Abstract
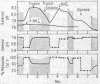
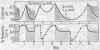
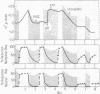
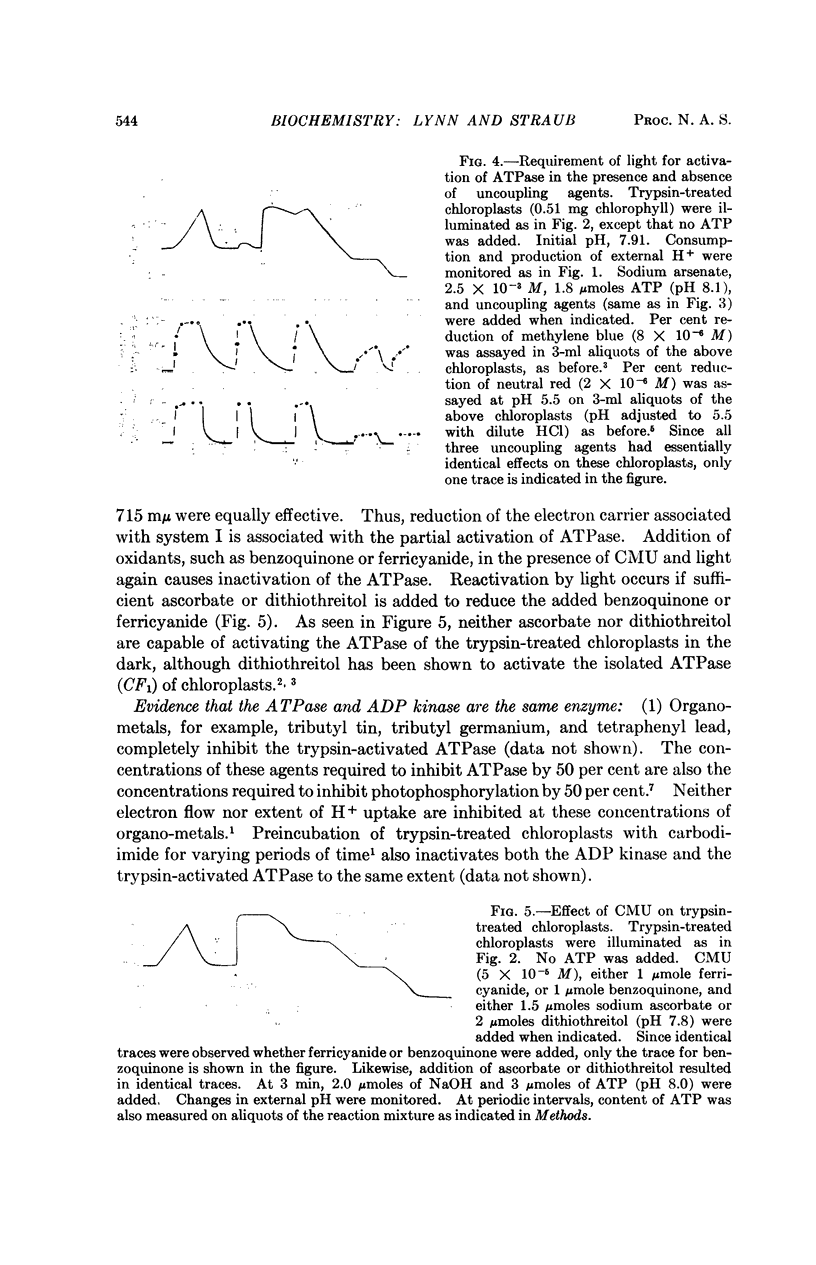
Treatment of chloroplasts with trypsin activates a light-requiring ATPase whose properties are strikingly similar to those of the light-requiring ADP kinase of chloroplasts. The observations here presented suggest that there exists, in chloroplasts, a reducible enzyme which, in its reduced state, catalyzes the reversible reaction: Pi-2 + ADP-3 + H+ ⇌ ATP-4 + H2O. By reduction and protonation of the catalytic site of this enzyme, light-driven electron flow in the chloroplast drives the reaction to the right. Hydrolysis of ATP proceeds only when the enzyme is reduced and when the proton concentration within the chloroplast is kept at low levels, viz., in the absence of light, in the presence of uncoupling agents which decrease the concentration of internal H+, or in the presence of electron acceptors which by oxidizing the internal electron acceptors also decrease the proton potential. Activation of the enzyme requires light; it remains active only in the presence of ATP. Hydrolysis of all the ATP results in inactivation of the ATPase. The membrane-bound protein CF2 limits the reversibility of the reaction by excluding ATP and H2O from the enzyme site. It also facilitates the ability of the chloroplasts to accumulate and to maintain high internal concentrations of such ions as ADP, Pi, PMS+, and imidazole.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Livne A., Racker E. A new coupling factor for photophosphorylation. Biochem Biophys Res Commun. 1968 Sep 30;32(6):1045–1049. doi: 10.1016/0006-291x(68)90135-6. [DOI] [PubMed] [Google Scholar]
- Lynn W. S., Brown R. H. P-2e-ratios approaching 4 in isolated chloroplasts. J Biol Chem. 1967 Feb 10;242(3):412–417. [PubMed] [Google Scholar]
- Lynn W. S. H+ and electron poising and photophosphorylation in chloroplasts. Biochemistry. 1968 Nov;7(11):3811–3820. doi: 10.1021/bi00851a005. [DOI] [PubMed] [Google Scholar]
- Martinez-Carrion M., Turano C., Riva F., Fasella P. Evidence of a critical histidine residue in soluble aspartic aminotransferase. J Biol Chem. 1967 Apr 10;242(7):1426–1430. [PubMed] [Google Scholar]
- Mitchell P., Moyle J. Stoichiometry of proton translocation through the respiratory chain and adenosine triphosphatase systems of rat liver mitochondria. Nature. 1965 Oct 9;208(5006):147–151. doi: 10.1038/208147a0. [DOI] [PubMed] [Google Scholar]
- Racker E. Resolution and reconstitution of the inner mitochondrial membrane. Fed Proc. 1967 Sep;26(5):1335–1340. [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]
- Wang J. H. The molecular mechanism of oxidative phosphorylation. Proc Natl Acad Sci U S A. 1967 Jul;58(1):37–44. doi: 10.1073/pnas.58.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]