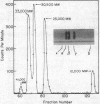
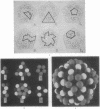
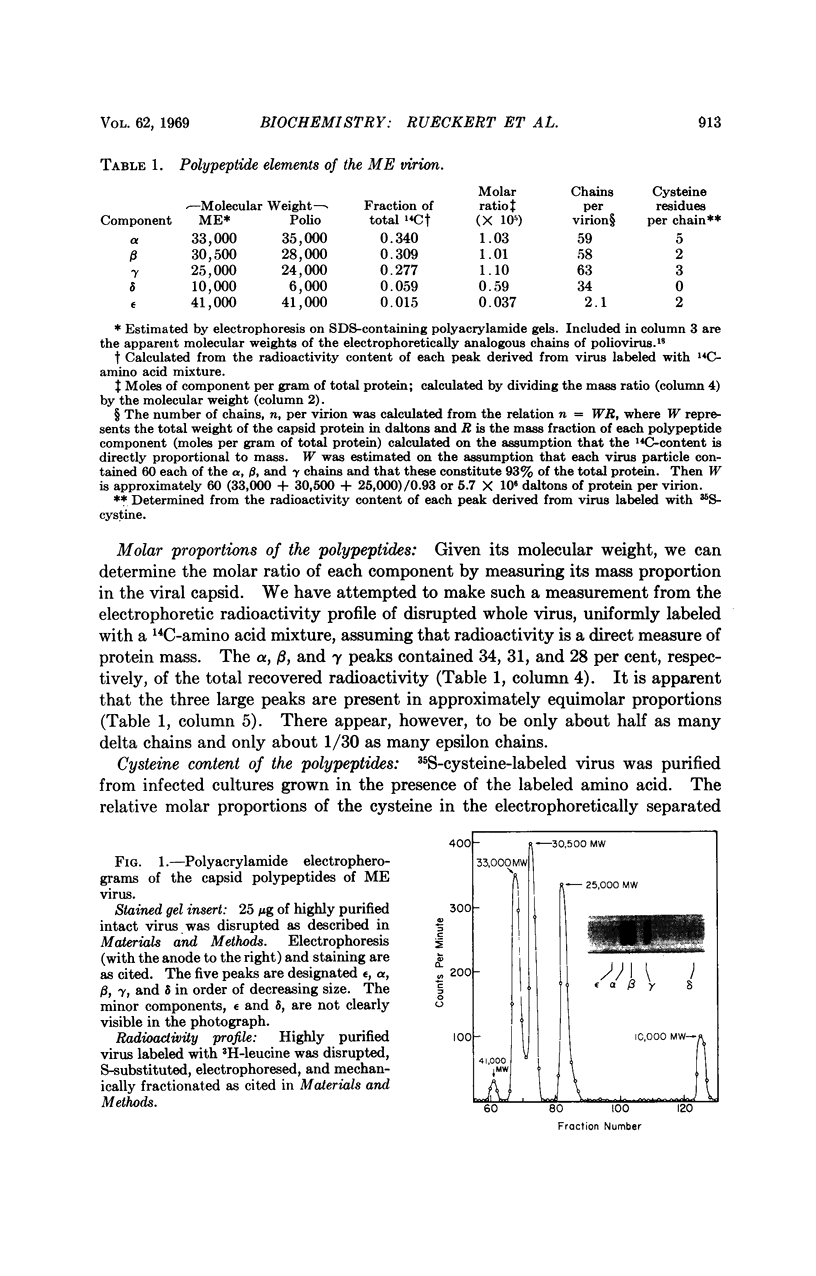
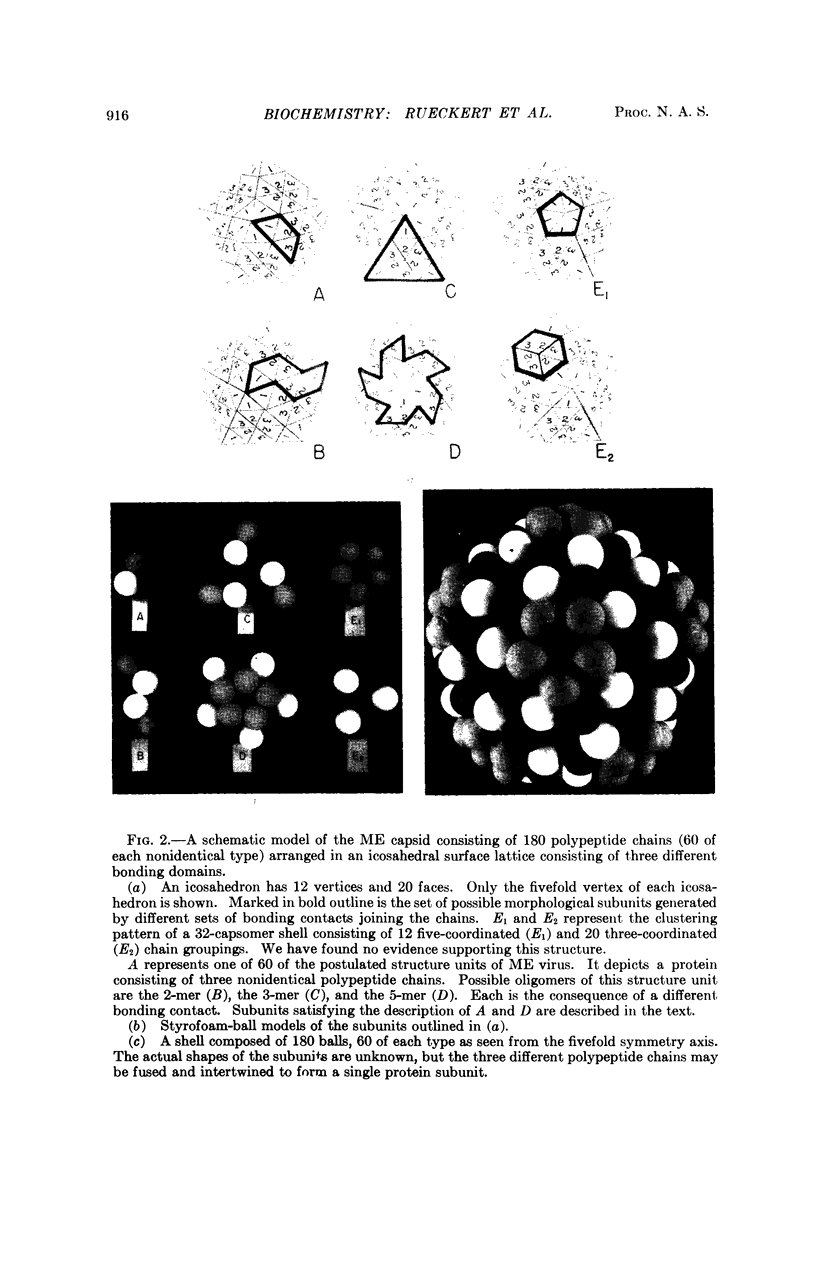
Abstract
It is proposed that the capsid structure of mouse-Elberfeld (ME) virus, a small icosahedral ribonucleic acid containing picornavirus, is determined by 60 identical protein subunits; each of the latter is composed of several non-identical polypeptide chains. Icosahedral symmetry in a 60-subunit shell allows three types of specific intersubunit bonding contacts which establish its axes of two-, three-, and fivefold symmetry, respectively. Of these three bonding types, two are sufficient to specify a complete shell. We interpret the possible identity of some discrete supramolecular structures involved in the biosynthesis of poliovirus and in the degradation of ME virus in the context of stepwise formation or disruption of two different types of specific intersubunit bonding contacts.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burness A. T., Walter D. S. Protein components of encephalomyocarditis virus. Nature. 1967 Sep 23;215(5108):1350–1352. doi: 10.1038/2151350a0. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., WATSON J. D. Structure of small viruses. Nature. 1956 Mar 10;177(4506):473–475. doi: 10.1038/177473a0. [DOI] [PubMed] [Google Scholar]
- FINCH J. T., KLUG A. Structure of poliomyelitis virus. Nature. 1959 Jun 20;183(4677):1709–1714. doi: 10.1038/1831709a0. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Klug A. Arrangement of protein subunits and the distribution of nucleic acid in turnip yellow mosaic virus. II. Electron microscopic studies. J Mol Biol. 1966 Jan;15(1):344–364. doi: 10.1016/s0022-2836(66)80231-0. [DOI] [PubMed] [Google Scholar]
- HAUSEN P., SCHAEFER W. [Studies on a mouse encephalomyelitis virus. Purification and physical-chemical properties of the virus]. Z Naturforsch B. 1962 Jan;17B:15–22. [PubMed] [Google Scholar]
- Holland J. J., Kiehn E. D. Specific cleavage of viral proteins as steps in the synthesis and maturation of enteroviruses. Proc Natl Acad Sci U S A. 1968 Jul;60(3):1015–1022. doi: 10.1073/pnas.60.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- LE BOUVIER G. L. The D to C change in poliovirus particles. Br J Exp Pathol. 1959 Dec;40:605–620. [PMC free article] [PubMed] [Google Scholar]
- Maizel J. V., Jr Acrylamide-gel electrophorograms by mechanical fractionation: radioactive adenovirus proteins. Science. 1966 Feb 25;151(3713):988–990. doi: 10.1126/science.151.3713.988. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Summers D. F. Evidence for differences in size and composition of the poliovirus-specific polypeptides in infected HeLa cells. Virology. 1968 Sep;36(1):48–54. doi: 10.1016/0042-6822(68)90115-3. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Summers D. F., Maizel J. V., Jr In vitro assembly of poliovirus-related particles. Virology. 1968 Jun;35(2):216–226. doi: 10.1016/0042-6822(68)90262-6. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., MAYER M. M., ROANE P. R., Jr Immunochemical studies of poliovirus. IV. Alteration of the immunologic specificity of purified poliomyelitis virus by heat and ultraviolet light. J Immunol. 1959 Jan;82(1):19–25. [PubMed] [Google Scholar]
- RUECKERT R. R. STUDIES ON THE STRUCTURE OF VIRUSES OF THE COLUMBIA SK GROUP. II. THE PROTEIN SUBUNITS OF ME-VIRUS AND OTHER MEMBERS OF THE COLUMBIA SK GROUP. Virology. 1965 Jun;26:345–358. doi: 10.1016/0042-6822(65)90282-5. [DOI] [PubMed] [Google Scholar]
- Rueckert R. R., Duesberg P. H. Non-identical peptide chains in mouse encephalitis virus. J Mol Biol. 1966 Jun;17(2):490–502. doi: 10.1016/s0022-2836(66)80159-6. [DOI] [PubMed] [Google Scholar]
- SCHARFF M. D., MAIZEL J. V., Jr, LEVINTOW L. PHYSICAL AND IMMUNOLOGICAL PROPERTIES OF A SOLUBLE PRECURSOR OF THE POLIOVIRUS CAPSID. Proc Natl Acad Sci U S A. 1964 Feb;51:329–337. doi: 10.1073/pnas.51.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- Smithies O. Disulfide-bond cleavage and formation in proteins. Science. 1965 Dec 17;150(3703):1595–1598. doi: 10.1126/science.150.3703.1595. [DOI] [PubMed] [Google Scholar]
- Summers D. F., Maizel J. V., Jr, Darnell J. E., Jr Evidence for virus-specific noncapsid proteins in poliovirus-infected HeLa cells. Proc Natl Acad Sci U S A. 1965 Aug;54(2):505–513. doi: 10.1073/pnas.54.2.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE Y., WATANABE K., HINUMA Y. Synthesis of poliovirus-specific proteins in HeLa cells. Biochim Biophys Acta. 1962 Dec 31;61:976–977. doi: 10.1016/0926-6550(62)90015-4. [DOI] [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]