Abstract
At high concentrations, the tubule poison paclitaxel is able to kill cancer cells that express Bcl-2; it inhibits the antiapoptotic activity of Bcl-2 by inducing its phosphorylation. To localize the site on Bcl-2 regulated by phosphorylation, mutant forms of Bcl-2 were constructed. Mutant forms of Bcl-2 with an alteration in serine at amino acid 70 (S70A) or with deletion of a 60-aa loop region between the α1 and α2 helices (Δloop Bcl-2, which also deletes amino acid 70) were unable to be phosphorylated by paclitaxel treatment of MDA-MB-231 cells into which the genes for the mutant proteins were transfected. The Δloop mutant completely inhibited paclitaxel-induced apoptosis. In cells expressing the S70A mutant, paclitaxel induced about one-third the level of apoptosis seen with wild-type Bcl-2. To evaluate the role of mitogen-activated protein kinases (MAPKs) in Bcl-2 phosphorylation, the activation of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and p38 was examined. Paclitaxel-induced apoptosis was associated with phosphorylation of Bcl-2 and activation of ERK and JNK MAPKs. If JNK activation was blocked by transfections with either a stress-activated protein kinase kinase dominant-negative (K→R) gene (which prevents the activation of a kinase upstream of JNK) or MAPK phosphatase-1 gene (which dephosphorylates and inactivates JNK), Bcl-2 phosphorylation did not occur, and the cells were not killed by paclitaxel. By contrast, neither an ERK inhibitor (PD098059) nor p38 inhibitors (SB203580 and SB202190) had an effect on Bcl-2 phosphorylation. Thus, our data show that the antiapoptotic effects of Bcl-2 can be overcome by phosphorylation of Ser-70; forms of Bcl-2 lacking the loop region are much more effective at preventing apoptosis than wild-type Bcl-2 because they cannot be phosphorylated. JNK, but not ERK or p38 MAPK, appear to be involved in the phosphorylation of Bcl-2 induced by paclitaxel.
Apoptosis is an evolutionarily conserved physiological process that ensures the elimination of unwanted or damaged cells from multicellular organisms (1, 2). The aberrant regulation of apoptosis has been observed in many disorders (such as neuronal diseases, AIDS, autoimmune diseases, and cancers) that result from an imbalance between positive and negative regulators of cell survival (2). In addition, many therapeutic agents eliminate tumor cells by inducing apoptotic cell death (2). Therefore, understanding the mechanism of apoptosis has important implications in the prevention and treatment of many diseases. The Bcl-2 family of proteins are apoptotic regulators that function as molecular rheostats to control cell survival (3, 4). These proteins are capable of protecting various cell types from experimentally induced cell death both in vitro and in vivo (5, 6). Expression of Bcl-2 rescues cell death induced by a variety of stresses, including depletion of trophic factors, antitumor drugs, oxygen free radicals, viral agents, and heat shock as well as neuronal axotomy (5–8). However, the molecular mechanism by which Bcl-2 prevents cell death remains incompletely defined.
Mitogen-activated protein kinases (MAPKs) transduce signals from the cell membrane to the nucleus in response to a variety of different stimuli and participate in various intracellular signaling pathways that control a wide spectrum of cellular processes, including cell growth, differentiation, and stress responses (9–13). In contrast to p42MAPK/extracellular signal-regulated kinase (ERK) 2 and p44MAPK/ERK1, which are activated by mitogenic stimuli, p46 c-Jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) and p54JNK/SAPK and p38MAPK are activated by inflammatory cytokines and cellular stresses such as osmotic and heat shock, UV and γ irradiation, protein synthesis inhibitors, metabolic poisons, lipopolysaccharide, proinflammatory cytokines, growth factor deprivation, and surface Ig crosslinking in human B lymphocytes (11, 13–18). In fact, the stimulation of JNK was a prerequisite for cell death under various conditions, and a blockade of JNK activation resulted in the prevention of cell death (18, 19). MAPKs can be dephosphorylated and inactivated by dual-specificity phosphatases (18, 19). These findings imply that under certain circumstances JNK may function in an intracellular signaling pathway, leading to cell death. Once activated, JNK/SAPK phosphorylates several transcription factors including c-Jun (11, 20), ATF-2 (12), and ELK-1 (21), thereby regulating gene expression.
The microtubule-stabilizing agents, such as paclitaxel and docetaxel, and microtubule-disrupting drugs, such as vincristine, vinblastine, and colchicine, have antimitotic and apoptosis-inducing activity (22–25). Human leukemic, breast cancer, and prostate cancer cells exposed to paclitaxel express a phosphorylated form of Bcl-2 and undergo apoptosis. The loop region of the Bcl-2 contains several phosphorylation sites, and deletion of the loop region negatively regulates Bcl-2 function (26, 27), suggesting that phosphorylation of Bcl-2 may inhibit Bcl-2 function.
These previous reports prompted us to examine whether the activities of JNK, ERK, and p38 are regulated by microtubule-damaging drugs (paclitaxel and vincristine) and whether they are involved in antagonizing the antiapoptotic function of Bcl-2 through phosphorylation. In addition, we examined the portions of the Bcl-2 molecule targeted by the function-inhibiting phosphorylation by creating a series of Bcl-2 structural mutants, which were made by changing serine residues to alanine. In this study we have shown that JNK is activated by paclitaxel treatment, and JNK activation leads to inactivation of antiapoptotic functions of Bcl-2 through phosphorylation. Deletion of the loop region completely abrogated paclitaxel-induced Bcl-2 phosphorylation, cytochrome c release from the mitochondria, caspase-3 activation, poly(ADP-ribose) polymerase (PARP) cleavage, and apoptosis. However, the S70A mutation was one-third as active as wild-type (WT) Bcl-2.
MATERIALS AND METHODS
Reagents.
Paclitaxel (from Taxus brevifolia) and vincristine were purchased from Sigma. Anti-Bcl2 antibody was purchased from Oncogene Science. Antibodies against JNK1, ERK2, p38, and PARP were purchased from Santa Cruz Biotechnology. Protein phosphatase I (PPase I, catalytic subunit) and protein tyrosine phosphatase (PTPase) were purchased from Boehringer Mannheim. Antibodies specific for phosphorylated forms of JNK/SAPK, ERK, and p38 were purchased from New England Biolabs. Enhanced chemiluminescence Western blot detection reagents were purchased from Amersham Pharmacia. Adenosine 5′-[γ32P]triphosphate (specific activity 3,000 Ci/mmol) was purchased from ICN. Anti-cytochrome c antibody was purchased from PharMingen. p38 inhibitors SB203580 and SB202190, mitogen/ERK kinase (MEK) inhibitor PD098059, and inactive inhibitor for MAPK SB202474 were purchased from Calbiochem. A dominant-negative SAPK kinase (SEK) 1 (Lys→Arg) and glutathione S-transferase (GST)-c-Jun vectors were provided by J. Kyriakis (Massachusetts General Hospital, Charlestown). The protein concentration was determined with the BCA reagent (Pierce). The caspase-3 assay kit was purchased from CLONTECH.
Cells and Culture Conditions.
MCF-7 and MDA-MB-231 cells were obtained from the American Type Culture Collection. MCF-7 and MDA-MB-231 cells were cultured in RPMI 1640 tissue culture media (Bio-Whittaker) supplemented with 10% FBS and 1% penicillin and streptomycin mixture. For short-wavelength UV light treatment of MCF-7 cells, the medium was removed, and the cells washed twice with PBS. Cells were irradiated at a dose rate of 1.3 J/m2 per s at 254 nm for 30 min, after which the original culture medium was added back to the cells.
Construction of Bcl-2 Mutants.
Human Bcl-2 cDNA was cloned in pMAX, and nucleotides corresponding to each serine residue were substituted to create a conservative alteration to alanine with a site-directed mutagenesis kit (Promega). Each single mutant was verified by sequencing the cDNA. The Δloop Bcl-2 plasmid was a gift from C.B. Thompson (University of Chicago).
Transient Transfection.
Cells were plated 24 h before transfection at a density of 1 × 106 in 100-mm dishes. Cells were cotransfected with the pCMV-gal plasmid carrying lacZ gene and plasmids for control vector, WT, or mutated Bcl-2 for 24 h. After removing the transfection mixture, the cells were incubated in complete medium for 8 h for recovery and then treated with or without paclitaxel or vincristine. Cells were harvested for analysis of Bcl-2 phosphorylation or apoptosis.
Lysate Preparation.
For determination of JNK activity, cells were collected by centrifugation at 300 g for 5 min at 4°C. The cell pellets were washed with cold PBS and solubilized with ice-cold JNK lysis buffer consisting of 25 mM Hepes (pH 7.5), 300 mM NaCl, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1% Triton X-100, 20 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 0.5 mM DTT, 100 μg/ml PMSF, and 2 μg/ml leupeptin. The cellular extract then was centrifuged for 30 min at 1,200 × g to remove debris. The supernatant was used immediately or aliquoted and stored at −70°C for future use.
For Western blotting, cells were lysed in a buffer containing 10 mM Tris⋅HCl (pH 7.6), 150 mM NaCl, 0.5 mM EDTA, 1 mM EGTA, 1% NP-40, 1 mM sodium orthovanadate, and a mixture of protease inhibitors (1 mM PMSF, 1 μg/ml pepstatin A, and 2 μg/ml aprotinin). The lysates then were sonicated for 10 s and centrifuged for 20 min at 900 × g. The supernatants were used to perform SDS/PAGE or stored at −70°C for further analyses.
Subcellular Fractionation.
Mitochondria and cytosolic (S100) fractions were prepared by resuspending cells in 0.8 ml of ice-cold buffer A (250 mM sucrose/20 mM Hepes/10 mM KCl/1.5 mM MgCl2/1 mM EDTA/1 mM EGTA/1 mM DTT/17 μg/ml PMSF/8 μg/ml aprotinin/2 μg/ml leupeptin, pH 7.4) (21). Cells were passed through an ice-cold cylinder cell homogenizer. Unlysed cells and nuclei were pelleted by centrifugation for 10 min at 750 × g. The supernatant was centrifuged at 10,000 × g for 25 min. This pellet was resuspended in buffer A and represents the mitochondrial fraction. The supernatant was centrifuged at 100,000 × g for 1 h. The supernatant from this final centrifugation represents the S100 fraction.
Measurement of JNK Activity.
JNK1 was immunoprecipitated, and kinase activity was measured by using an immunokinase complex assay with GST-c-Jun as a substrate as described (11). Briefly, cell lysates (200 μg protein) first were incubated overnight at 4°C with 10 μg of polyclonal anti-JNK1 antibody and then incubated with 20 μl of protein A Sepharose for an additional 1 h. The beads were pelleted and washed three times with cold PBS containing 1% NP-40 and 2 mM sodium orthovanadate, once with cold 100 mM Tris⋅HCl (pH 7.5) buffer containing 0.5 M LiCl, and once with cold kinase reaction buffer consisting of 12.5 mM Mops (pH 7.5), 12.5 mM β-glycerophosphate, 7.5 mM MgCl2, 0.5 mM EGTA, 0.5 mM NaF, and 0.5 mM sodium orthovanadate. The kinase reaction was performed in the presence of 1 μCi of [γ-32P]ATP, 20 μM ATP, 3.3 μM DTT, and 3 μg of substrate GST-c-Jun-(1–135) in kinase reaction buffer for 30 min at 30°C and stopped by addition of 10 μl of 5× Laemmli loading buffer. The samples were heated for 5 min at 95°C and analyzed by 12% SDS/PAGE. Phosphorylated substrate c-Jun was visualized by autoradiography. The OD of autoradiograms was determined by using the National Institutes of Health image program. The kinase activity was expressed as fold increase over control.
In Vitro Phosphorylation.
In vitro phosphorylation was performed on the GST-Bcl-2 protein. Briefly, JNK1 was immunoprecipitated from either paclitaxel-treated or untreated (control) MCF-7 cells. Cell lysates (200 μg protein) first were incubated overnight at 4°C with either 5 or 10 μg of polyclonal anti-JNK1 antibody and then incubated with 20 μl of protein A Sepharose for an additional 1 h. The beads were pelleted and washed as described in the kinase assay (above). The reaction was performed in 50 μl assay buffer (12.5 mM Mops, pH 7.5/12.5 mM β-glycerophosphate/7.5 mM MgCl2/3.3 μM DTT/0.5 mM EGTA/0.5 mM NaF/0.5 mM sodium orthovanadate) containing 1 μCi of [γ-32P]ATP, 20 μM ATP, and 10 μg of GST-Bcl-2 for 30 min at 30°C and stopped by addition of 10 μl of 5× Laemmli loading buffer. The samples were heated for 5 min at 95°C and analyzed by 6–20% SDS/PAGE. Phosphorylated Bcl-2 was visualized by autoradiography.
Western Blot Analysis.
Equal amounts of lysate protein (40 μg/lane) were run on either 10% or 8–16% SDS/PAGE and electrophoretically transferred to nitrocellulose. Nitrocellulose blots first were blocked with 5% BSA in TBS buffer (20 mM Tris⋅HCl, pH 7.4/500 mM NaCl) and then incubated with primary antibody in TBS containing 0.1% BSA overnight at 4°C. Immunoreactivity was detected by incubation with horseradish peroxidase-conjugated secondary antibody, and specific complexes were detected by the ECL technique.
Apoptosis.
Transfection was done as described above. The cells first were washed twice with cold PBS and then fixed with 75% methanol for 30 min. The fixed cells were washed again with cold PBS and stained with 1 μg/ml of 4′,6-diamidino-2-phenylindole solution for 30 min. The cells were washed three times in PBS, mounted on slides, and examined by fluorescence microscopy. In transfection experiments, we calculated apoptosis only in lacZ gene-positive cells. By cotransfection with pCMV carrying lacZ gene and staining with 5-bromo-4-chloro-3-indolyl d-galactopyranoside, we calculated that the transfection efficiency was about 70%. Cells were scored as apoptotic if they exhibited margination and condensation of the chromatin and cell shrinkage. For the quantitation of apoptotic cells, more than 500 nuclei from 20 fields were evaluated for each point.
RESULTS
Activation of MAPKs by Paclitaxel.
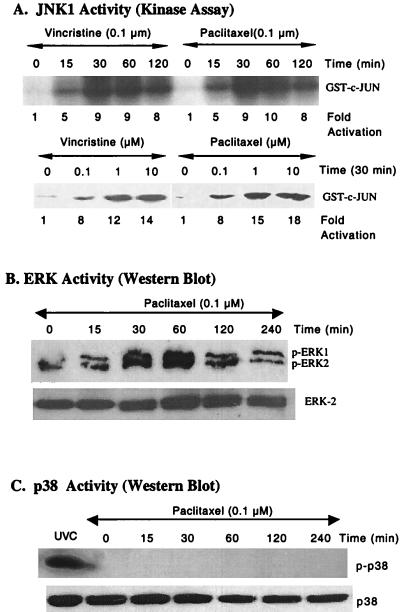
To test the hypothesis that paclitaxel-induced apoptosis may involve the MAPKs, we examined the effects of paclitaxel on the activation of JNK, ERK, and p38. We first characterized JNK activity by using MCF-7 cells (Fig. 1). Cells were treated with vincristine or paclitaxel for various times. The kinase activity increased to a maximum level at 30 min (Fig. 1A). The kinase activity also was increased with increasing doses of paclitaxel or vincristine treatment at 30 min (Fig. 1A). Similarly, paclitaxel induced JNK activation in MDA-MB-231 cells (data not shown).
Figure 1.
Effects of vincristine and paclitaxel on MAPK activation. (A) Vincristine and paclitaxel induced JNK activation in a time- and dose-dependent manner. MCF-7 cells were treated with vincristine or paclitaxel for various times. The cell lysates were prepared and immunoprecipitated with 10 μg of polyclonal anti-JNK1 antibody followed by 20 μl of protein A Sepharose. The kinase reaction was performed by the procedures described in Materials and Methods. (Upper) The time course of kinase reaction. Represented is the autoradiograph of [γ-32P]ATP incorporation into exogenous GST-c-Jun-(1–135). (Lower) The dose response of JNK activation. Anti-JNK1 immunocomplexes were obtained with lysates of cells treated with various doses of vincristine or paclitaxel for 30 min. (B) Paclitaxel-induced ERK activity. Cells were treated with paclitaxel (0.1 μM) for various time points, and Western blots were performed by using antibody specific for phosphorylated ERK (Upper) and anti-ERK2 antibody (Lower). (C) Paclitaxel had no effect on p38 activity. Cells were treated with paclitaxel (0.1 μM) for various time points, and Western blots were performed by using antibody specific for phosphorylated p38 (Upper) and anti-p38 antibody (Lower). For a positive control, cells were treated with short-wavelength UV light.
We next determined ERK activity by using antibody specific for phosphorylated ERK that recognizes activated forms of both ERK1 and ERK2. ERK activity increased in MCF-7 cells over a period of 60 min and thereafter declined (Fig. 1B). The immunoblot using ERK2 specific antibody revealed that the level of ERK2 protein did not change in MCF-7 cells (Fig. 1B). Interestingly, p38 was not activated in response to 0.1 μM paclitaxel treatment, and total p38 levels did not change in MCF-7 cells (Fig. 1C). These data suggest that paclitaxel activates the JNK and ERK, but not p38, pathways.
JNK/SAPK Signaling Mediates Paclitaxel-Induced Bcl-2 Phosphorylation and Apoptosis.
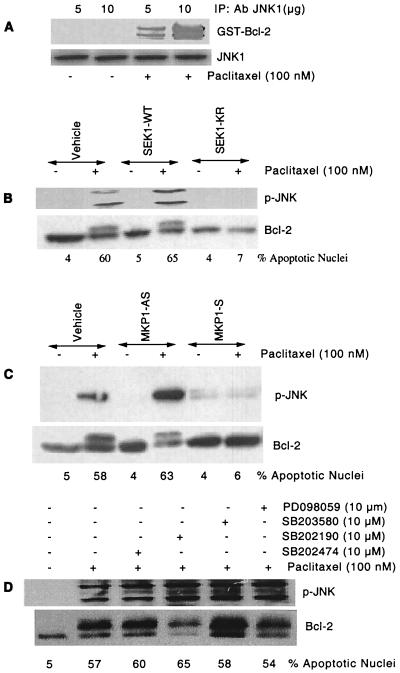
Because the MAPK pathways were activated in response to paclitaxel treatment, we sought to evaluate the contribution of these pathways to Bcl-2 phosphorylation. We first evaluated in vitro phosphorylation of GST-Bcl-2 by activated JNK1 (Fig. 2A). The JNK1 was immunoprecipitated either from paclitaxel-treated (100 nM) or untreated MCF-7 cells. The immunoprecipitated JNK1 then was incubated with GST-Bcl-2. These results demonstrate that activated JNK1 can phosphorylate Bcl-2 in vitro (Fig. 2A). In addition, treatment of cells with paclitaxel had no effect on total JNK1 as determined by the Western blotting (Fig. 2A).
Figure 2.
Block of JNK pathway inhibits paclitaxel-induced Bcl-2 phosphorylation and apoptosis. (A) JNK phosphorylates GST-Bcl-2 in vitro (Upper). MCF-7 cells were treated with paclitaxel (100 nM) for 4 h, and activated JNK1 was immunoprecipitated with either 5 μg or 10 μg anti-JNK1 antibody. GST-Bcl-2 (10 μg) was incubated with immunoprecipitated JNK1 in vitro (see Materials and Methods). The same blot was reprobed with anti-JNK1 antibody (Lower). (B) A dominant-negative mutant SEK1 (K→R) inhibits activation of the JNK pathway, Bcl-2 phosphorylation, and apoptosis induced by paclitaxel (100 nM). MCF-7 cells were transfected with a total of 2 μg/ml DNA of either empty vector (−) or mutated SEK1 (+) and incubated for 1 day. Cells were washed and incubated with fresh medium. After 8 h, the cells were incubated with 100 nM paclitaxel (+) or vehicle (−) for 30 min, 4 h, and 24 h for the measurement of JNK activation, Bcl-2 phosphorylation, and apoptosis, respectively. The JNK activity and immunoblottings were determined as described. (C) Effect of MKP-1 on JNK activation and Bcl-2 phosphorylation. MCF-7 cells were transfected with 30 μg anti-MKP-1 antisense (MKP-1-AS) or sense vector (MKP1-S) with Lipofectamine and incubated for 1 day. Cells were washed, and fresh medium was replaced. After 8 h, the cells were incubated with 100 nM paclitaxel (+) or vehicle (−) for 30 min, 4 h, and 24 h for the measurement of JNK activation, Bcl-2 phosphorylation, and apoptosis, respectively. (D) Effect of ERK and p38 inhibitors on JNK activation and Bcl-2 phosphorylation. MCF-7 cells were pretreated with inhibitors of ERK (PD098059), p38 (SB203580, SB202190), or negative control (SB202474) for 1 h. They were exposed to paclitaxel for 30 min, 4 h, and 24 h to determine JNK activation, Bcl-2 phosphorylation, and apoptosis, respectively.
We next assessed the role of the JNK pathway in paclitaxel-induced Bcl-2 phosphorylation and apoptosis by using a dominant-negative SEK1 (K→R) mutant. Transfection of cells with SEK1 (WT) had no effect on paclitaxel-induced JNK activation, Bcl-2 phosphorylation, and apoptosis (Fig. 2B). By comparison, transfection of cells with SEK1 (K→R) abolished paclitaxel-induced JNK activation, Bcl-2 phosphorylation, and apoptosis (Fig. 2B). Thus, in the absence of JNK activation, Bcl-2 phosphorylation and cell apoptosis do not occur.
We next examined the effect of dephosphorylation of activated JNK, by MAPK phosphatase (MKP) 1 on Bcl-2 phosphorylation. These MKP-1 sense and antisense expression plasmids have been described (15). As shown in Fig. 2C, transient transfection of cells with MKP1-AS plasmid had no significant effect on paclitaxel-induced JNK activation, Bcl-2 phosphorylation, and apoptosis. By comparison, transfection of cells with MKP-1 sense plasmid abolished paclitaxel-induced JNK activation, Bcl-2 phosphorylation, and apoptosis (Fig. 2C). Thus, dephosphorylation of activated JNK appears to prevent Bcl-2 phosphorylation and apoptosis.
To evaluate the involvement of ERK and p38 in Bcl-2 phosphorylation and apoptosis, we used the specific inhibitors of MEK1/2 (PD098059) and p38 (SB203580 and SB2022190). Inhibition of MEK1/2 by PD098059 resulted in the inhibition of ERK1/2 as MEK1/2 are upstream kinases of ERK1/2 (data not shown). MCF-7 cells were pretreated for 1 h with or without 10 μM of MEK1/2 inhibitor (PD098059) or p38 inhibitors (SB203580 and SB2022190), followed by incubation with 100 nM paclitaxel for 24 h (Fig. 2D). We used SB202474 (inactive inhibitor of p38) as a negative control. As expected, the ERK inhibitor (PD098059) and p38 inhibitors (SB203580 and SB202190) had no effect on paclitaxel-induced JNK activation and Bcl-2 phosphorylation (Fig. 2D). We also confirmed that neither the ERK inhibitor (PD098059) nor the p38 inhibitors (SB203580 and SB2022190) had any effect on paclitaxel-induced apoptosis (Fig. 2D). These data confirm our findings that JNK, but not ERK and p38, is involved in Bcl-2 phosphorylation and apoptosis.
Effects of Deletion of the Loop Region and Single-Point Mutations of Serine to Alanine within the Loop Region on Bcl-2 Phosphorylation and Apoptosis.
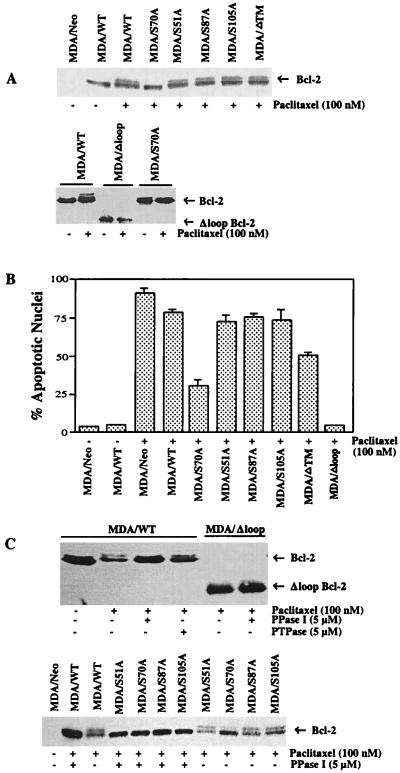
We previously have demonstrated that okadaic acid, but not vanadate, led to phosphorylation of Bcl-2 in response to paclitaxel treatment (24), suggesting that Bcl-2 is phosphorylated on serine/threonine residue(s). Recent reports confirmed the involvement of serine residues in Bcl-2 phosphorylation (25, 26). However, it is not yet clear how many sites are phosphorylated in response to paclitaxel treatment. To understand the specific serine residue(s) on Bcl-2 protein responsible for paclitaxel-induced phosphorylation, we mutated several serine residues of Bcl-2 protein to alanine residue(s) by site-directed mutagenesis. This homologous substitution usually does not affect the conformation of the protein but prevents phosphorylation. After conservative Ser→Ala mutagenesis, the resulting plasmids were used to transfect MDA-MB-231 cells, which do not express endogenous Bcl-2 and thus represent a sensitive indicator system within which to test the function of Bcl-2 mutant phenotypes with paclitaxel treatment. As shown in Fig. 3A, mutation of Ser-70 to alanine (S70A) abolished the ability of paclitaxel to induce Bcl-2 phosphorylation. Interestingly, mutations on other possible sites (S51A, S87A, and S105A) did not appear to affect paclitaxel-induced Bcl-2 phosphorylation (Fig. 3A). Deletion of the transmembrane region of Bcl-2 (MDA/ΔTM) also had no effects on Bcl-2 phosphorylation. At base line most Bcl-2 molecules are not phosphorylated. Interestingly, deletion of the loop region of Bcl-2 (MDA/Δloop) abolished paclitaxel-induced Bcl-2 phosphorylation (Fig. 3A).
Figure 3.
Loop deletion mutant of Bcl-2 blocks paclitaxel-induced Bcl-2 phosphorylation and apoptosis. MDA-MB-231 cells were transiently transfected with a total of 2 μg/ml DNA of either empty vector (−) or Bcl-2 (WT, S70A, S51A, S87A, S105A, ΔTM, or Δloop) expression plasmid with Lipofectamine and incubated for 1 day. Cells were washed, and fresh medium was added. After 8 h, the cells were incubated with 100 nM paclitaxel (+) or vehicle (−) for 4 h and 24 h for the measurements of Bcl-2 phosphorylation (A) and apoptosis (B), respectively. Cells were stained with 4′,6-diamidino-2-phenylindole to examine apoptotic nuclei. (C) Phosphorylated Bcl-2 is dephosphorylated by PPase I, but not by PTPase in MDA-MB-231 cells. Cells were transfected with Bcl-2 (WT and mutants) as described above and treated with paclitaxel (100 nM) for 4 h. Cell lysates then were incubated with either PPase I (5 μM) or PTPase (5 μM).
Previous studies have demonstrated that, as compared with its full-length counterpart, the loop deletion mutant of Bcl-2 displays a more potent inhibition of apoptosis induced by IL-3 deprivation in murine pro-B FL5.12 cells (28). Similarly, anti-IgM-induced apoptosis of WEHI-231 cells was blocked by the loop deletion mutant but not the full-length Bcl-2. In the present study, the death suppressor activity of the Bcl-2 was assessed by transfecting Bcl-2 (WT and mutants) into MDA-MB-231 cells. (Fig. 3B). More than 70% apoptosis was observed for paclitaxel-treated cells expressing WT Bcl-2 (MDA/WT) or any of the three mutants (MDA/S51A, MDA/S87A, or MDA/S105A). However, about 27% of the treated cells bearing S70A mutation underwent apoptosis even though Bcl-2 was not phosphorylated (Fig. 3 A and B). Transfection of cells with transmembrane region deletion mutant (MDA/ΔTM) significantly reduced paclitaxel-induced apoptosis (50%). By contrast, the loop region deletion mutant (MDA/Δloop) was completely resistant to paclitaxel-induced apoptosis (Fig. 3B), suggesting that the loop region is crucial to the down-regulation of Bcl-2 function. However, in contrast to phosphorylated normal Bcl-2, about one-third of the antiapoptotic effect was retained by phosphorylated Bcl-2 that could not be inserted into the membrane (Fig. 3B). Thus when phosphorylated Bcl-2 does not associate with the membrane it retains some capacity to prevent apoptosis. In addition, the apoptosis seen in cells bearing the S70A mutant suggest that phosphorylation of the Ser-70 site is not sufficient to antagonize all of the antiapoptotic functions of Bcl-2.
We previously have observed phosphorylation of Bcl-2 in cells treated with okadaic acid and paclitaxel, but not by vanadate (24). We therefore examined the nature of Bcl-2 phosphorylation in WT and mutants of Bcl-2 by using PPase I or PTPase (Fig. 3C). Lysates from Bcl-2 transfected and paclitaxel-treated cells were incubated with protein phosphatase. PPase I, but not PTPase, dephosphorylated phosphorylated WT Bcl-2 (MDA/WT), suggesting that Bcl-2 protein is phosphorylated on serine/threonine residues (Fig. 3C). Incubation of lysates from MDA/S51A, MDA/S87A, MDA/S105A, and MDA/ΔTM cells with PPase I resulted in dephosphorylation of phosphorylated Bcl-2 (Fig. 3C). As shown above, paclitaxel did not cause Bcl-2 phosphorylation in MDA/Δloop and MDA/S70A cells. Thus, in vivo Bcl-2 protein appears to be phosphorylated on serine/threonine residues, and Ser-70 is the most important site. These results suggest that the phosphorylation state of Bcl-2 is determined by the dynamic interplay of the level of kinase activation and the level of phosphatase activation. However, stimuli that lead to kinase activation (e.g., paclitaxel) or phosphatase inhibition can lead to Bcl-2 phosphorylation and a loss of its antiapoptotic activity. Thus these data demonstrate that kinase and phosphatase activities influence phosphorylation of Ser-70 and the loop region of Bcl-2.
Bcl-2 Phosphorylation Is Linked to Mitochondrial Functions.
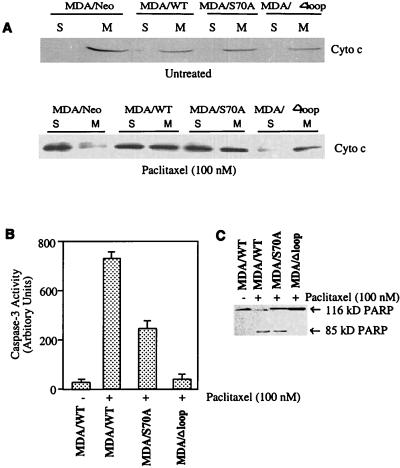
Mitochondria appear to play an important role in the early events of apoptosis (29). The loss of cytochrome c from the mitochondrial intermembrane space has been proposed as an early central event in apoptotic cell death (30, 31). Because Bcl-2 has been shown to localize to the outer mitochondrial membrane and found to inhibit cytochrome c release from the mitochondria (30, 31), the effects of Bcl-2 (WT and mutants) on cytochrome c redistribution in response to paclitaxel treatment were investigated. The presence of cytochrome c in the cytosolic and mitochondrial fractions of Bcl-2-transfected cells treated with paclitaxel was assessed. Fig. 4A (Upper) clearly demonstrates that cytochrome c was mainly localized to mitochondrial fractions in untreated MDA/Neo, MDA/WT, MDA/S70A, and MDA/Δloop cells. Paclitaxel treatment caused a marked increase in cytochrome c levels in the cytosolic fraction (S) of MDA/Neo cells (Fig. 4A, Lower). By comparison, cytochrome c release from mitochondria to cytosol was partially blocked in MDA/WT and MDA/S70A cells. In contrast, cytochrome c largely remained in the mitochondria of MDA/Δloop cells after paclitaxel treatment (Fig. 4A, Lower). These data suggest that the absence of the loop region of Bcl-2 prevents the inhibition of Bcl-2 function; Bcl-2 must be inhibited for paclitaxel to promote the release of cytochrome c from mitochondria to cytosol.
Figure 4.
Bcl-2 phosphorylation is linked to mitochondrial functions. MDA-MB-231 cells were transiently transfected with a total of 2 μg/ml of DNA of either empty vector (−) or Bcl-2 (WT, S70A, Δloop) expressing plasmid with Lipofectamine and incubated for 1 day. Cells were washed, and fresh medium was replaced. After 8 h, the cells were incubated with 100 nM paclitaxel (+) or vehicle (−) for 6, 12, and 24 h for the measurements of cytochrome c release (A), caspase-3 activation (B), and PARP cleavage (C), respectively. (A) The redistribution of cytochrome c in MDA/Neo, MDA/WT, MDA/S70A, and MDA/Δloop cells. The cells were mechanically lysed and separated into mitochondrial (M) and S100 (S) fractions. The amount of cytochrome c present in each fraction was determined by Western blot analysis. (B) Loop deletion mutant of Bcl-2 blocks paclitaxel-induced caspase-3 activation. Caspase-3 activity was measured as per manufacturer’s instructions. (C) Loop deletion mutant of Bcl-2 inhibits paclitaxel-induced PARP cleavage. Cell lysates were run on SDS/PAGE, and immunoblottings were performed by using anti-PARP antibody.
Because cytochrome c has been shown to activate caspase-3, which in turn cleaves PARP (32, 33), we compared the nature of caspase-3 activation and PARP cleavage in MDA cells transfected with WT and mutants (S70 A and Δloop) of Bcl-2. Treatment of cells with paclitaxel (100 nM) resulted in caspase-3 activation in MDA/WT cells (Fig. 4B). Caspase-3 activity was reduced to 50% in MDA/S70A cells compared with MDA/WT cells. Interestingly, no significant caspase-3 activity (over control) was observed in MDA/Δloop cells (Fig. 4B). We next examined the cleavage of PARP in Bcl-2 transfectants in response to paclitaxel treatment (Fig. 4C). Treatment of cells with paclitaxel resulted in PARP cleavage in MDA/WT and to a lesser degree (about one-third) in MDA/S70A cells (Fig. 4C). In contrast, cleavage of PARP was not observed in MDA/Δloop cells after paclitaxel treatment (Fig. 4C). Overall, these data suggest that the loop region deletion mutants of Bcl-2 completely block cytochrome c redistribution, caspase-3 activation, and PARP cleavage in MDA cells. The downstream events are only partially blocked in S70A-expressing cells. Without the loop region, the signal transduction cascade that normally interferes with Bcl-2 function and leads to apoptosis is ineffective.
DISCUSSION
In this study, we report that the activation of JNK, but not ERK and p38, plays a pivotal role in mediating apoptosis via the inactivation of the antiapoptotic protein Bcl-2 at least in part through phosphorylation of Ser-70 on Bcl-2. Bcl-2 can prevent or delay apoptosis induced by a wide variety of stimuli and insults, suggesting that Bcl-2 controls a crucial step in the final common pathway for cell death (2). Recent data support that the posttranslational modification of Bcl-2 by phosphorylation is important for the regulation of Bcl-2 function (22, 24). Here, we demonstrate that JNK is involved in the phosphorylation of Bcl-2, and inhibition of JNK activity, either by overexpression of MKP-1 or by a dominant-negative SEK1 (K→R), blocks the phosphorylation of Bcl-2 and apoptosis in breast cancer cells. An alternative pathway to apoptosis involves the inhibition of a serine phosphatase that leads to phosphorylation of Bcl-2. In addition, deletion of the loop region completely blocks paclitaxel-induced Bcl-2 phosphorylation, cytochrome c release, caspase-3 activity, PARP cleavage, and apoptosis. On the other hand, the S70A mutant could not be phosphorylated, but some activation of the downstream steps of apoptosis nevertheless occurred. Thus, Bcl-2 that cannot be phosphorylated on Ser-70 retains some, but not all, of its antiapoptotic efficacy. This result implies either that paclitaxel inhibits Bcl-2 function in some fashion in addition to phosphorylation or that the S70A mutant Bcl-2 is less effective than WT Bcl-2 at preventing apoptosis.
Our results suggest that treatment of cells with paclitaxel leads to the activation of ERK and JNK, but has no effect on p38 MAPK. JNK often is stimulated in response to many cellular stresses (11, 14, 18), but the downstream effectors involved in mediating its apoptotic effects have not been identified. Furthermore, JNK is thought to mediate an intracellular signal for stress-activated apoptosis (18, 19). The mechanism by which Bcl-2 prevents apoptosis is incompletely defined, even though Bcl-2 has been proposed to function as an antioxidant or free-radical scavenger or to modulate calcium efflux through the endoplasmic reticulum in some experimental models (34–36). Our findings suggest that paclitaxel-mediated JNK activation is closely linked to increases in the phosphorylation of Bcl-2, resulting in the cell death signal. Similar to our findings, a recent report demonstrated that JNK was capable of phosphorylating Bcl-2 (27), suggesting the possibility that JNK could play a role in paclitaxel-induced Bcl-2 phosphorylation. We previously have demonstrated that the cAMP-dependent protein kinase (PKA) is activated in response to paclitaxel treatment, and the inhibition of PKA activation can block Bcl-2 phosphorylation and apoptosis (24). Although our prior data indicated that activated PKA could directly phosphorylate Bcl-2, we believe that JNK plays a dominant role in Bcl-2 phosphorylation and apoptosis. Both kinases are capable of phosphorylating Ser-70 but some stimuli that lead to Bcl-2 phosphorylation (e.g., short-wavelength UV light and retinoic acid) do not activate PKA. In contrast, we have not observed any proapoptotic stimuli that do not activate JNK.
Bcl-2 can prevent or delay apoptosis induced by a wide variety of stimuli and insults (6). Recent studies have demonstrated that Bcl-2 is the target for the posttranslational modification, such as phosphorylation (24, 25, 28, 37). The loop domain of Bcl-2 contains the serine phosphorylation sites, and phosphorylation in this region has been reported to impair the antiapoptotic effect of Bcl-2 (28, 38). Contrary to our data and others’ data (28, 39, 40), it has been suggested that phosphorylation of Bcl-2 protein is necessary for antiapoptotic function in some models (26, 41). The effects of the loop domain-deleted mutants have not yet been assessed in those models. If phosphorylation were essential for Bcl-2 function, loop deletion mutants should be nonfunctional rather than hyperfunctional, as we have seen. However, consistent with our findings, the deletion of the loop domain potentiates the protective effect of Bcl-2 against growth factor withdrawal and anti-IgM-induced apoptosis (28, 39). We are unable to explain the differences in the role of Bcl-2 phosphorylation in the two systems. Interestingly, a recent study has demonstrated that the phosphorylation of Bcl-2 is a marker for M phase events rather than a determinant of apoptosis (42). Paclitaxel produces cell cycle arrest in M phase. However, our data would argue that phosphorylation of Bcl-2 is functionally linked to apoptosis because in its absence (e.g., loop deletion mutants), apoptosis does not occur, but growth arrest does.
It has been shown that Bcl-2, which is located in part in the outer mitochondrial membrane, inhibits drug-induced mitochondrial permeability transition and the release of cytochrome c (30, 31). Cytochrome c has been shown to bind APAF-1, and in the presence of dATP, promotes the activity of caspase-9, which in turn activates caspase-3 (33). Our findings show that paclitaxel causes the cytosolic accumulation of cytochrome c, resulting in caspase-3 activation and apoptosis in MDA/Neo and to a lesser extent in MDA/S70A, but not in MDA/Δloop cells. The loop deletion mutation of Bcl-2 (MDA/Δloop) was not phosphorylated by paclitaxel, and cytochrome c redistribution, caspase-3 activation, and PARP cleavage did not occur. Bcl-2 overexpression has been shown to inhibit caspase-3 activation by blocking the mitochondrial cytochrome c release (30, 31). This finding suggests that the paclitaxel-induced phosphorylation of the loop domain of Bcl-2 may be important for the ability of Bcl-2 to permit release of cytochrome c from the mitochondria to the cytosol.
It is not completely clear from these studies what role the transmembrane domain of Bcl-2 plays in its function. Transfection of cells with a transmembrane deletion mutant Bcl-2 (ΔTM Bcl-2) resulted in phosphorylation of ΔTM Bcl-2 and apoptosis in response to paclitaxel treatment, despite the mutant’s inability to attach to membranes. When Bcl-2 is phosphorylated, apoptotic protein Bax is released from the Bcl-2/Bax complex (24), and free Bax can induce apoptosis (3, 4). Thus, phosphorylated Bcl-2 mediates some antiapoptotic activity even when it does not insert in membranes. It is possible that the soluble molecule can interfere with Bax activity to some extent.
Our results suggest that paclitaxel-mediated JNK activation is closely linked to increases in the phosphorylation of Bcl-2, resulting in the cell death signal. Because the Ser-70 residue is conserved through phylogeny in Bcl-2, including in human, rat, chicken, and mouse (43), it may contribute some of the physiologically relevant regulation of Bcl-2 functions related to prevention of apoptosis. Our data suggest a model whereby proapoptotic signals such as paclitaxel treatment activate members of the JNK/SAPK MAPK family, which then disable Bcl-2 through phosphorylation within its flexible loop region. Such Bcl-2 inactivation would be expected to leave unopposed proapoptotic processes such as mitochondrial membrane depolarization, generation of reactive oxygen species, and activation of caspases leading to apoptosis. It is possible that alterations in the loop region of Bcl-2 could account for some cases of chemotherapy resistance. Inability to turn off Bcl-2 could enable a cell to survive a chemotherapy assault rather than proceed through apoptosis.
Acknowledgments
We thank Dr. J. Kyriakis (Massachusetts General Hospital, Charlestown, MA) for dominant-negative SEK1 (Lys→Arg) plasmid, Dr. Stanley J. Korsmeyer (Dana-Farber Cancer Institute, Boston) for Bcl-2 plasmid, and Dr. C. Thompson (University of Chicago) for Δloop Bcl-2 plasmid. We also thank Drs. N. Holbrook and Y.S. Liu (National Institute on Aging, Baltimore) for MKP (sense and antisense) expression vectors.
ABBREVIATIONS
- JNK
c-Jun N-terminal kinase
- SAPK
stress-activated protein kinase
- MAPK
mitogen-activated protein kinase
- ERK
extracellular signal-regulated kinase
- SEK
SAPK kinase
- MKP
MAPK phosphatase
- PARP
poly(ADP-ribose) polymerase
- PPase I
phosphatase I
- PTPase
protein tyrosine phosphatase
- GST
glutathione S-transferase
- WT
wild type
- MEK
mitogen/ERK kinase
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Wyllie A H. Br J Cancer. 1993;67:205–208. doi: 10.1038/bjc.1993.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thompson C B. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 3.Yang E, Korsmeyer S J. Blood. 1996;88:386–401. [PubMed] [Google Scholar]
- 4.White E. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- 5.Dubois-Dauphin M, Frankowski H, Tsujimoto Y, Huarte J, Martinou J C. Proc Natl Acad Sci USA. 1994;91:3309–3313. doi: 10.1073/pnas.91.8.3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reed J C. J Cell Biol. 1994;124:1–6. doi: 10.1083/jcb.124.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reed J C, Miyashita T, Takayama S, Wang H G, Sato T, Krajewski S, Aime-Sempe C, Bodrug S, Kitada S, Hanada M. J Cell Biochem. 1996;60:23–32. doi: 10.1002/(SICI)1097-4644(19960101)60:1%3C23::AID-JCB5%3E3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 8.Park J R, Hockenbery D M. J Cell Biochem. 1996;60:12–17. doi: 10.1002/(sici)1097-4644(19960101)60:1<12::aid-jcb3>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 9.Cowley S, Paterson H, Kemp P, Marshall C J. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 10.Davis R J. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakis J M, Banerjee P, Nikolakaki E, Dai T, Rubie E A, Ahmad M F, Avruch J, Woodjett J R. Nature (London) 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- 12.Gupta S, Campbell D, Derijard B, Davis R J. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- 13.Raingeaud J, Gupta S, Rogers J S, Dickens M, Han J, Ulevitch R J, Davis R J. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- 14.Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis R. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu Y, Gorospe M, Yang C, Holbrook N J. J Biol Chem. 1995;270:8377–8380. doi: 10.1074/jbc.270.15.8377. [DOI] [PubMed] [Google Scholar]
- 16.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 17.Sluss H K, Barrett T, Derijard B, Davis R J. Mol Cell Biol. 1994;14:8376–8384. doi: 10.1128/mcb.14.12.8376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verheij M, Bose R, Lin X H, Yao B, Jarvis W D, Grant S, Birrer M J, Szabo E, Zon L I, Kyriakis J M, et al. Nature (London) 1996;380:75–79. doi: 10.1038/380075a0. [DOI] [PubMed] [Google Scholar]
- 19.Xia Z, Dickens M, Raingeaud J, Davis R, Greenberg M E. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- 20.Kallunki T, Su B, Tsigelny I, Sluss H K, Derijard B, Moore G, Davis R, Karin M. Genes Dev. 1994;8:2996–3007. doi: 10.1101/gad.8.24.2996. [DOI] [PubMed] [Google Scholar]
- 21.Cavigelli M, Dolfi F, Claret F-X, Karin M. EMBO J. 1995;14:5957–5964. doi: 10.1002/j.1460-2075.1995.tb00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haldar S, Basu A, Croce C M. Cancer Res. 1997;57:229–233. [PubMed] [Google Scholar]
- 23.Ham J, Babij C, Whitfield J, Pfarr C M, Lallemand D, Yaniv M, Rubin L L. Neuron. 1995;14:927–939. doi: 10.1016/0896-6273(95)90331-3. [DOI] [PubMed] [Google Scholar]
- 24.Srivastava R K, Srivastava A R, Korsmeyer S J, Nesterova M, Cho-Chung Y S, Longo D L. Mol Cell Biol. 1998;18:3509–3517. doi: 10.1128/mcb.18.6.3509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haldar S, Basu A, Croce C M. Cancer Res. 1998;58:1609–1615. [PubMed] [Google Scholar]
- 26.Ito T, Deng X, Carr B, May W S. J Biol Chem. 1997;272:11671–11673. doi: 10.1074/jbc.272.18.11671. [DOI] [PubMed] [Google Scholar]
- 27.Maundrell K, Antonsson B, Magnenat E, Camps M, Muda M, Chabert C, Gillieron C, Boschert U, Vial-Knecht E, Martinou J-C, Arkinstall S. J Biol Chem. 1997;272:25238–25242. doi: 10.1074/jbc.272.40.25238. [DOI] [PubMed] [Google Scholar]
- 28.Chang B S, Minn A J, Muchmore S W, Fesik S W, Thompson C B. EMBO J. 1997;16:968–977. doi: 10.1093/emboj/16.5.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bernardi P, Broekemeier K M, Pfeiffer D R. J Bioenerg Biomembr. 1994;26:509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 30.Kluck R M, Bossy-Wetzel E, Green D R, Newmeyer D D. Science. 1997;275:1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Liu X, Bhalla K, Kim C N, Ibrado A M, Cai J, Peng T-I, Jones D P, Wang X. Science. 1997;275:1126–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 32.Lazebnik Y A, Kaufman S H, Desnoyers S, Poirier G G, Earnshaw W C. Nature (London) 1994;371:346–347. doi: 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- 33.Zou H, Henzel W J, Liu X, Lutschg A, Wang X. Cell. 1997;90:405–413. doi: 10.1016/s0092-8674(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 34.Hockenbery D M, Oltvai Z N, Yin S-M, Milliman C L, Korsmeyer S J. Cell. 1993;75:241–251. doi: 10.1016/0092-8674(93)80066-n. [DOI] [PubMed] [Google Scholar]
- 35.Kane D J, Saraffian T A, Anton R, Hahn H, Gralla E B, Valentine S J, Ord T, Bredesen D E. Science. 1993;262:1274–1277. doi: 10.1126/science.8235659. [DOI] [PubMed] [Google Scholar]
- 36.Lam M, Dubyak G, Chen L, Nunez G, Miesfeld R L, Distelhorst C W. Proc Natl Acad Sci USA. 1994;91:6569–6573. doi: 10.1073/pnas.91.14.6569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu Z-B, Minden M D, McCulloch E A. Blood. 1998;92:1768–1775. [PubMed] [Google Scholar]
- 38.Basu A, Haldar S. Int J Oncol. 1998;13:659–664. doi: 10.3892/ijo.13.4.659. [DOI] [PubMed] [Google Scholar]
- 39.Uhlmann E J, D’Sa-Eipper C, Subramanian T, Wagner A J, Hay N, Chinnadurai G. Cancer Res. 1996;56:2506–2509. [PubMed] [Google Scholar]
- 40.Fang G, Chang B S, Kim C N, Perkins C, Thompson C B, Bhalla K N. Cancer Res. 1998;58:3202–3208. [PubMed] [Google Scholar]
- 41.Ruvolo P P, Deng X, Carr B K, May W S. J Biol Chem. 1998;273:25436–25442. doi: 10.1074/jbc.273.39.25436. [DOI] [PubMed] [Google Scholar]
- 42.Ling Y-H, Tornos C, Perez-Soler R. J Biol Chem. 1998;273:18984–18991. doi: 10.1074/jbc.273.30.18984. [DOI] [PubMed] [Google Scholar]
- 43.Takayama S, Cazals-Hatem D L, Kitada S, Tanaka S, Miyashita T, Hovey L R, 3rd, Huen D, Rickinson A, Veerapandian P, Krajewski S, et al. DNA Cell Biol. 1994;13:679–692. doi: 10.1089/dna.1994.13.679. [DOI] [PubMed] [Google Scholar]