Abstract
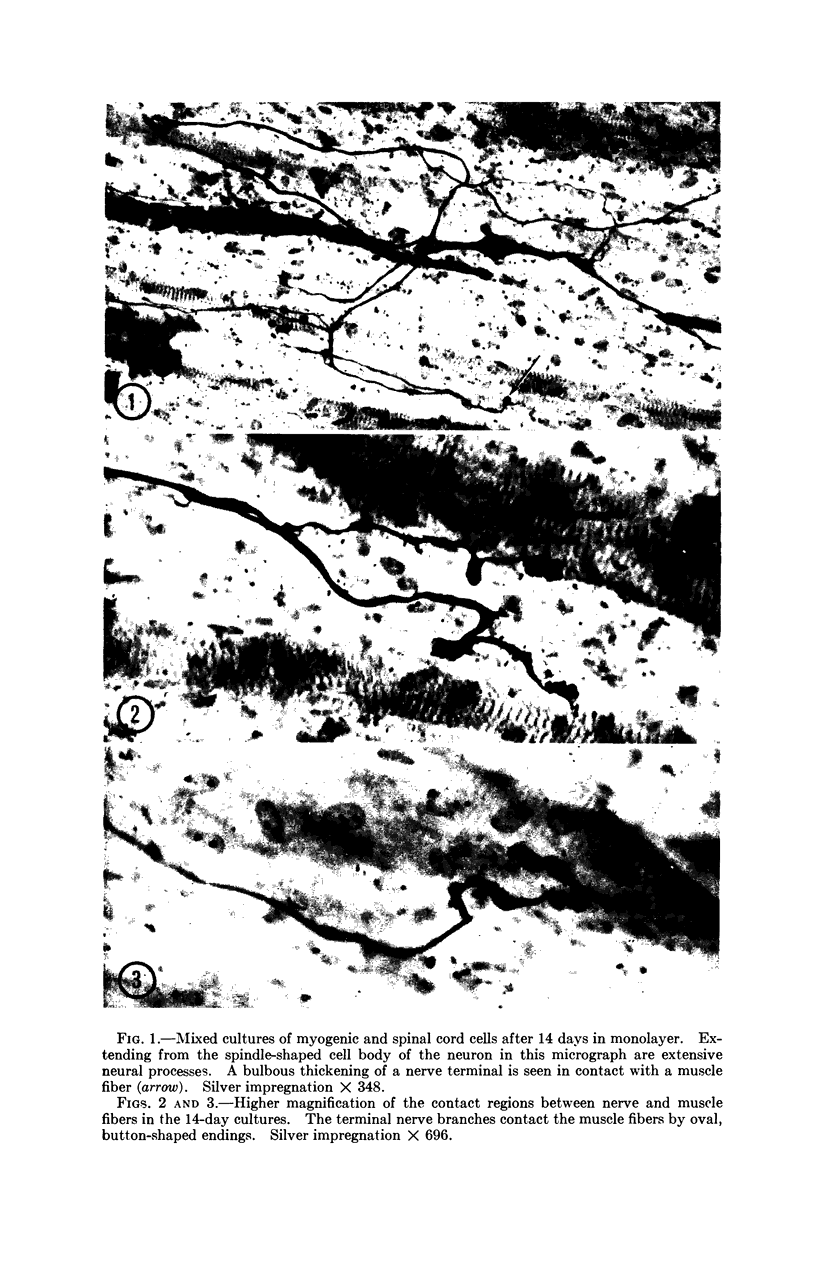
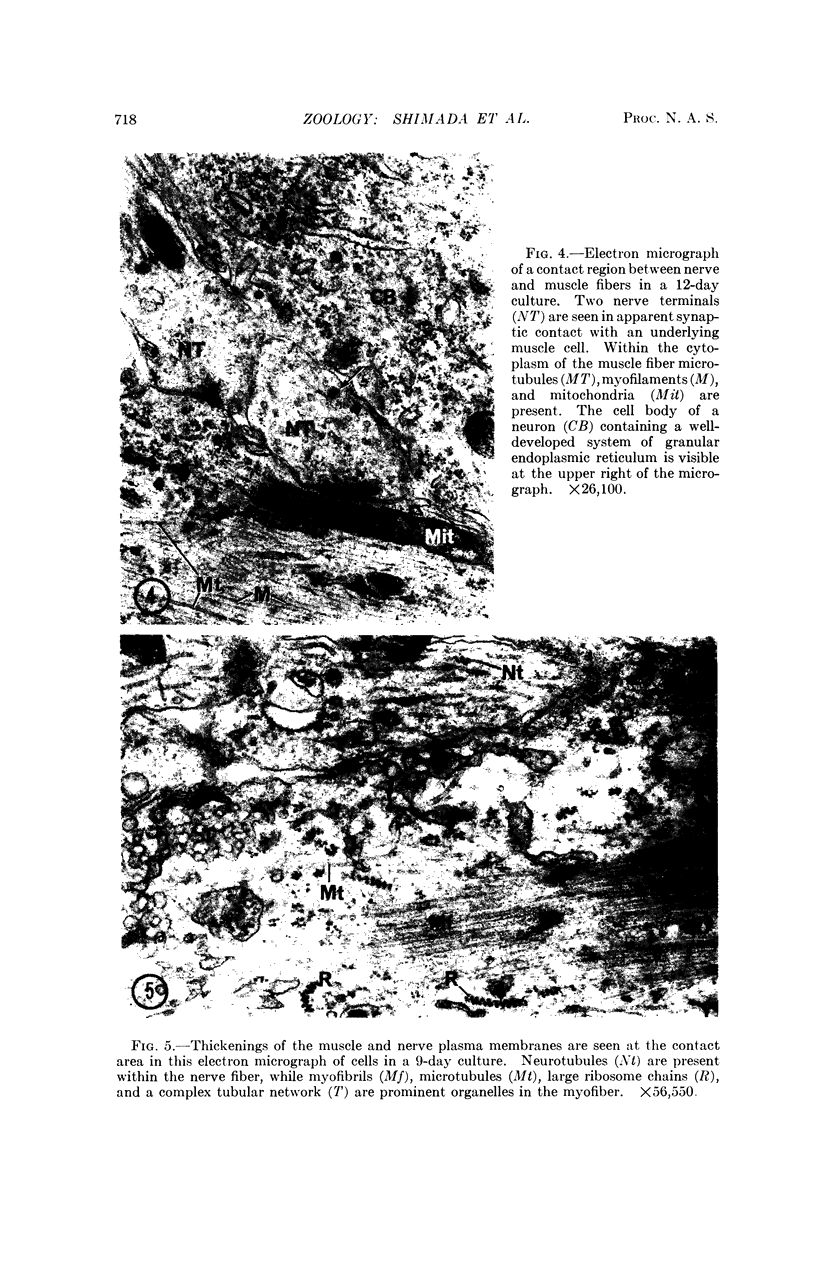
The formation of nerve-muscle junctions in monolayer cultures of embryonic muscle and spinal cord cells is described. Muscle-forming cells (myoblasts) from leg muscles of 12-day chick embryos were separated with trypsin and cultured on a collagen substrate for two days. Suspensions of ventral spinal cord cells from six-day chick embryos were then plated over the differentiating muscle cells. The cultures were subsequently examined by electron microscopy and in silver-stained light microscopic preparations. After 10-12 days in culture, irregular thickenings were observed along the nerve cell processes in contact with the muscle fibers which by that time had undergone advanced differentiation (myogenesis). By electron microscopy it was demonstrated that many of the nerve-muscle contacts had the characteristics of a synapse. Some aspects of the fine structure of these junctions are described. The possibilities raised by these findings with respect to innervation mechanisms and specificities in nerve-muscle interactions are briefly discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABERCROMBIE M., HEAYSMAN J. E. Observations on the social behaviour of cells in tissue culture. II. Monolayering of fibroblasts. Exp Cell Res. 1954 May;6(2):293–306. doi: 10.1016/0014-4827(54)90176-7. [DOI] [PubMed] [Google Scholar]
- Corner M. A., Crain S. M. Spontaneous contractions and bioelectric activity after differentiation in culture of presumptive neuromuscular tissues of the early frog embryo. Experientia. 1965 Jul 15;21(7):422–424. doi: 10.1007/BF02139785. [DOI] [PubMed] [Google Scholar]
- Fischman D. A. An electron microscope study of myofibril formation in embryonic chick skeletal muscle. J Cell Biol. 1967 Mar;32(3):557–575. doi: 10.1083/jcb.32.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HESS A. Structural differences of fast and slow extrafusal muscle fibres and their nerve endings in chickens. J Physiol. 1961 Jul;157:221–231. doi: 10.1113/jphysiol.1961.sp006717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano H. Ultrastructural study on the morphogenesis of the neuromuscular junction in the skeletal muscle of the chick. Z Zellforsch Mikrosk Anat. 1967;79(2):198–208. [PubMed] [Google Scholar]
- James D. W., Tresman R. L. De novo formation of neuro-muscular junctions in tissue culture. Nature. 1968 Oct 26;220(5165):384–385. doi: 10.1038/220384a0. [DOI] [PubMed] [Google Scholar]
- KONIGSBERG I. R. Clonal analysis of myogenesis. Science. 1963 Jun 21;140(3573):1273–1284. doi: 10.1126/science.140.3573.1273. [DOI] [PubMed] [Google Scholar]
- KRUGER P., GUNTHER P. G. Innervation und pharmakologisches Verhalten des M. gastrocnemius und M. pectoralis maior der Vogel. Acta Anat (Basel) 1958;33(4):325–338. [PubMed] [Google Scholar]
- MOSCONA A. Rotation-mediated histogenetic aggregation of dissociated cells. A quantifiable approach to cell interactions in vitro. Exp Cell Res. 1961 Jan;22:455–475. doi: 10.1016/0014-4827(61)90122-7. [DOI] [PubMed] [Google Scholar]
- Okazaki K., Holtzer H. An analysis of myogenesis in vitro using fluorescein-labeled antimyosin. J Histochem Cytochem. 1965 Nov-Dec;13(8):726–739. doi: 10.1177/13.8.726. [DOI] [PubMed] [Google Scholar]
- PETERSON E. R., CRAIN S. M., MURRAY M. R. DIFFERENTIATION AND PROLONGED MAINTENANCE OF BIOELECTRICALLY ACTIVE SPINAL CORD CULTURES (RAT, CHICK AND HUMAN). Z Zellforsch Mikrosk Anat. 1965 Mar 25;66(1):130–154. doi: 10.1007/BF00339322. [DOI] [PubMed] [Google Scholar]
- SEVIER A. C., MUNGER B. L. TECHNICAL NOTE: A SILVER METHOD FOR PARAFFIN SECTIONS OF NEURAL TISSUE. J Neuropathol Exp Neurol. 1965 Jan;24:130–135. doi: 10.1097/00005072-196501000-00012. [DOI] [PubMed] [Google Scholar]
- Shimada Y., Fischman D. A., Moscona A. A. The fine structure of embryonic chick skeletal muscle cells differentiated in vitro. J Cell Biol. 1967 Nov;35(2):445–453. doi: 10.1083/jcb.35.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada Y. Suppression of myogenesis by heterotypic and heterospecific cells in monolayer cultures. Exp Cell Res. 1968 Aug-Sep;51(2-3):564–578. doi: 10.1016/0014-4827(68)90144-4. [DOI] [PubMed] [Google Scholar]
- WADDINGTON C. H., PERRY M. M. Helical arrangement of ribosomes in differentiating muscle cells. Exp Cell Res. 1963 May;30:599–600. doi: 10.1016/0014-4827(63)90339-2. [DOI] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci U S A. 1968 Oct;61(2):477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]