1 | 1.0 | 0.3 | 0.1 | 0.0008 M | 0.0256 MS M | |
| PLP 89−106 | 1.2 | 0.8 | 0.3 | 0.1 | 1.5 | 0.8 | 0.6 | 0.4 | 0.037 M | 0.4899 | |
| PLP 103−120 | 1.2 | 0.6 | 0.4 | 0.1 | 1.1 | 0.4 | 0.4 | 0.1 | 0.0064 M | 0.1602 | |
| PLP 139−158 | 1.2 | 0.7 | 0.3 | 0.1 | 1.3 | 0.7 | 0.4 | 0.1 | 0.0023 M | 0.0351 MS M | |
| PLP 172−191 | 1.3 | 0.7 | 0.3 | 0.1 | 0.5 | 0.2 | 0.5 | 0.2 | 0.0999 | 0.8925 | |
| PLP 195−206 | 1.8 | 0.8 | 0.5 | 0.1 | 1.5 | 0.7 | 0.2 | 0.1 | 0.0001 M | 0.0016 MS M | |
The measurements in this study represent the mean number and standard error (SEM) of IFNγ- or IL-5-secreting cells present in duplicate culture wells after the background spot counts (media alone) have been subtracted.
Numbers represent P values for the negative binomial regression. Numbers in bold are significant P values at p < 0.05. Each bold value has a column to the right describing the direction of the response (e.g. males greater than females = M; MS Males greater than MS Females = MS M; MS Females greater than MS Males = MS F). P values have been adjusted for multiple comparisons (Bonferroni Correction). The negative binomial regression model also adjusted for disease duration and age.
Whole PLP was tested at either 10 μg/ml or 50 μg/ml.
Interestingly, 11/13 myelin antigens showed statistically significant IL-5 skewing in all males, whereas no control antigens or mitogens showed IL-5 gender skewing (Table 3, All M vs. F). To examine the male IL-5 skewing further, we separated the responses by disease status and sex. Table 4 shows 9 of the myelin antigens with the most significant male IL-5 skewing, and of these, 7/9 demonstrated that the men with MS gave the highest mean IL-5 response (Table 4, bold numbers marked with asterisks).
Table 4.
IL-5 significant gender responses are male skewed
| Male vs. Female |
By DX and Sex |
||||||
|---|---|---|---|---|---|---|---|
| Antigen |
Sex |
Avga |
SEa |
P valueb |
Dx,Sexc |
Avga |
SEa |
| MOG 1−22 | F | 0.59 | 0.22 | C M | 1.15**d | 0.50 | |
| M | 1.56 | 0.57 | <0.0001 | C F | 0.32 | 0.09 | |
| MS M | 2.26*** | 1.32 | |||||
| MS F | 0.91 | 0.46 | |||||
| MOG 34−56 | F | 0.28 | 0.07 | C M | 0.39 | 0.14 | |
| M | 0.83 | 0.33 | 0.0069 | C F | 0.22 | 0.07 | |
| MS M | 1.58*** | 0.85 | |||||
| MS F | 0.34 | 0.13 | |||||
| MBP 87−106 | F | 0.51 | 0.14 | C M | 1.33 | 0.64 | |
| M | 1.48 | 0.65 | 0.0094 | C F | 0.63 | 0.12 | |
| MS M | 1.74* | 0.38 | |||||
| MS F | 0.37 | 0.14 | |||||
| PLP 40−60 | F | 0.29 | 0.07 | C M | 0.85* | 0.49 | |
| M | 1.29 | 0.48 | 0.0008 | C F | 0.32 | 0.10 | |
| MS M | 2.05** | 1.01 | |||||
| MS F | 0.26 | 0.09 | |||||
| PLP 89−106 | F | 0.46 | 0.19 | C M | 1.21 | 0.81 | |
| M | 1.33 | 0.59 | 0.037 | C F | 0.32 | 0.11 | |
| MS M | 1.53 | 0.79 | |||||
| MS F | 0.63 | 0.40 | |||||
| PLP 103−120 | F | 0.41 | 0.09 | C M | 1.24* | 0.64 | |
| M | 1.17 | 0.43 | 0.0064 | C F | 0.39 | 0.12 | |
| MS M | 1.05 | 0.38 | |||||
| MS F | 0.43 | 0.14 | |||||
| PLP 139−158 | F | 0.34 | 0.09 | C M | 1.15 | 0.70 | |
| M | 1.21 | 0.50 | 0.0023 | C F | 0.32 | 0.11 | |
| MS M | 1.32* | 0.68 | |||||
| MS F | 0.37 | 0.15 | |||||
| PLP 195−206 | F | 0.38 | 0.09 | C M | 1.76 | 0.76 | |
| M | 1.65 | 0.53 | 0.0001 | C F | 0.54 | 0.14 | |
| MS M | 1.47*** | 0.65 | |||||
| MS F | 0.20 | 0.08 | |||||
| MBP | F | 2.1 | 1.64 | 0.0106 | C M | 0.94 | 0.29 |
| M | 2.1 | 0.78 | C F | 3.46 | 3.04 | ||
| MS M | 4.16*** | 2.04 | |||||
| MS F | 0.51 | 0.18 | |||||
Mean and standard error (SE) of IL-5 secreting cells. Bold numbers highlight where MS Males (MS M, right side) showed the highest IL-5 responses of any disease (DX) or sex combination.
Negative binomial regression P values showing significant difference between all male vs. all female responses.
DX, Sex: C M, control males (n=33); C F, control females (n=41); MS M, MS males (n= 19); MS F, MS females (n=35).
Asterisks represent P values in pairwise comparisons where MS M were significantly greater than MS F (*** p≤0.001, **p≤0.01, *p≤0.05) or C M greater than C F.
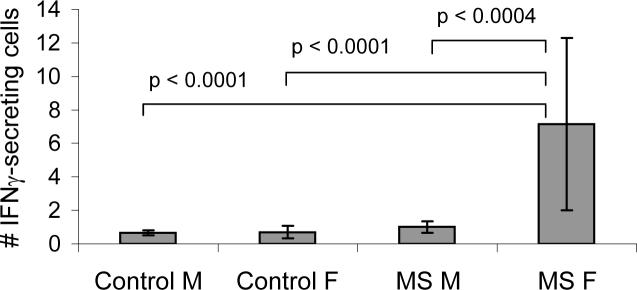
We also examined sex-by-disease interactions, where neither sex nor diagnosis (DX) alone were sufficient to explain the cytokine outcome. For PLP 40−60, IFNγ responses were highly skewed only to women with MS (Sex vs. DX, p = 0.001). Comparisons between groups show the MS female IFNγ response to PLP 40−60 is significantly greater compared to men with MS, control men and control women (Fig. 1). Although we predicted that TNFα and IL-10 responses would show similar gender skewing, TNFα responses did not show any female skewing compared to IFNγ, with the exception of the TNFα response to tetanus toxoid and its peptide TT 830−844 (TT, p < 0.0048; TT 830−844, p < 0.018). With one exception, MOG 1−22 (p < 0.05), IL-10 responses did not show significant male skewing similar to IL-5 (data not shown).
Figure 1. IFNγ response to PLP 40−60 is significantly greater in women with MS.
ELISPOT responses to PLP 40−60 (mean ± SEM) are shown for healthy controls and MS patients divided by sex. Chi Square P values show pair-wise comparisons between groups and are adjusted for multiple comparisons. MS F, n = 38; MS M, n = 22; Control F, n = 40; Control M, n = 32.
Within MS patients, women with MS show IFNγ skewing and men with MS show IL--5 skewing in response to peptides of PLP, MOG and MBP
To understand the contribution of sex more clearly, we divided the subjects into 4 categories: Control males, Control females, MS males and MS females. Although we observed significant female skewing in the IFNγ response to multiple myelin peptides and male skewing in IL-5 responses to the same myelin epitopes (Table 3), this data is not able to show the interplay between IFNγ and IL-5 responses that we observed in the women with MS, nor is it able to show the important cross-regulation that occurs between Th1 and Th2 cytokines. Th1 and Th2 cytokines rarely occur in high amounts in the same cell or in the same person. This is because they often have opposing immune functions as well as cross-regulatory function on the other type of cytokine. The ratio between IFNγ/IL5 demonstrates this Th1 skewing in women with MS, revealing IFNγ skewing in MS females' responses to 11/12 myelin epitopes (only whole PLP protein did not show this skewing)(Table 5). Five of these showed highly significant increased ratios in women with MS compared to the men with MS: PLP 40−60, PLP 195−206, MOG 64−96, p<0.0001; PLP 172−191, MOG 34−56, p = 0.01 (Table 5). Of these myelin antigens, only PLP 195−206 also showed an increased ratio in control women compared to control men.
Table 5.
IFNγ/IL-5 Ratios a
| Controls |
MS Patients |
|||||
|---|---|---|---|---|---|---|
| Stimulus |
C M |
C F |
P-value |
MS M |
MS F |
P-valueb |
| Candida | 5.8 | 3.0 | NS | 1.1 | 14.4 | <.0001c |
| Diphtheria | 6.3 | 4.7 | NS | 1.2 | 6.0 | NS |
| Streptokinase | 8.5 | 5.4 | NS | 2.3 | 6.7 | <.0001 |
| Tetanus tox. | 1.2 | 1.5 | NS | 2.8 | 2.4 | NS |
| TT 830−844 | 0.9 | 5.0 | <.0001 | 2.5 | 10.3 | <.0001 |
| TT 947−967 | 2.5 | 2.7 | NS | 1.5 | 9.9 | <.0001 |
| anti-CD3 | 7.2 | 6.6 | NS | 5.2 | 7.1 | <.0001 |
| PHA | 7.3 | 6.1 | NS | 5.4 | 8.9 | <.0001 |
| MBP | 13.3 | 8.7 | NS | 12.0 | 13.9 | NS |
| MBP 87−106 | 1.2 | 0.6 | NS | 0.5 | 1.3 | NS |
| MOG 1−22 | 2.5 | 4.8 | NS | 0.7 | 1.5 | NS |
| MOG 34−56 | 9.4 | 2.2 | NS | 1.5 | 6.5 | 0.0148 |
| MOG 64−96 | 8.1 | 3.0 | NS | 1.2 | 8.3 | <.0001 |
| PLP-10 | 0.1 | 1.8 | NS | 9.9 | 0.8 | NS |
| PLP-50 | 0.9 | 3.2 | NS | 1.2 | 0.5 | NS |
| PLP 40−60 | 0.8 | 2.2 | NS | 0.5 | 27.8 | <.0001 |
| PLP 89−106 | 8.8 | 0.9 | NS | 1.7 | 2.1 | NS |
| PLP 103−120 | 0.6 | 6.8 | 0.0010 | 6.2 | 10.2 | NS |
| PLP 139−158 | 0.5 | 2.3 | NS | 2.6 | 2.8 | NS |
| PLP 172−191 | 0.5 | 2.4 | NS | 2.5 | 5.5 | 0.0100 |
| PLP 195−206 | 0.5 | 7.1 | 0.0005 | 1.4 | 21.8 | <.0001 |
Ratios are the mean IFNγ response divided by the mean IL-5 response for all subjects in that group.
P values were calculated using a bootstrap analysis.
P values in bold represent IFNγ/IL-5 ratios where the female ratio is significantly increased compared to the male ratio (P ≤ .01) by a one-way students' T test. NS = not significant.
Control antigens/mitogens do not show significant gender skewing
To determine if the IFNγ-skewed responses in MS females were unique to myelin antigens, we also tested cytokine responses simultaneously to mitogens and a series of control antigens consisting of non-MS-associated antigens including tetanus toxoid, diphtheria toxoid, Candida albicans extract, and streptokinase. In contrast to the myelin epitopes, mitogen-stimulation with anti-CD3 mAb and PHA failed to show significant differences between Male vs. Female IFNγ (Table 3, p = 0.18 and 0.65, respectively). Like wise, IL-5 responses to mitogens and control antigens did not reveal sex differences (Table 3). When we examined the IFNγ/IL-5 ratio for sex/disease combinations, MS females did show a significantly elevated IFNγ/IL-5 ratio compared to MS males for anti-CD3 and PHA (Table 5). Tetanus toxoid stimulation resulted in virtually identical ratios between women and men (Table 5). Although some control antigen responses showed IFNγ-skewed responses in women (e.g. diphtheria, p = 0.0001 vs. males) none showed parallel IL-5 skewing in men (Table 3). For 4/6 control antigens, IFNγ/IL-5 ratios still showed significantly higher ratios in women with MS compared to men with MS. This was not true for control females vs. control males, with the exception of TT 830−844 (Table 5).
The HLA DR composition of our study subjects does not explain the gender differences
To determine whether the observed sex differences might be explained by particular HLA types, we genotyped HLA DR/DQ in the majority of our study subjects (Tables 1 and 2). As expected, HLA DRB1*1501 (DR15) was more highly represented among the MS patients, with 22/43 (51%) of DR15+ MS patients and 23/72 (32%) of DR15+ healthy controls. We performed a negative binomial regression analysis examining whether the presence of DR15, DR4 or DR3, or any combination of those alleles, correlates with cytokine responses, while controlling for gender, disease and age. This allowed us to see whether gender-, disease-, or age- effects were confounded by disproportionate representation of particular HLA types among the responders. Almost no cytokine responses could be attributed exclusively to HLA type (data not shown). As might be expected, the IFNγ response to MBP was highly associated with the presence of all 3 HLA types (DR3 p<.0001; DR4 p < .0001; DR15 p < .0003), with MBP 87−106 strongly associated with DR15 (p < 0.003). Neither MBP nor MBP 87−106 showed IFNγ gender or -disease associations (Table 3, All M vs. F, and not shown). IFNγ responses to PLP 40−60 also appeared to be associated with DR15 (p < 0.0007). The IL-5 response to MBP showed an association with DR3 (p<0.0001). Other DR3 associations included MOG 1−22, PLP 139−158, PLP 195−206 (all p ≤ 0.004). These very few associations do not appear to be sufficient to explain our gender differences. Thus, these data suggest that the HLA DR composition of our study subjects does not explain the gender differences observed for the majority of the myelin antigens.
Discussion
Previously, we have shown significant differences in women versus men in inflammatory cytokine responses to the major protein component of myelin, proteolipid protein (PLP), which is thought to be a target in MS patients (Pelfrey et al., 2002). In the present study, we examined sex differences in single-cell secretion of Th1 and Th2 cytokines from freshly isolated PBMC between MS patients and healthy individuals. Our data show a sex bias in the cytokine responses to multiple potentially MS-relevant myelin antigens: Women with MS show IFNγ-skewed responses and men with MS patients IL-5-skewed responses. These data extend our previous findings (Pelfrey et al., 2002): (1) by demonstrating gender skewing in cytokine responses to an expanded myelin antigen repertoire, which includes MBP, MOG and PLP; (2) by showing TNFα and IL-10 do not display comparable gender skewing compared to IFNγ and IL5; (3) by defining the patient population as early, untreated RR MS patients to avoid confounding factors, such as different disease duration, age and immunomodulatory therapy; and (4) by showing HLA type does not appear to underlie the gender differences.
Previous studies have examined sex differences, but have not observed similar skewing as we report here. One study showed no significant differences for any cytokines between men and women with MS (Eikelenboom et al., 2005). A possible explanation for these results is that the authors used PMA to stimulate cells before measuring cytokines, and the very strong, non-specific, mitogenic stimulus is not able to distinguish between the sexes. Our data agree, since neither anti-CD3 nor PHA was able to distinguish sex differences with any of the cytokines we tested, although ratios of IFNγ/IL-5 did reveal some skewing with mitogenic stimulation, but only in women with MS. In another study, women were more likely than men to have increased T-cell reactivity to immunodominant PLP peptides (PLP 184−199 and 190−209), but did not show increased responses to MBP or MBP 82−100 (Greer et al., 2004). We also were unable to show significant female IFNγ reactivity to MBP or MBP 87−106. Instead, our results suggest that the primary factor in observing a significant MBP IFNγ response is the HLA type of the responders. Jacobs and coworkers used PMA to stimulate PBMC and analyzed cytokines by flow cytometry (Nguyen et al., 2003). The percentage of TNFα-producing CD3 positive cells was significantly higher in male compared to female RR-MS patients. In contrast, we observed increased TNFα in men only for whole PLP, but were not able to detect mitogen-induced differences in any cytokine responses. These authors also observed that the percentage of CD3 positive cells producing IFNγ was significantly correlated with EDSS in females but not in males (Nguyen et al., 2003). This agrees with our previous observation showing a positive correlation between IFNγ responses in MS patients and disability measured by the MS Functional Composite (Moldovan et al., 2003). Notably, 75% of the MS patients in our study were women. One other group has observed a sex bias in the cytokine responses among MS patients (Greer et al., 2004). The common feature between these studies that relates to sex differences in cytokine expression is the use of myelin peptides rather than mitogen stimulation. Thus, the key to observing sex differences among cytokines that distinguish between Th1 responses and Th2 responses in MS is the use of myelin-specific antigens. It is important to note that several studies have reported an association between HLA DR2 (DRB1*1502 and *0501) and female gender in MS (Al-Shammri et al., 2004; Duquette et al., 1992; Fukazawa et al., 2000; Van et al., 1986), highlighting the importance of studying male and female patients separately. We were not trying to repeat these studies, but rather to assess whether our gender-skewed cytokine responses can be explained by a disproportionate representation of HLA alleles in our MS patient cohort. Our results do not support HLA as a critical factor in the gender-specific cytokine skewing.
Men with MS tend to have a more aggressive disease course. Disability is more severe in men and mortality is higher, even among men with an initially relapsing remitting course (Weinshenker et al., 1989; Wynn et al., 1990). It is now clear that changes on MRI reflect the underlying pathological process typical of MS, with contrast-enhancing lesions representing areas of active inflammation with blood-brain barrier disruption, whereas T1- hypointense lesions (known as “black holes”) indicate axonal loss (Truyen et al., 1996). In our hands, men with MS showed highly IL-5 skewed responses to a long list of myelin peptides, suggesting that men respond in a Th2/IL-5-dominated manner, which most likely serves to suppress Th1-IFNγ-dominated responses. In men, this may alter the pathological course of MS in a more destructive manner than for women. This theory is supported by a recent MRI study, in which men demonstrated fewer gadolinium contrast-enhancing lesions, but a higher proportion of black holes compared with women, indicating that men with MS are more prone to develop destructive and less inflammatory lesions than women (Pozzilli et al., 2003). Recently, the same group presented another study of gender-related modulation of pathological changes in RRMS where they related serum sex hormone levels to MRI characteristics of brain lesions in MS. In men, they reported a positive correlation between estradiol concentrations and brain damage as measured by both T1 and T2- lesion load (Tomassini et al., 2005). Greater brain damage, as documented by black holes, was associated with higher testosterone in women and higher estradiol levels in men, indicating that sex hormones (both estradiol and testosterone) are involved in the process leading to irreversible tissue damage, but that their role might differ between the sexes. There are other supporting studies that suggest men may have a more destructive and potentially Th2-type, Ab-mediated disease. A recent study of therapeutic plasma exchange as a therapy for MS (Keegan et al., 2002; Keegan et al., 2005; Weinshenker et al., 1999) showed that MS patients with a pattern II pathology, characterized by antibody/complement-associated demyelination, showed substantial neurological improvement following plasma exchange. One of the factors associated with a favorable response in that study was male sex (Keegan et al., 2002). Thus, it appears that men are more likely to develop a Th2/IL-5 skewed immune response to myelin proteins. Evidently, there are more factors involved that remain to be determined, since glatiramer acetate treatment can induce IL-5 and does not induce more severe disease (Duda et al., 2000). We speculate that under certain conditions in the brain, the increased IL-5 response may predispose men with MS to Ab-mediated demyelination.
The concept of ‘neuroprotective immunity’ might explain why the MS disease course is often milder and less disabling in women than in men. The significantly decreased IFNγ/IL-5 ratio in men may suppress the regulatory or neuroprotective effects provided by IFNγ. A beneficial role for IFNγ in MS has been suggested by several animal models. IFNγ receptor knockout mice developed more severe EAE (Ferber et al., 1996) and neutralizing antibodies against IFNγ increase Theiler's virus-induced demyelination and viral persistence (Rodriguez et al., 2003). Alternatively, genetic variants that affect expression or function of IFNγ might influence the susceptibility to MS and severity of the disease. Weinshenker and colleagues showed that being a carrier of the A allele of a 3′(325)*G→A single nucleotide polymorphism just 3′ to the IFNG gene is associated with increased susceptibility to MS in men (Kantarci et al., 2005). More recently, we have shown that this polymorphism is associated with decreased IFNγ protein and mRNA expression in men with MS (Kantarci et al., 2007). Thus, genetic variation in IFNγ may partially account for gender differences in susceptibility to MS.
In addition to some protective effects, IFNγ is known for its detrimental activities associated with MS. IFNγ has been strongly linked to MS pathogenesis through increased production of IFNγ prior to clinical attacks (Beck et al., 1988; Lu et al., 1993), treatment of MS patients with rIFNγ induced exacerbations (Panitch et al., 1987), and the CNS inflammatory process is characterized by increased IFNγ expression (Woodroofe and Cuzner, 1993). Thus, IFNγ displays a complex set of activities that may differ according to location in the body, time of expression in the disease, concomitant expression of sex hormones and activity on different subsets of cells.
Sex hormones can alter the immune response in many ways. What is poorly understood is how endogenous hormone levels affect the quantity and quality of a particular immune response. Cytokine gene expression may be affected directly or indirectly by sex hormones. The IFNγ gene appears to be directly affected by 17-beta-estradiol, which markedly increases activity of the IFNγ promoter in lymphoid cells that express the appropriate hormone receptor, and can augment the effect of T-cell-activating agents (Fox et al., 1991). We are examining endogenous levels of sex hormones to determine whether hormones play a role in the immune response to myelin proteins in MS. There are still many avenues of research to discover with respect to the role of sex differences and the immune response in MS.
Acknowledgements
We thank Dr. Richard Rudick, Director, and the physicians and research staff at the Mellen Center for Multiple Sclerosis Treatment and Research at the Cleveland Clinic, without whom MS patients for these studies would not be available.
Funding: Supported in part by the National Institutes of Health, National Center for Research Resources, General Clinical Research Center Grant M01 RR-018390; NMSS FA 1459-A-1 (I.R.M.); NMSS RG 3263-A-3 (C.M.P.); NIH RO1 NS 41972 (C.M.P.); Nancy Davis Center Without Walls.
Abbreviations
- MS
multiple sclerosis
- MBP
myelin basic protein
- MOG
myelin oligodendrocyte glycoprotein
- RRMS
relapsing remitting multiple sclerosis
- PLP
proteolipid protein
- IL-5
interleukin-5
- IL-10
interleukin-10
- TNFα
tumor necrosis factor alpha
- IFNγ
interferon gamma
- Th1
T-helper-1
- Th2
T-helper-2
- EAE
experimental autoimmune encephalomyelitis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Al-Shammri S, Nelson RF, Al-Muzairi I, Akanji AO. HLA determinants of susceptibility to multiple sclerosis in an Arabian Gulf population. Mult. Scler. 2004;10:381–386. doi: 10.1191/1352458504ms1065oa. [DOI] [PubMed] [Google Scholar]
- Beck J, Rondot P, Catinot L, Falcoff E, Kirchner H, Wietzerbin J. Increased production of interferon gamma and tumor necrosis factor precedes clinical manifestation in multiple sclerosis: do cytokines trigger off exacerbations? Acta Neurol. Scand. 1988;78:318–323. doi: 10.1111/j.1600-0404.1988.tb03663.x. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J. Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Chou YK, Bourdette DN, Offner H, Whitham R, Wang RY, Hashim GA, Vandenbark AA. Frequency of T cells specific for myelin basic protein and myelin proteolipid protein in blood and cerebrospinal fluid in multiple sclerosis. J. Neuroimmunol. 1992;38:105–113. doi: 10.1016/0165-5728(92)90095-3. [DOI] [PubMed] [Google Scholar]
- Correale J, McMillan M, McCarthy K, Le T, Weiner LP. Isolation and characterization of autoreactive proteolipid protein- peptide specific T-cell clones from multiple sclerosis patients. Neurology. 1995;45:1370–1378. doi: 10.1212/wnl.45.7.1370. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J. Exp. Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda PW, Schmied MC, Cook SL, Krieger JI, Hafler DA. Glatiramer acetate (Copaxone) induces degenerate, Th2-polarized immune responses in patients with multiple sclerosis. J. Clin. Invest. 2000;105:967–976. doi: 10.1172/JCI8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquette P, Pleines J, Girard M, Charest L, Senecal-Quevillon M, Masse C. The increased susceptibility of women to multiple sclerosis. Can. J. Neurol. Sci. 1992;19:466–471. [PubMed] [Google Scholar]
- Eikelenboom MJ, Killestein J, Uitdehaag BM, Polman CH. Sex differences in proinflammatory cytokine profiles of progressive patients in multiple sclerosis. Mult. Scler. 2005;11:520–523. doi: 10.1191/1352458505ms1195oa. [DOI] [PubMed] [Google Scholar]
- Ferber IA, Brocke S, Taylor-Edwards C, Ridgway W, Dinisco C, Steinman L, Dalton D, Fathman CG. Mice with a disrupted IFN-gamma gene are susceptible to the induction of experimental autoimmune encephalomyelitis (EAE) J. Immunol. 1996;156:5–7. [PubMed] [Google Scholar]
- Folch J, Lees MB, Sloane Stanley G. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- Fox HS, Bond BL, Parslow TG. Estrogen regulates the IFN-gamma promoter. J. Immunol. 1991;146:4362–4367. [PubMed] [Google Scholar]
- Fukazawa T, Yamasaki K, Ito H, Kikuchi S, Minohara M, Horiuchi I, Tsukishima E, Sasaki H, Hamada T, Nishimura Y, Tashiro K, Kira J. Both the HLA-CPB1 and -DRB1 alleles correlate with risk for multiple sclerosis in Japanese: clinical phenotypes and gender as important factors. Tissue Antigens. 2000;55:199–205. doi: 10.1034/j.1399-0039.2000.550302.x. [DOI] [PubMed] [Google Scholar]
- Greer JM, Csurhes PA, Pender MP, McCombe PA. Effect of gender on T-cell proliferative responses to myelin proteolipid protein antigens in patients with multiple sclerosis and controls. J. Autoimmun. 2004;22:345–352. doi: 10.1016/j.jaut.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Kantarci OH, Goris A, Hebrink DD, Heggarty S, Cunningham S, Alloza I, Atkinson EJ, de Andrade M, McMurray CT, Graham CA, Hawkins SA, Billiau A, Dubois B, Weinshenker BG, Vandenbroeck K. IFNG polymorphisms are associated with gender differences in susceptibility to multiple sclerosis. Genes Immun. 2005;6:153–161. doi: 10.1038/sj.gene.6364164. [DOI] [PubMed] [Google Scholar]
- Kantarci OH, Hebrink DD, Schaefer-Klein J, Yulong S, Achenback S, Atkinson EJ, Heggarty S, Cotleur AC, de Andrade M, Vandenbroeck K, Pelfrey CM, Weinshenker B. IFNG polymorphisms are associated with sex bias in multiple sclerosis susceptibility and also with interferon gamma expression. Arch. Neurol. 2007 IN PRESS. [Google Scholar]
- Keegan M, Konig F, McClelland R, Bruck W, Morales Y, Bitsch A, Panitch H, Lassmann H, Weinshenker B, Rodriguez M, Parisi J, Lucchinetti CF. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. doi: 10.1016/S0140-6736(05)67102-4. [DOI] [PubMed] [Google Scholar]
- Keegan M, Pineda AA, McClelland RL, Darby CH, Rodriguez M, Weinshenker BG. Plasma exchange for severe attacks of CNS demyelination: predictors of response. Neurology. 2002;58:143–146. doi: 10.1212/wnl.58.1.143. [DOI] [PubMed] [Google Scholar]
- Kerlero d.R., Hoffman M, Mendel I, Yust I, Kaye J, Bakimer R, Flechter S, Abramsky O, Milo R, Karni A, Ben Nun A. Predominance of the autoimmune response to myelin oligodendrocyte glycoprotein (MOG) in multiple sclerosis: reactivity to the extracellular domain of MOG is directed against three main regions. Eur. J. Immunol. 1997;27:3059–3069. doi: 10.1002/eji.1830271144. [DOI] [PubMed] [Google Scholar]
- Lu CZ, Jensen MA, Arnason BG. Interferon gamma- and interleukin-4-secreting cells in multiple sclerosis. J. Neuroimmunol. 1993;46:123–128. doi: 10.1016/0165-5728(93)90241-p. [DOI] [PubMed] [Google Scholar]
- Lucey DR, Clerici M, Shearer GM. Type 1 and type 2 cytokine dysregulation in human infectious, neoplastic, and inflammatory diseases. Clin. Microbiol. Rev. 1996;9:532–562. doi: 10.1128/cmr.9.4.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markovic-Plese S, Fukaura H, Zhang J, al-Sabbagh A, Southwood S, Sette A, Kuchroo VK, Hafler DA. T cell recognition of immunodominant and cryptic proteolipid protein epitopes in humans. J. Immunol. 1995;155:982–992. [PubMed] [Google Scholar]
- Martin R, Jaraquemada D, Flerlage M, Richert J, Whitaker J, Long EO, McFarlin DE, McFarland HF. Fine specificity and HLA restriction of myelin basic protein- specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J. Immunol. 1990;145:540–548. [PubMed] [Google Scholar]
- Mathisen PM, Yu M, Johnson JM, Drazba JA, Tuohy VK. Treatment of experimental autoimmune encephalomyelitis with genetically modified memory T cells. J. Exp. Med. 1997;186:159–164. doi: 10.1084/jem.186.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan IR, Rudick RA, Cotleur AC, Born SE, Lee JC, Karafa MT, Pelfrey CM. Interferon gamma responses to myelin peptides in multiple sclerosis correlate with a new clinical measure of disease progression. J. Neuroimmunol. 2003;141:132–140. doi: 10.1016/s0165-5728(03)00221-2. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Ramanathan M, Weinstock-Guttman B, Baier M, Brownscheidle C, Jacobs LD. Sex differences in in vitro pro-inflammatory cytokine production from peripheral blood of multiple sclerosis patients. J. Neurol. Sci. 2003;209:93–99. doi: 10.1016/s0022-510x(03)00004-2. [DOI] [PubMed] [Google Scholar]
- Ohashi T, Yamamura T, Inobe J, Kondo T, Kunishita T, Tabira T. Analysis of proteolipid protein (PLP)-specific T cells in multiple sclerosis: identification of PLP 95−116 as an HLA-DR2,w15-associated determinant. Int. Immunol. 1995;7:1771–1778. doi: 10.1093/intimm/7.11.1771. [DOI] [PubMed] [Google Scholar]
- Panitch HS, Hirsch RL, Schindler J, Johnson KP. Treatment of multiple sclerosis with gamma interferon: exacerbations associated with activation of the immune system. Neurology. 1987;37:1097–1102. doi: 10.1212/wnl.37.7.1097. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Cotleur AC, Lee JC, Rudick RA. Sex differences in cytokine responses to myelin peptides in multiple sclerosis. J. Neuroimmunol. 2002;130:211–223. doi: 10.1016/s0165-5728(02)00224-2. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Moldovan IR. Epitope mapping in multiple sclerosis (MS) using the ELISPOT assay. In: Kalyuzhny AE, editor. Methods in Molecular Biology. Handbook of ELISPOT: Methods and Protocols. Humana Press Inc.; Totowa, NJ: 2005. pp. 219–235. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Rudick RA, Cotleur AC, Lee JC, Tary-Lehmann M, Lehmann PV. Quantification of Self-Recognition in Multiple Sclerosis by Single-Cell Analysis of Cytokine Production. J. Immunol. 2000;165:1641–1651. doi: 10.4049/jimmunol.165.3.1641. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Trotter JL, Tranquill LR, McFarland HF. Identification of a novel T cell epitope of human proteolipid protein (residues 40−60) recognized by proliferative and cytolytic CD4+ T cells from multiple sclerosis patients. J. Neuroimmunol. 1993;46:33–42. doi: 10.1016/0165-5728(93)90231-m. [DOI] [PubMed] [Google Scholar]
- Pelfrey CM, Trotter JL, Tranquill LR, McFarland HF. Identification of a second T cell epitope of human proteolipid protein (residues 89−106) recognized by proliferative and cytolytic CD4+ T cells from multiple sclerosis patients. J. Neuroimmunol. 1994;53:153–161. doi: 10.1016/0165-5728(94)90025-6. [DOI] [PubMed] [Google Scholar]
- Pozzilli C, Tomassini V, Marinelli F, Paolillo A, Gasperini C, Bastianello S. 'Gender gap' in multiple sclerosis: magnetic resonance imaging evidence. Eur. J. Neurol. 2003;10:95–97. doi: 10.1046/j.1468-1331.2003.00519.x. [DOI] [PubMed] [Google Scholar]
- Racke MK, Bonomo A, Scott DE, Cannella B, Levine A, Raine CS, Shevach EM, Rocken M. Cytokine-induced immune deviation as a therapy for inflammatory autoimmune disease. J. Exp. Med. 1994;180:1961–1966. doi: 10.1084/jem.180.5.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez M, Zoecklein LJ, Howe CL, Pavelko KD, Gamez JD, Nakane S, Papke LM. Gamma interferon is critical for neuronal viral clearance and protection in a susceptible mouse strain following early intracranial Theiler's murine encephalomyelitis virus infection. J. Virol. 2003;77:12252–12265. doi: 10.1128/JVI.77.22.12252-12265.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudick RA, Ransohoff RM, Lee JC, Peppler R, Yu M, Mathisen PM, Tuohy VK. In vivo effects of interferon beta-1a on immunosuppressive cytokines in multiple sclerosis. Neurology. 1998;50:1294–1300. doi: 10.1212/wnl.50.5.1294. [published erratum appears in Neurology 1998 Jul;51(1):332]. [DOI] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Cannella B, Brosnan CF. Identification of lymphotoxin and tumor necrosis factor in multiple sclerosis lesions. J. Clin. Invest. 1991a;87:949–954. doi: 10.1172/JCI115102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selmaj K, Raine CS, Cross AH. Anti-tumor necrosis factor therapy abrogates autoimmune demyelination. Ann. Neurol. 1991b;30:694–700. doi: 10.1002/ana.410300510. [DOI] [PubMed] [Google Scholar]
- Selmaj KW, Raine CS. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann. Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. N. Engl. J. Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Tomassini V, Onesti E, Mainero C, Giugni E, Paolillo A, Salvetti M, Nicoletti F, Pozzilli C. Sex hormones modulate brain damage in multiple sclerosis: MRI evidence. J. Neurol. Neurosurg. Psychiatry. 2005;76:272–275. doi: 10.1136/jnnp.2003.033324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotter JL, Hickey WF, van der Veen RC, Sulze L. Peripheral blood mononuclear cells from multiple sclerosis patients recognize myelin proteolipid protein and selected peptides. J. Neuroimmunol. 1991;33:55–62. doi: 10.1016/0165-5728(91)90034-5. [DOI] [PubMed] [Google Scholar]
- Truyen L, van Waesberghe JH, van Walderveen MA, van Oosten BW, Polman CH, Hommes OR, Ader HJ, Barkhof F. Accumulation of hypointense lesions (“black holes”) on T1 spin-echo MRI correlates with disease progression in multiple sclerosis. Neurology. 1996;47:1469–1476. doi: 10.1212/wnl.47.6.1469. [DOI] [PubMed] [Google Scholar]
- Tuohy VK, Lu Z, Sobel RA, Laursen RA, Lees MB. Identification of an encephalitogenic determinant of myelin proteolipid protein for SJL mice. J. Immunol. 1989;142:1523–1527. [PubMed] [Google Scholar]
- Tuohy VK, Yu M, Weinstock-Guttman B, Kinkel RP. Diversity and plasticity of self recognition during the development of multiple sclerosis. J. Clin. Invest. 1997;99:1682–1690. doi: 10.1172/JCI119331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Veen RC, Kapp JA, Trotter JL. Fine-specificity differences in the recognition of an encephalitogenic peptide by T helper 1 and 2 cells. J. Neuroimmunol. 1993;48:221–226. doi: 10.1016/0165-5728(93)90195-5. [DOI] [PubMed] [Google Scholar]
- Van LR, Sanders EA, D'Amaro J. Sex distribution, age of onset and HLA profiles in two types of multiple sclerosis. A role for sex hormones and microbial infections in the development of autoimmunity? J. Neurol. Sci. 1986;76:13–21. doi: 10.1016/0022-510x(86)90138-3. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, Bass B, Rice GP, Noseworthy J, Carriere W, Baskerville J, Ebers GC. The natural history of multiple sclerosis: a geographically based study. I. Clinical course and disability. Brain. 1989;112:133–146. doi: 10.1093/brain/112.1.133. [DOI] [PubMed] [Google Scholar]
- Weinshenker BG, O'Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, Pineda AA, Stevens LN, Rodriguez M. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann. Neurol. 1999;46:878–886. doi: 10.1002/1531-8249(199912)46:6<878::aid-ana10>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Whitacre CC, Reingold SC, O'Looney PA. A gender gap in autoimmunity. Science. 1999;283:1277–1278. doi: 10.1126/science.283.5406.1277. [DOI] [PubMed] [Google Scholar]
- Woodroofe MN, Cuzner ML. Cytokine mRNA expression in inflammatory multiple sclerosis lesions: detection by non-radioactive in situ hybridization. Cytokine. 1993;5:583–588. doi: 10.1016/s1043-4666(05)80008-0. [DOI] [PubMed] [Google Scholar]
- Wynn DR, Rodriguez M, O'Fallon WM, Kurland LT. A reappraisal of the epidemiology of multiple sclerosis in Olmsted County, Minnesota. Neurology. 1990;40:780–786. doi: 10.1212/wnl.40.5.780. [DOI] [PubMed] [Google Scholar]
- Zhang J, Markovic-Plese S, Lacet B, Raus J, Weiner HL, Hafler DA. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J. Exp. Med. 1994;179:973–984. doi: 10.1084/jem.179.3.973. [DOI] [PMC free article] [PubMed] [Google Scholar]