Abstract
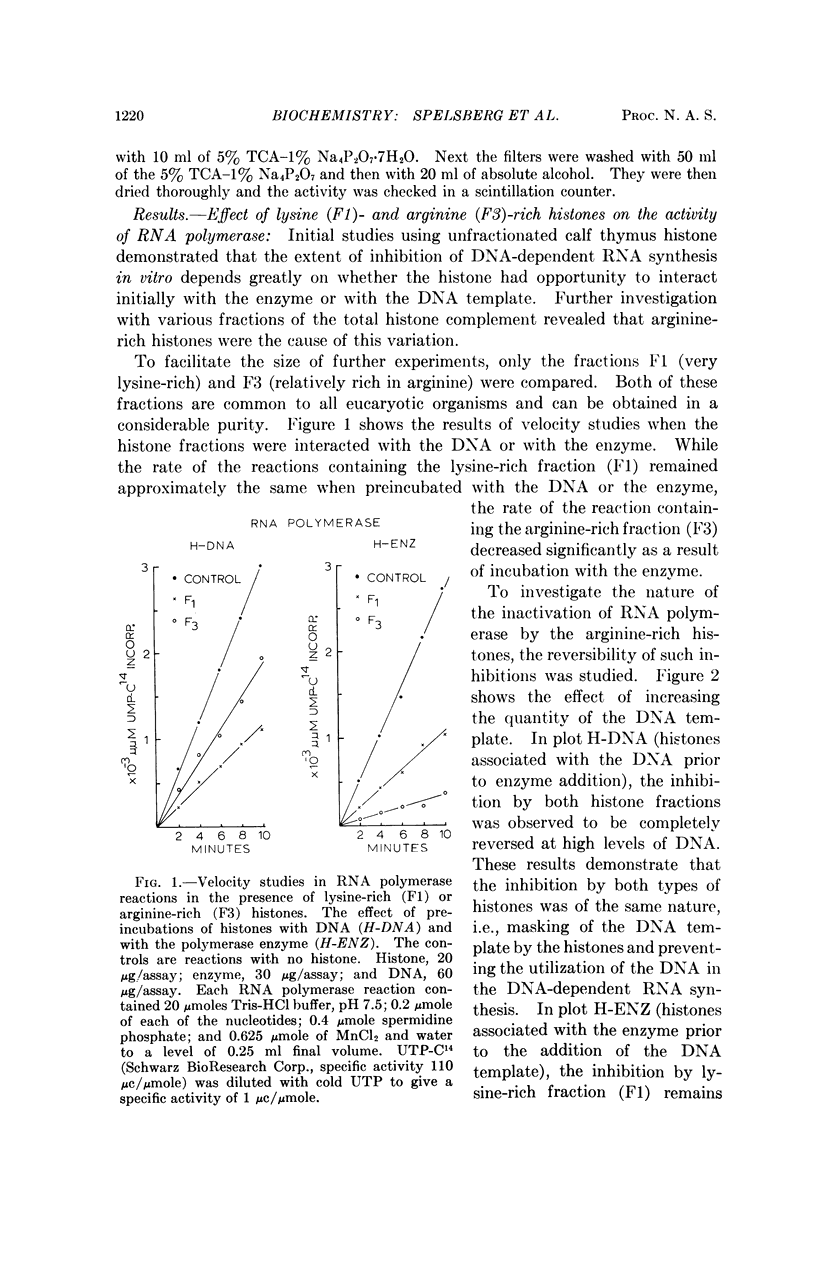
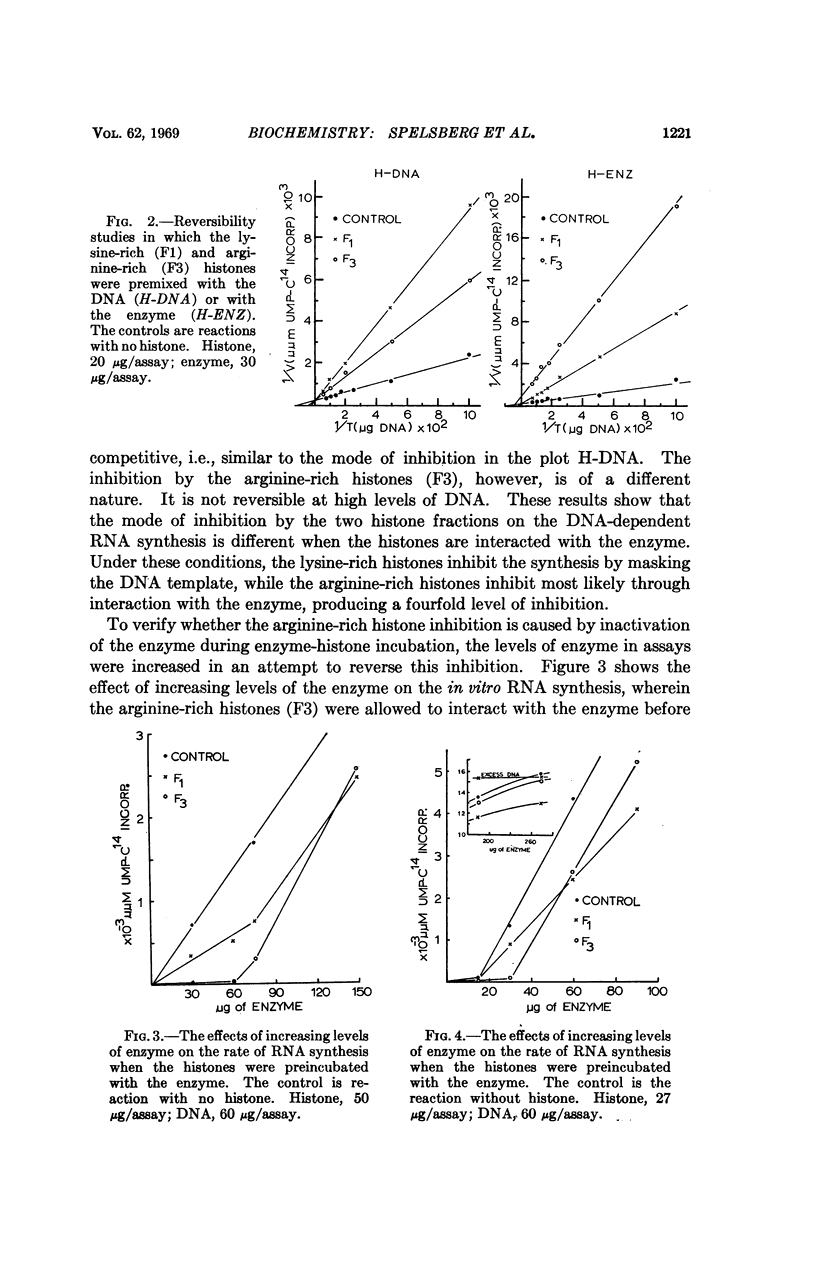
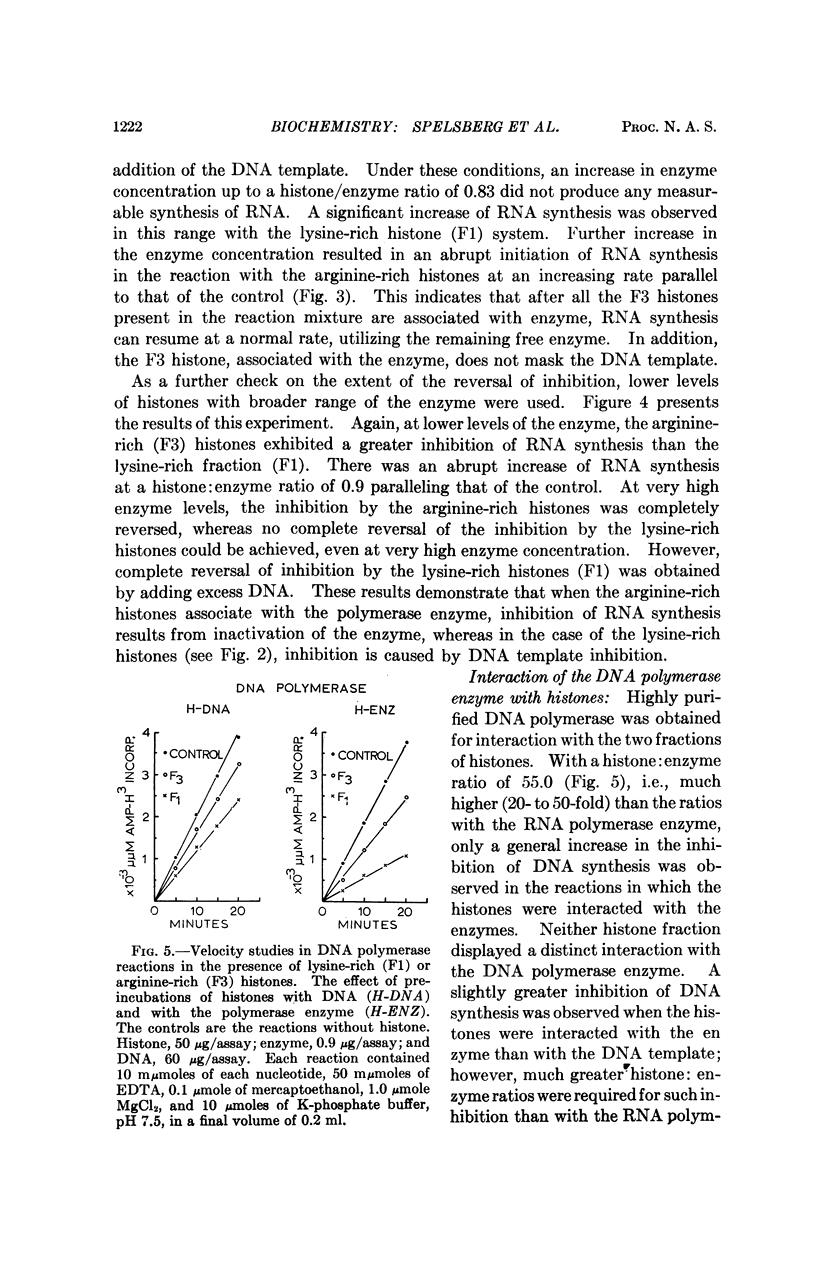
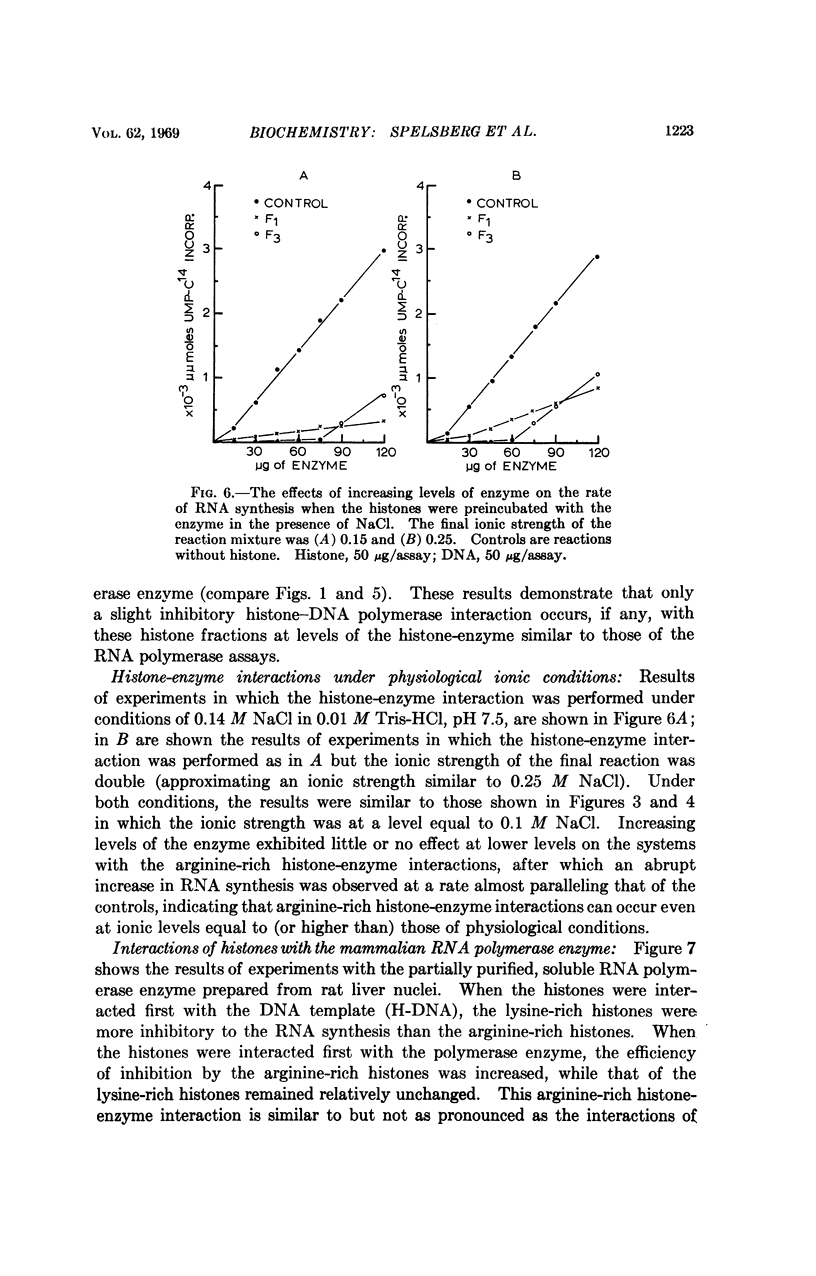
Arginine-rich histones (F3) interact with both the bacterial and mammalian RNA polymerase and inhibit the in vitro RNA synthesis to a much greater extent than when associated with the DNA template. The lysine-rich histones (F1) inhibit the RNA synthesis mainly through template inhibition. Neither histone fraction displayed such an interaction with DNA polymerase. The RNA polymerase-F3 histone interaction takes place at ionic strength equal to or greater than those occurring in living cells, suggesting a possible role of arginine-rich histones in the regulation of RNA synthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner J., Dahmus M. E., Fambrough D., Huang R. C., Marushige K., Tuan D. Y. The Biology of Isolated Chromatin: Chromosomes, biologically active in the test tube, provide a powerful tool for the study of gene action. Science. 1968 Jan 5;159(3810):47–56. doi: 10.1126/science.159.3810.47. [DOI] [PubMed] [Google Scholar]
- Cunningham D. D., Steiner D. F. Extraction of RNA polymerase from rat liver nuclei in a soluble form. Biochim Biophys Acta. 1967;145(3):834–836. doi: 10.1016/0005-2787(67)90144-x. [DOI] [PubMed] [Google Scholar]
- FURTH J. J., HO P. THE ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID IN ANIMAL TISSUE. I. THE DEOXYRIBONUCLEIC ACID-DIRECTED SYNTHESIS OF RIBONUCLEIC ACID AS CATALYZED BY AN ENZYME OBTAINED FROM BOVINE LYMPHOSARCOMA TISSUE. J Biol Chem. 1965 Jun;240:2602–2606. [PubMed] [Google Scholar]
- Farmbrough D. M., Fujimura F., Bonner J. Quantitative distribution of histone components in the pea plant. Biochemistry. 1968 Feb;7(2):575–585. doi: 10.1021/bi00842a010. [DOI] [PubMed] [Google Scholar]
- Georgiev G. P., Ananieva L. N., Kozlov J. V. Stepwise removal of protein from a deoxyribonucleoprotein complex and de-repression of the genome. J Mol Biol. 1966 Dec 28;22(2):365–371. doi: 10.1016/0022-2836(66)90140-9. [DOI] [PubMed] [Google Scholar]
- Hnilica L. S., Bess L. G. The heterogeneity of arginine-rich histones. Anal Biochem. 1965 Sep;12(3):421–436. doi: 10.1016/0003-2697(65)90209-5. [DOI] [PubMed] [Google Scholar]
- Hnilica L. S., Edwards L. J., Hey A. E. Studies on nuclear proteins. II. Quantitative distribution of histone fractions in various tissues. Biochim Biophys Acta. 1966 Jul 27;124(1):109–117. doi: 10.1016/0304-4165(66)90318-7. [DOI] [PubMed] [Google Scholar]
- Hnilica L. S. Studies on nuclear proteins. I. Observations on the tissue and species specificity of the moderately lysine-rich histone fraction 2b. Biochim Biophys Acta. 1966 Mar 28;117(1):163–175. doi: 10.1016/0304-4165(66)90163-2. [DOI] [PubMed] [Google Scholar]
- Jacob S. T., Sajdel E. M., Munro H. N. Altered characteristics of mammalian RNA polymerase following solubilization from nuclei. Biochem Biophys Res Commun. 1968 Sep 6;32(5):831–838. doi: 10.1016/0006-291x(68)90316-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKAMOTO T., FOX C. F., WEISS S. B. ENZYMATIC SYNTHESIS OF RIBONUCLEIC ACID. I. PREPARATION OF RIBONUCLEIC ACID POLYMERASE FROM EXTRACTS OF MICROCOCCUS LYSODEIKTICUS. J Biol Chem. 1964 Jan;239:167–174. [PubMed] [Google Scholar]
- STEDMAN E. Cell specificity of histones. Nature. 1950 Nov 4;166(4227):780–781. doi: 10.1038/166780a0. [DOI] [PubMed] [Google Scholar]
- Seifart K., Sekeris C. E. Extraction of DNA-dependent RNA polymerase from rat liver nuclei. Hoppe Seylers Z Physiol Chem. 1967 Nov;348(11):1555–1557. [PubMed] [Google Scholar]
- Stellwagen R. H., Cole R. D. Danger of contamination in chromatographically prepared arginine-rich histone. J Biol Chem. 1968 Sep 10;243(17):4452–4455. [PubMed] [Google Scholar]



