Abstract
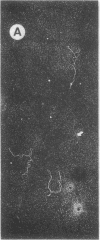
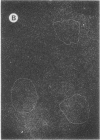
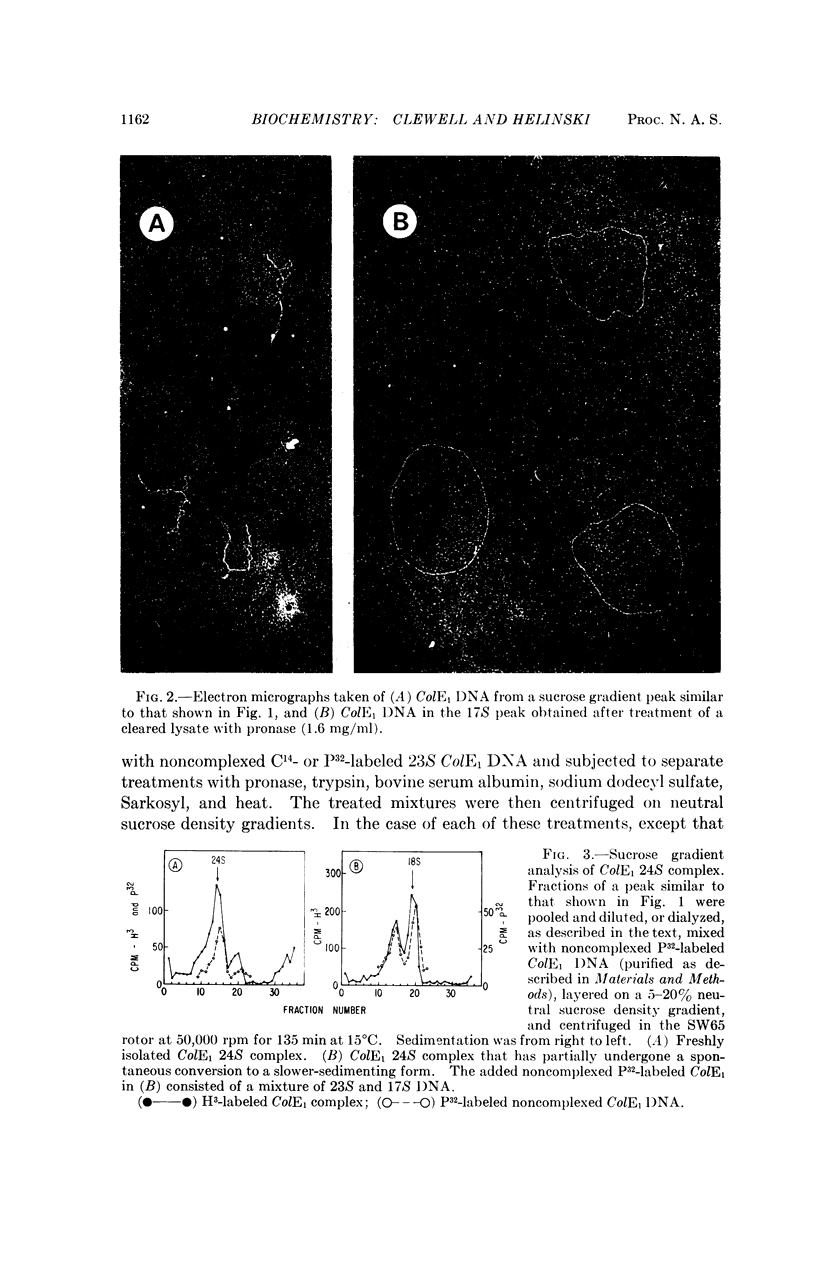
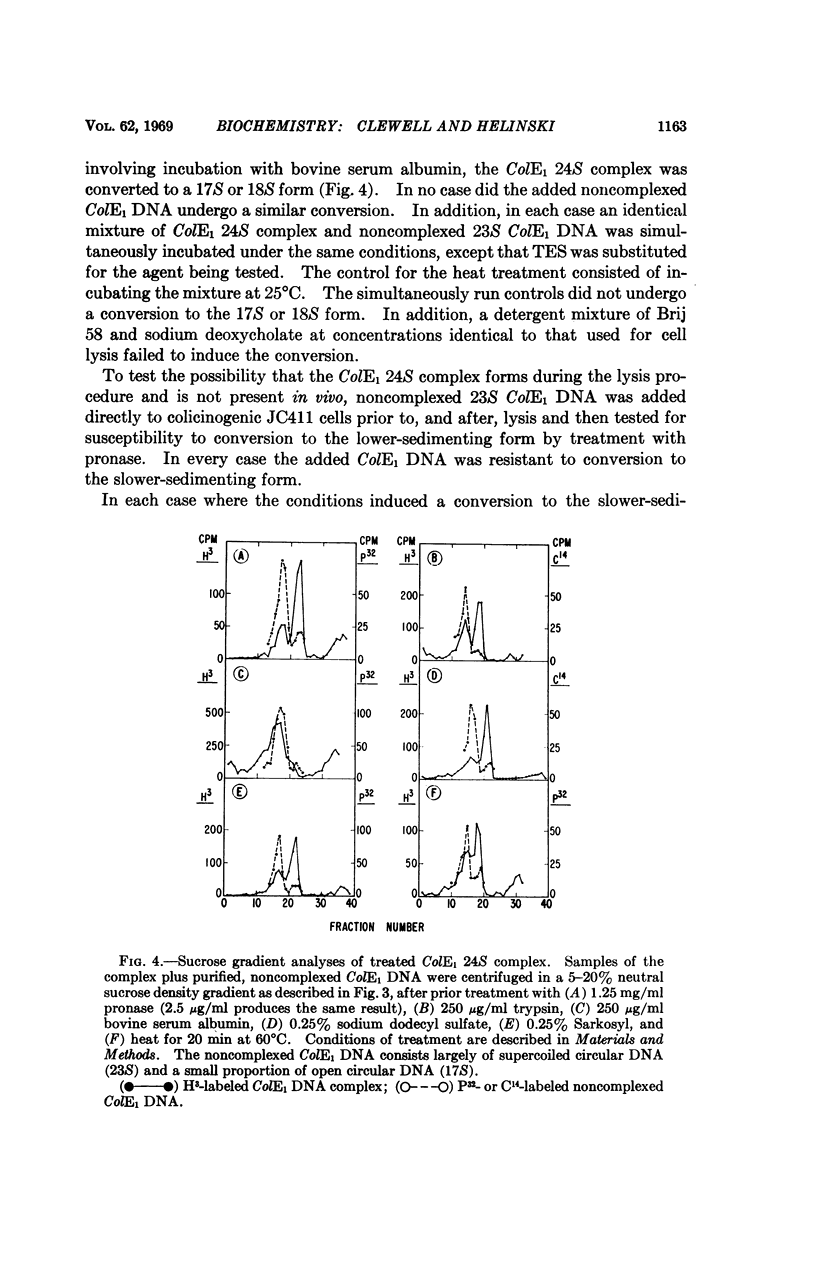
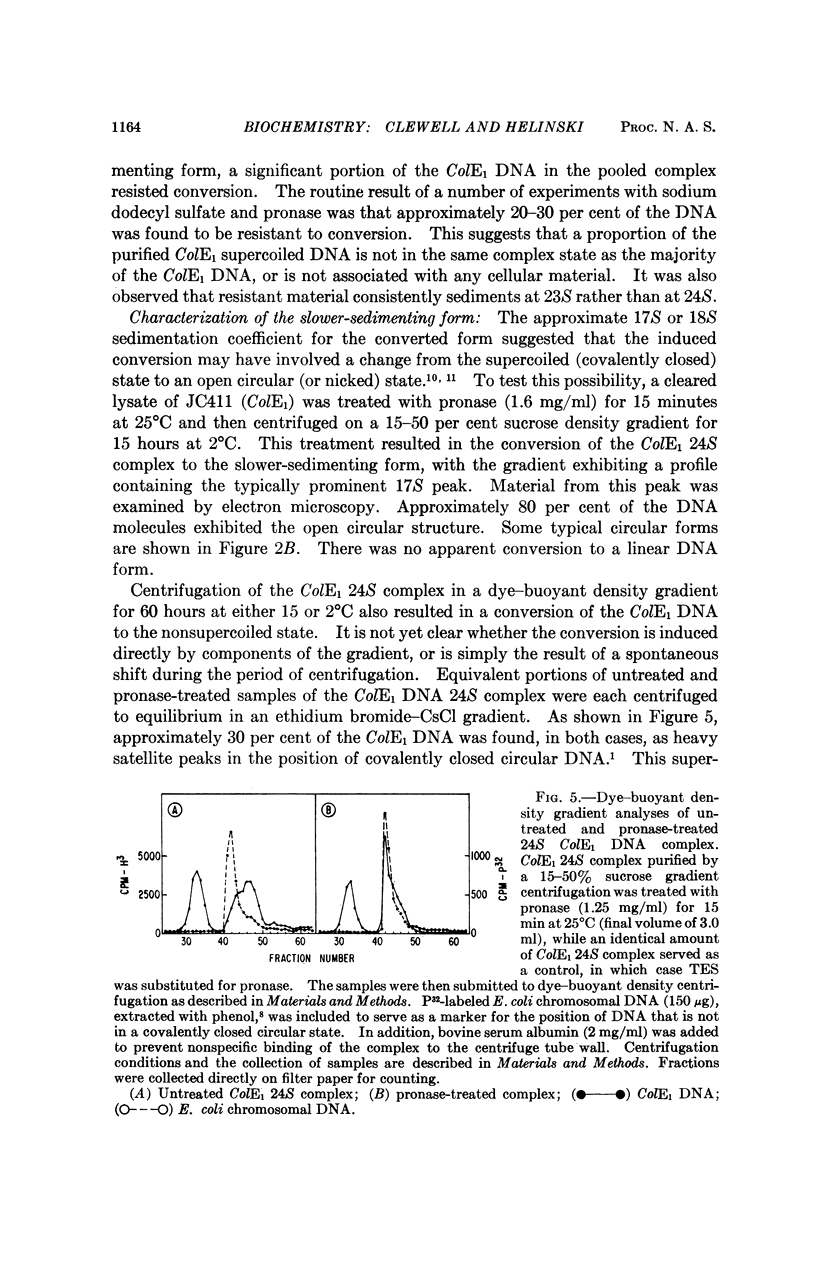
The 23S twisted circular form of ColE1 DNA has been isolated from Escherichia coli as a tightly associated DNA-protein complex with a sedimentation coefficient of approximately 24S. Treatment of this complex with pronase, trypsin, sodium dodecyl sulfate, Sarkosyl, or heat results in a conversion to a slower sedimenting form of 17S or 18S, as determined by centrifugation in neutral sucrose gradients. These treatments do not alter the sedimentation properties of noncomplexes supercoiled ColE1 DNA even in the presence of the ColE1-protein complex. Electron microscopic analyses indicate that the decrease in sedimentation rate of the ColE1-protein complex after treatment with these various agents is largely owing to an induced transition of ColE1 DNA from the supercoiled to the open circular state.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Circular DNA forms of colicinogenic factors E1, E2 and E3 from Escherichia coli. J Mol Biol. 1968 Sep 14;36(2):185–194. doi: 10.1016/0022-2836(68)90374-4. [DOI] [PubMed] [Google Scholar]
- Godson G. N., Sinsheimer R. L. Lysis of Escherichia coli with a neutral detergent. Biochim Biophys Acta. 1967 Dec 19;149(2):476–488. doi: 10.1016/0005-2787(67)90175-x. [DOI] [PubMed] [Google Scholar]
- Helinski D. R., Herschman H. R. Effect of Rec mutations on the activity of colicinogenic factors. J Bacteriol. 1967 Sep;94(3):700–706. doi: 10.1128/jb.94.3.700-706.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickson F. T., Roth T. F., Helinski D. R. Circular DNA forms of a bacterial sex factor. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1731–1738. doi: 10.1073/pnas.58.4.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knippers R., Sinsheimer R. L. Process of infection with bacteriophage phiX174. XX. Attachment of the parental DNA of bacteriophage phiX174 to a fast-sedimenting cell component. J Mol Biol. 1968 May 28;34(1):17–29. doi: 10.1016/0022-2836(68)90231-3. [DOI] [PubMed] [Google Scholar]
- Komano T., Sinsheimer R. L. Preparation and purification of phi X-RF component I. Biochim Biophys Acta. 1968 Jan 29;155(1):295–298. doi: 10.1016/0005-2787(68)90360-2. [DOI] [PubMed] [Google Scholar]
- Lee Y. P., Wang M. H. Studies of the nature of the inhibitory action of inorganic phosphate, fluoride, and detergents on 5'-adenylic acid deaminase activity and on the activation by adenosine triphosphate. J Biol Chem. 1968 May 10;243(9):2260–2265. [PubMed] [Google Scholar]
- McClintock D. K., Markus G. Conformational changes in aspartate transcarbamylase. I. Proteolysis of the intact enzyme. J Biol Chem. 1968 Jun 10;243(11):2855–2862. [PubMed] [Google Scholar]
- Roth T. F., Helinski D. R. Evidence for circular DNA forms of a bacterial plasmid. Proc Natl Acad Sci U S A. 1967 Aug;58(2):650–657. doi: 10.1073/pnas.58.2.650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKETA K., POGELL B. M. ALLOSTERIC INHIBITION OF RAT LIVER FRUCTOSE 1,6-DIPHOSPHATASE BY ADENOSINE 5'-MONOPHOSPHATE. J Biol Chem. 1965 Feb;240:651–662. [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J. Physical and topological properties of circular DNA. J Gen Physiol. 1966 Jul;49(6):103–125. doi: 10.1085/jgp.49.6.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]