Abstract
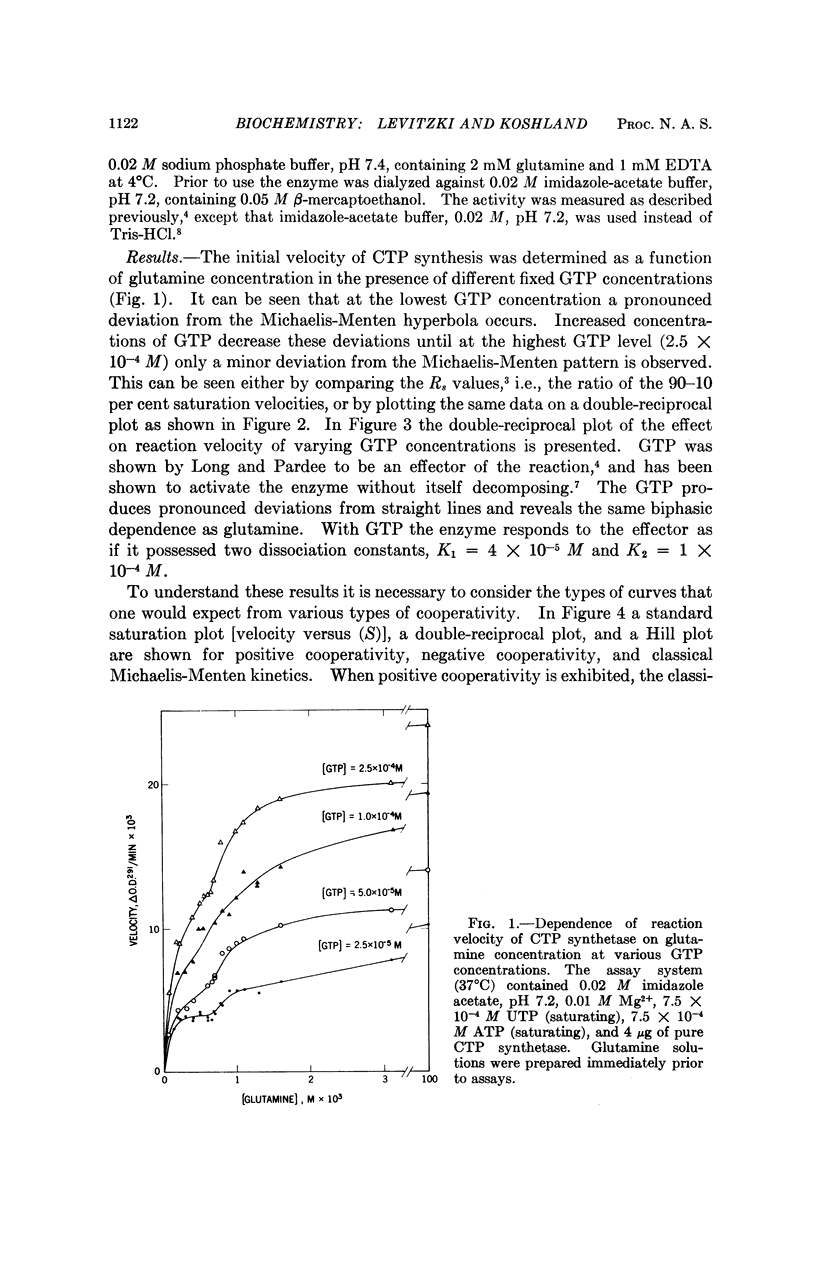
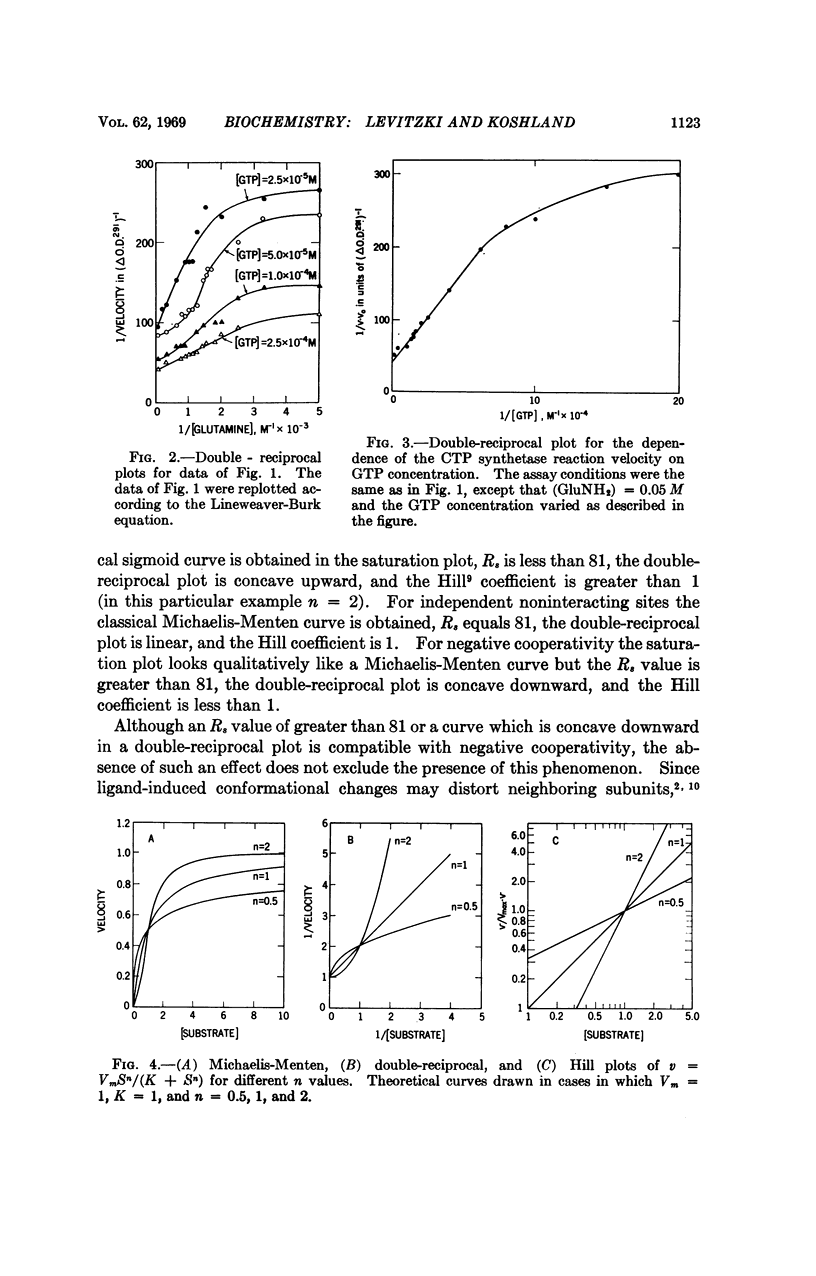
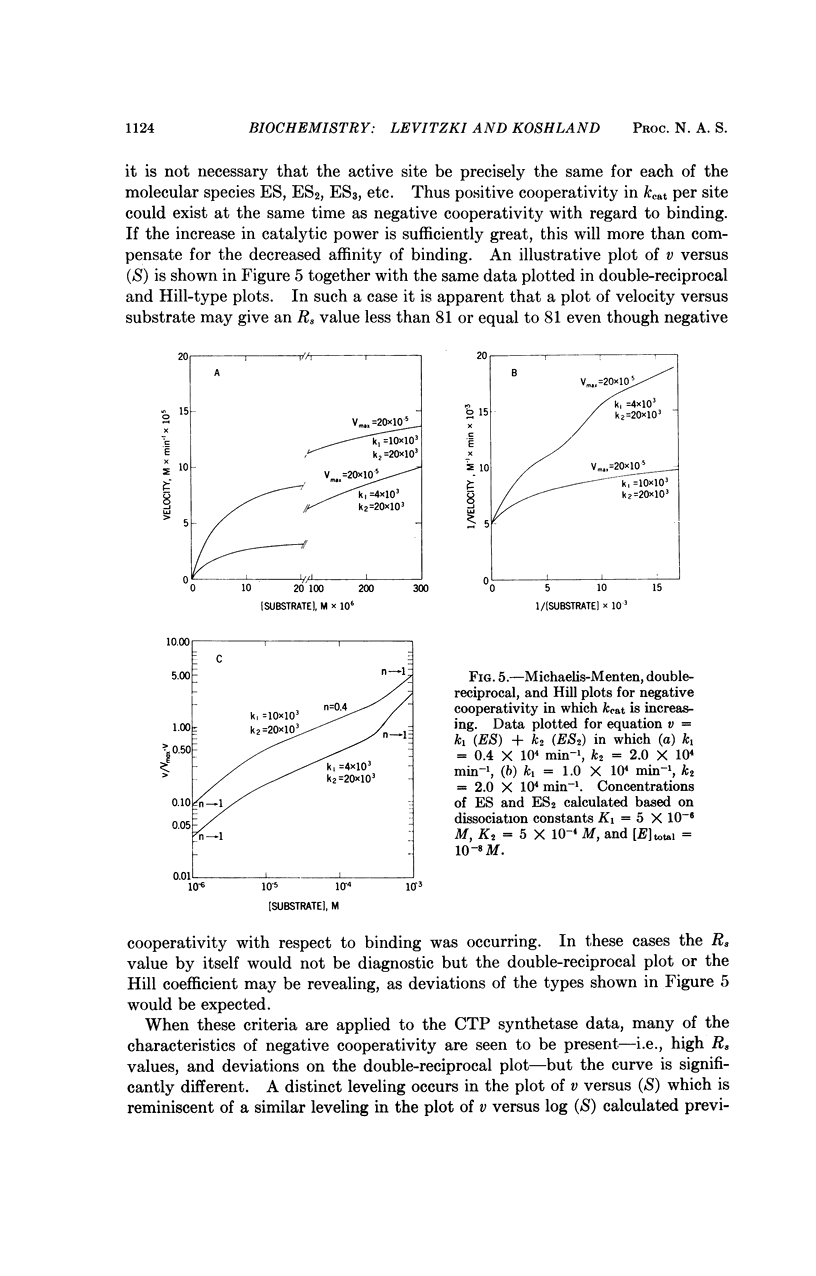
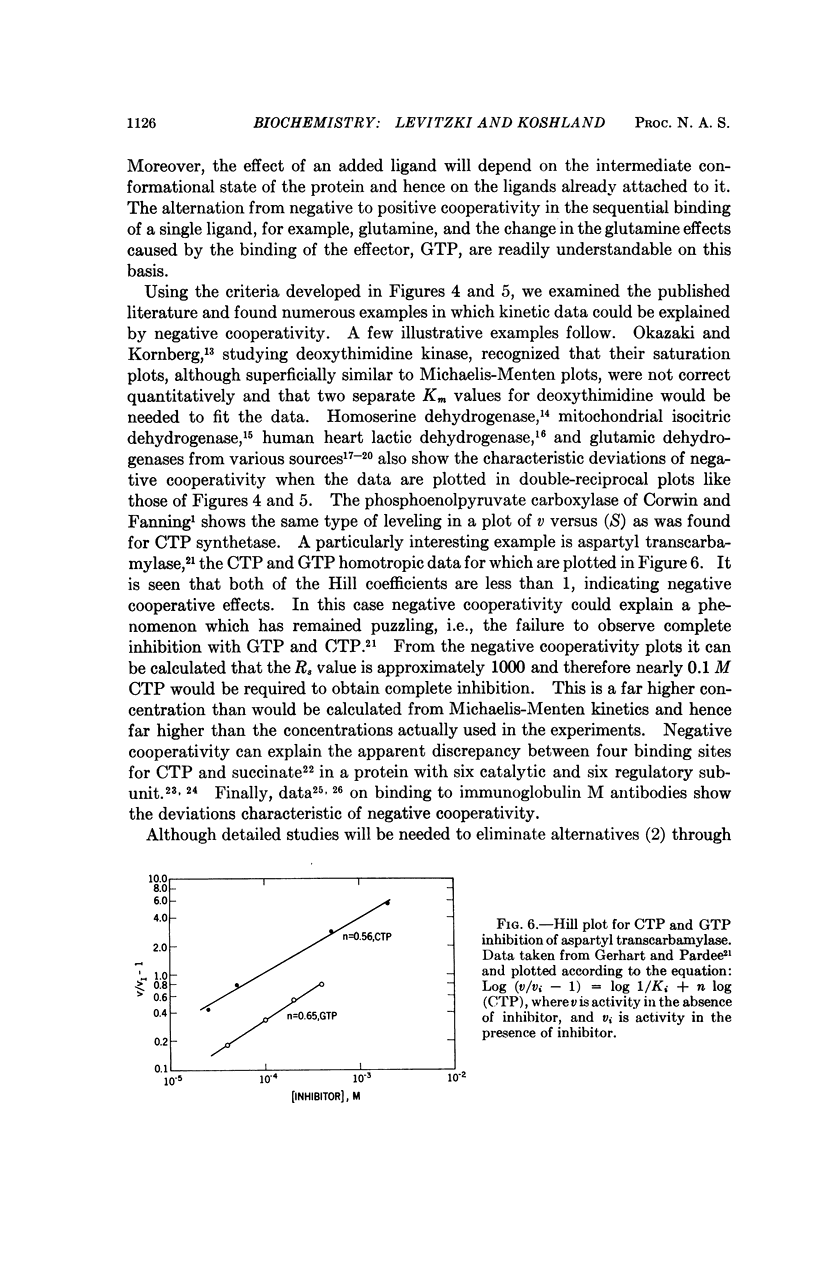
Negative cooperativity has been observed in CTP synthetase, an allosteric enzyme which contains a regulatory site. Thus, the same enzyme exhibits negative cooperativity for GTP (an effector) and glutamine (a substrate) and positive cooperativity for ATP and UTP (both substrates). In the process of the delineation of these phenomena, diagnostic procedures for negative cooperativity were developed. Application of these procedures to other enzymes indicates that negative cooperativity is a characteristic of many of them. These findings add strong support for the sequential model of subunit interactions which postulates that ligand-induced conformational changes are responsible for regulatory and cooperative phenomena in enzymes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernofsky C., Utter M. F. Secondary activation effects of mitochondrial isocitrate dehydrogenases from yeast. Biochim Biophys Acta. 1967 Mar 15;132(2):244–255. doi: 10.1016/0005-2744(67)90143-x. [DOI] [PubMed] [Google Scholar]
- Changeux J. P., Gerhart J. C., Schachman H. K. Allosteric interactions in aspartate transcarbamylase. I. Binding of specific ligands to the native enzyme and its isolated subunits. Biochemistry. 1968 Feb;7(2):531–538. doi: 10.1021/bi00842a007. [DOI] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- Corman L., Kaplan N. O. Kinetic studies of dogfish liver glutamate dehydrogenase with diphosphopyridine nucleotide and the effect of added salts. J Biol Chem. 1967 Jun 25;242(12):2840–2846. [PubMed] [Google Scholar]
- Corwin L. M., Fanning G. R. Studies of parameters affecting the allosteric nature of phosphoenolpyruvate carboxylase of Escherichia coli. J Biol Chem. 1968 Jun 25;243(12):3517–3525. [PubMed] [Google Scholar]
- DATTA P., GEST H. HOMOSERINE DEHYDROGENASE OF RHODOSPIRILLUM RUBRUM. PURIFICATION, PROPERTIES, AND FEEDBACK CONTROL OF ACTIVITY. J Biol Chem. 1965 Jul;240:3023–3033. [PubMed] [Google Scholar]
- GERHART J. C., PARDEE A. B. ASPARTATE TRANSCARBAMYLASE, AN ENZYME DESIGNED FOR FEEDBACK INHIBITION. Fed Proc. 1964 May-Jun;23:727–735. [PubMed] [Google Scholar]
- Haber J. E., Koshland D. E., Jr Relation of protein subunit interactions to the molecular species observed during cooperative binding of ligands. Proc Natl Acad Sci U S A. 1967 Nov;58(5):2087–2093. doi: 10.1073/pnas.58.5.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill A. V. The Combinations of Haemoglobin with Oxygen and with Carbon Monoxide. I. Biochem J. 1913 Oct;7(5):471–480. doi: 10.1042/bj0070471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirtley M. E., Koshland D. E., Jr Models for cooperative effects in proteins containing subunits. Effects of two interacting ligands. J Biol Chem. 1967 Sep 25;242(18):4192–4205. [PubMed] [Google Scholar]
- Koshland D. E., Jr, Némethy G., Filmer D. Comparison of experimental binding data and theoretical models in proteins containing subunits. Biochemistry. 1966 Jan;5(1):365–385. doi: 10.1021/bi00865a047. [DOI] [PubMed] [Google Scholar]
- LIEBERMAN I. Enzymatic amination of uridine triphosphate to cytidine triphosphate. J Biol Chem. 1956 Oct;222(2):765–775. [PubMed] [Google Scholar]
- Long C. W., Pardee A. B. Cytidine triphosphate synthetase of Escherichia coli B. I. Purification and kinetics. J Biol Chem. 1967 Oct 25;242(20):4715–4721. [PubMed] [Google Scholar]
- LéJohn H. B., Jackson S. Allosteric interactions of a regulatory nicotinamide adenine dinucleotide-specific glutamate dehydrogenase from Blastocladiella. A molecular model for the enzyme. J Biol Chem. 1968 Jun 25;243(12):3447–3457. [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- NISSELBAUM J. S., BODANSKY O. Purification and properties of human heart lactic dehydrogenase. J Biol Chem. 1961 Feb;236:323–327. [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. II. KINETICS AND FEEDBACK CONTROL. J Biol Chem. 1964 Jan;239:275–284. [PubMed] [Google Scholar]
- Onoue K., Grossberg A. L., Yagi Y., Pressman D. Immunoglobulin M antibodies with ten combining sites. Science. 1968 Nov 1;162(3853):574–576. doi: 10.1126/science.162.3853.574. [DOI] [PubMed] [Google Scholar]
- Onoue K., Yagi Y., Grossberg A. L., Pressman D. Number of binding sites of rabbit macroglobulin antibody and its subunits. Immunochemistry. 1965 Dec;2(4):401–415. doi: 10.1016/0019-2791(65)90039-x. [DOI] [PubMed] [Google Scholar]
- Weber K. New structural model of E. coli aspartate transcarbamylase and the amino-acid sequence of the regulatory polypeptide chain. Nature. 1968 Jun 22;218(5147):1116–1119. doi: 10.1038/2181116a0. [DOI] [PubMed] [Google Scholar]
- Wiggert B. O., Cohen P. P. Comparative study of tadpole and frog glutamate dehydrogenases. J Biol Chem. 1966 Jan 10;241(1):210–216. [PubMed] [Google Scholar]


