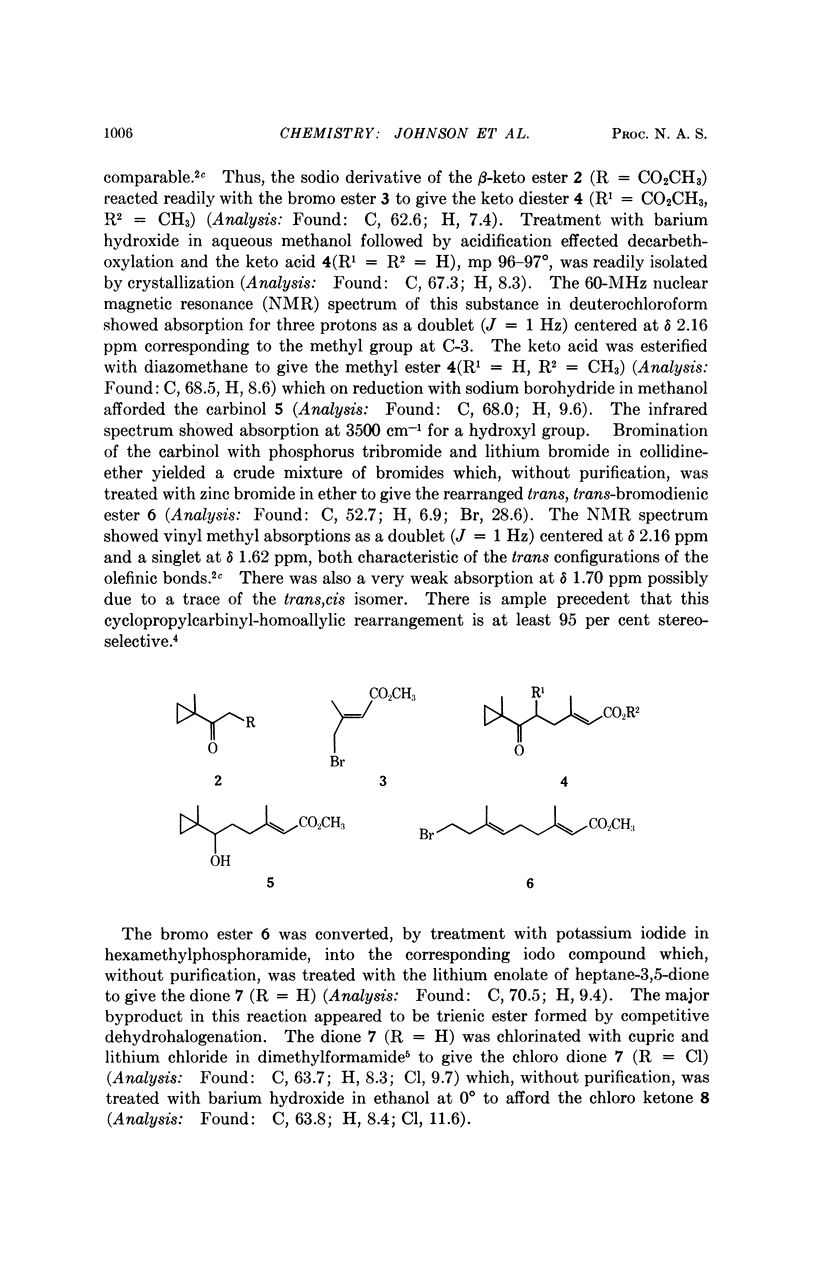
Abstract
Methyl cis-10-epoxy-3,7,11-trimethyl-trans,trans-2,6-tridecadienoate has been stereoselectively synthesized in 12 steps starting from methyl trans-γ-bromo-β,β-dimethylacrylate and 1-acetyl-1-methylcyclopropane. The final product proved to be identical with the second, less abundant juvenile hormone (methyl 12-homojuvenate) isolated from the Cecropia silk moth. The biological activities of the compound were evaluated in various insects.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Krishnakumaran A., Schneiderman H. A. Prothoracotrophic activity of compounds that mimic juvenile hormone. J Insect Physiol. 1965 Dec;11(12):1517–1532. doi: 10.1016/0022-1910(65)90019-3. [DOI] [PubMed] [Google Scholar]
- Meyer A. S., Schneiderman H. A., Gilbert L. I. A highly purified preparation of juvenile hormone from the silk moth Hyalophora cecropia L. Nature. 1965 Apr 17;206(981):272–275. doi: 10.1038/206272a0. [DOI] [PubMed] [Google Scholar]
- Meyer A. S., Schneiderman H. A., Hanzmann E., Ko J. H. The two juvenile hormones from the cecropia silk moth. Proc Natl Acad Sci U S A. 1968 Jul;60(3):853–860. doi: 10.1073/pnas.60.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneiderman H. A., Krishnakumaran A., Kulkarni V. G., Friedman L. Juvenile hormone activity of structurally unrelated compounds. J Insect Physiol. 1965 Dec;11(12):1641–1649. doi: 10.1016/0022-1910(65)90031-4. [DOI] [PubMed] [Google Scholar]



