Abstract
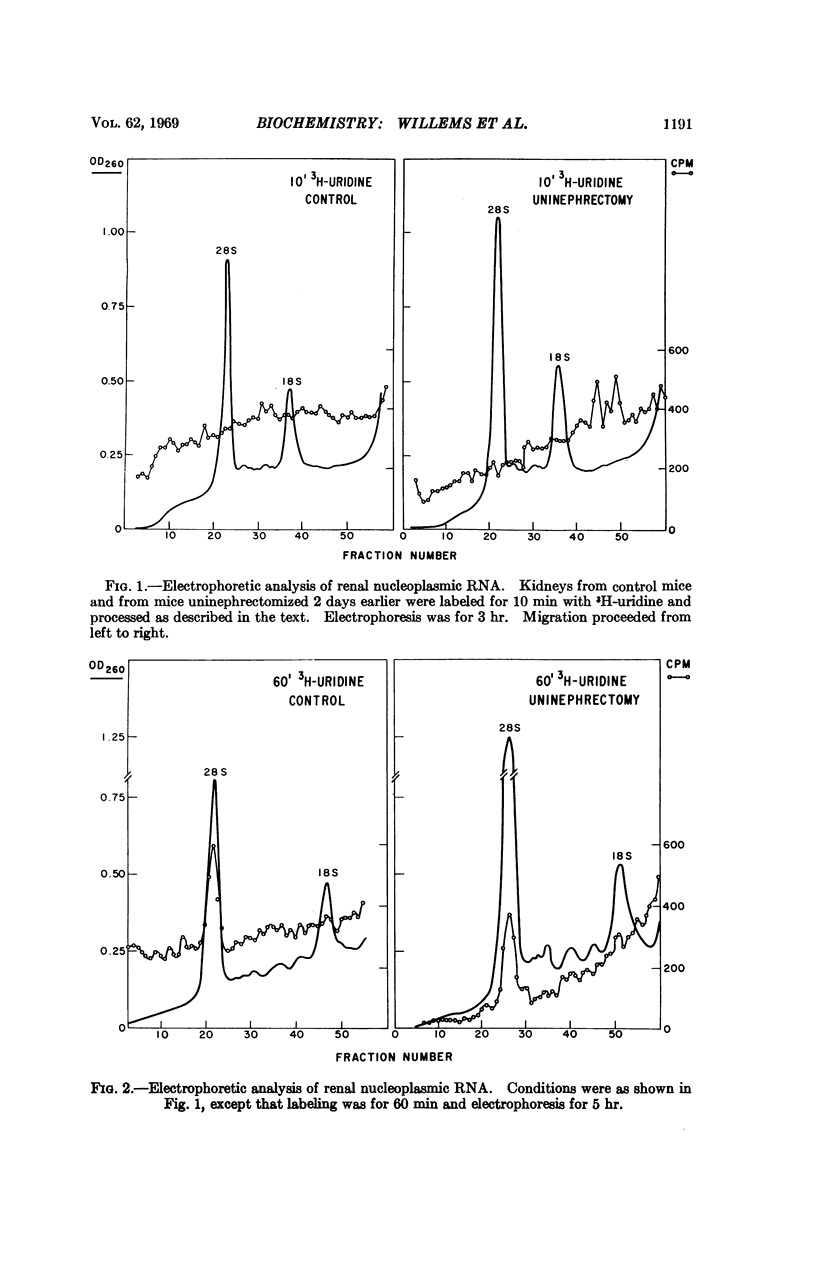
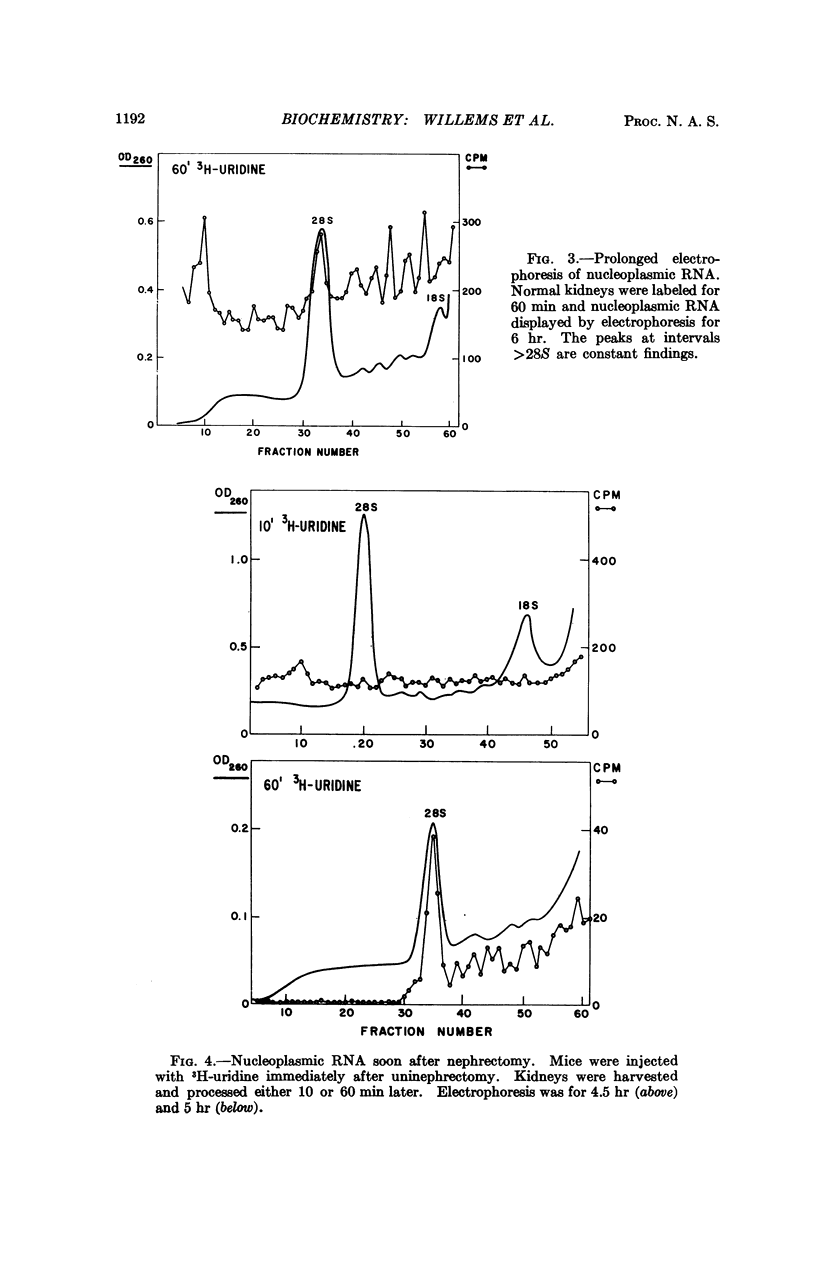
Heterogeneous RNA (HnRNA) (ca. 10S-70S) labeled for ten minutes with 3H-uridine was identified by polyacrylamide-gel electrophoresis in the nucleoplasm of kidney cells. In the region >28S several discretely migrating species were present. Ten minutes after the removal of one kidney, labeled HnRNA >28S (“giant” HnRNA) in the remaining kidney began to decrease, and by 60 minutes it had fallen drastically. The presence of labeled giant HnRNA during a pulse label after uninephrectomy and its disappearance after longer labeling suggest that the decrease is a consequence of faster processing. Accelerated processing of giant HnRNA is antecedent to the increased synthesis of renal cytoplasmic RNA that follows uninephrectomy.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi G., Parnas H., Hwang M. I., Attardi B. Giant-size rapidly labeled nuclear ribonucleic acid and cytoplasmic messenger ribonucleic acid in immature duck erythrocytes. J Mol Biol. 1966 Sep;20(1):145–182. doi: 10.1016/0022-2836(66)90123-9. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Pène J. J., Darnell J. E. Studies on HeLa cell nuclear DNA-like RNA by RNA-DNA hybridization. Proc Natl Acad Sci U S A. 1967 Jul;58(1):320–327. doi: 10.1073/pnas.58.1.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. D., Gurdon J. B. Size distribution and stability of DNA-like RNA synthesized during development of anucleolate embryos of Xenopus laevis. J Mol Biol. 1966 Aug;19(2):399–422. doi: 10.1016/s0022-2836(66)80013-x. [DOI] [PubMed] [Google Scholar]
- CHAUVEAU J., MOULE Y., ROUILLER C. Isolation of pure and unaltered liver nuclei morphology and biochemical composition. Exp Cell Res. 1956 Aug;11(2):317–321. doi: 10.1016/0014-4827(56)90107-0. [DOI] [PubMed] [Google Scholar]
- Church R. B., McCarthy B. J. Ribonucleic acid synthesis in regenerating and embryonic liver. I. The synthesis of new species of RNA during regeneration of mouse liver after partial hepatectomy. J Mol Biol. 1967 Feb 14;23(3):459–475. doi: 10.1016/s0022-2836(67)80118-9. [DOI] [PubMed] [Google Scholar]
- Denis H. Gene expression in amphibian development II. Release of the genetic information in growing embryos. J Mol Biol. 1966 Dec 28;22(2):285–304. doi: 10.1016/0022-2836(66)90133-1. [DOI] [PubMed] [Google Scholar]
- Dingman C. W., Peacock A. C. Analytical studies on nuclear ribonucleic acid using polyacrylamide gel electrophoresis. Biochemistry. 1968 Feb;7(2):659–668. doi: 10.1021/bi00842a022. [DOI] [PubMed] [Google Scholar]
- Drews J., Brawerman G., Morris H. P. Nucleotide sequence homologies in nuclear and cytoplasmic ribonucleic acid from rat liver and hepatomas. Eur J Biochem. 1968 Jan;3(3):284–292. doi: 10.1111/j.1432-1033.1968.tb19528.x. [DOI] [PubMed] [Google Scholar]
- Edström J. E., Daneholt B. Sedimentation properties of the newly synthesized RNA from isolated nuclear components of Chironomus tentans salivary gland cells. J Mol Biol. 1967 Sep 14;28(2):331–343. doi: 10.1016/s0022-2836(67)80013-5. [DOI] [PubMed] [Google Scholar]
- Halliburton I. W., Thomson R. Y. Chemical aspects of compensatory renal hypertrophy. Cancer Res. 1965 Dec;25(11):1882–1887. [PubMed] [Google Scholar]
- Malt R. A., LeMaitre D. A. Differential labeling of 28-S RNA in free and membrane-bound ribosomes of kidney. Biochim Biophys Acta. 1967 Aug 22;145(1):190–192. [PubMed] [Google Scholar]
- Malt R. A., Lemaitre D. A. Accretion and turnover of RNA in the renoprival kidney. Am J Physiol. 1968 May;214(5):1041–1047. doi: 10.1152/ajplegacy.1968.214.5.1041. [DOI] [PubMed] [Google Scholar]
- Malt R. A., Miller W. I. Sequential changes in classes of RNA during compensatory growth of the kidney. J Exp Med. 1967 Jul 1;126(1):1–13. doi: 10.1084/jem.126.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malt R. A. Rapidly labeled nuclear and cytoplasmic renal RNA. J Exp Med. 1966 Oct 1;124(4):679–688. doi: 10.1084/jem.124.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M., Fujisawa T. Methylation of ribosomal RNA precursor and tRNA in rat liver. Biochim Biophys Acta. 1968 May 21;157(3):476–492. doi: 10.1016/0005-2787(68)90147-0. [DOI] [PubMed] [Google Scholar]
- Muramatsu M., Hodnett J. L., Steele W. J., Busch H. Synthesis of 28-S RNA in the nucleolus. Biochim Biophys Acta. 1966 Jul 20;123(1):116–125. doi: 10.1016/0005-2787(66)90164-x. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Penman S., Smith I., Holtzman E. Ribosomal RNA synthesis and processing in a particulate site in the HeLa cell nucleus. Science. 1966 Nov 11;154(3750):786–789. doi: 10.1126/science.154.3750.786. [DOI] [PubMed] [Google Scholar]
- Penman S., Vesco C., Penman M. Localization and kinetics of formation of nuclear heterodisperse RNA, cytoplasmic heterodisperse RNA and polyribosome-associated messenger RNA in HeLa cells. J Mol Biol. 1968 May 28;34(1):49–60. doi: 10.1016/0022-2836(68)90234-9. [DOI] [PubMed] [Google Scholar]
- Retèl J., Planta R. J. Ribosomal precursor RNA in Saccharomyces carlsbergensis. Eur J Biochem. 1967 Dec;3(2):248–258. doi: 10.1111/j.1432-1033.1967.tb19524.x. [DOI] [PubMed] [Google Scholar]
- Scherrer K., Marcaud L., Zajdela F., London I. M., Gros F. Patterns of RNA metabolism in a differentiated cell: a rapidly labeled, unstable 60S RNA with messenger properties in duck erythroblasts. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1571–1578. doi: 10.1073/pnas.56.5.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz G., Gallwitz D., Sekeris C. E. Rapidly labelled high molecular RNA from rat liver. Eur J Biochem. 1968 Apr 3;4(2):149–156. doi: 10.1111/j.1432-1033.1968.tb00186.x. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Birnboim H. C., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. II. Base composition and cellular localization of a heterogeneous RNA fraction. J Mol Biol. 1966 Aug;19(2):362–372. doi: 10.1016/s0022-2836(66)80010-4. [DOI] [PubMed] [Google Scholar]
- Soeiro R., Vaughan M. H., Warner J. R., Darnell J. E., Jr The turnover of nuclear DNA-like RNA in HeLa cells. J Cell Biol. 1968 Oct;39(1):112–118. doi: 10.1083/jcb.39.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner J. R., Soeiro R., Birnboim H. C., Girard M., Darnell J. E. Rapidly labeled HeLa cell nuclear RNA. I. Identification by zone sedimentation of a heterogeneous fraction separate from ribosomal precursor RNA. J Mol Biol. 1966 Aug;19(2):349–361. doi: 10.1016/s0022-2836(66)80009-8. [DOI] [PubMed] [Google Scholar]
- Weinberg R. A., Loening U., Willems M., Penman S. Acrylamide gel electrophoresis of HeLa cell nucleolar RNA. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1088–1095. doi: 10.1073/pnas.58.3.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]


