Abstract
The SH2 domain-containing tyrosine phosphatase Shp2 plays a pivotal role during the gastrulation of vertebrate embryos. However, because of the complex phenotype observed in mouse mutant embryos, the precise role of Shp2 during development is unclear. To define the specific functions of this phosphatase, Shp2 homozygous mutant embryonic stem cells bearing the Rosa-26 LacZ transgene were isolated and used to perform a chimeric analysis. Here, we show that Shp2 mutant cells amass in the tail bud of embryonic day 10.5 chimeric mouse embryos and that this accumulation begins at the onset of gastrulation. At this early stage, Shp2 mutant cells collect in the primitive streak of the epiblast and thus show deficiencies in their contribution to the mesoderm lineage. In high-contribution chimeras, we show that overaccumulation of Shp2 mutant cells at the posterior end of the embryo results in two abnormal phenotypes: spina bifida and secondary neural tubes. Consistent with a failure to undergo morphogenic movements at gastrulation, Shp2 is required for embryo fibroblast cells to mount a positive chemotactic response to acidic fibroblast growth factor in vitro. Our results demonstrate that Shp2 is required at the initial steps of gastrulation, as nascent mesodermal cells form and migrate away from the primitive streak. The aberrant behavior of Shp2 mutant cells at gastrulation may result from their inability to properly respond to signals initiated by fibroblast growth factors.
Proteins with Src homology 2 (SH2) domains mediate signaling by tyrosine kinases through their ability to bind specific phosphotyrosine-containing sites on activated receptors or cytoplasmic docking proteins (1, 2). One such SH2-containing protein is the tyrosine phosphatase Shp2, which commonly appears to augment signaling initiated by receptor tyrosine kinases (3–8). Shp2 possesses two amino-terminal SH2 domains, a central catalytic domain, and a short carboxyl-terminal tail. The binding of the SH2 domains to phosphotyrosine-containing peptides serves the dual function of both localizing the enzyme to the vicinity of its substrates and directly stimulating the activity of the enzyme (9, 10). To address the biological requirements for Shp2 and to investigate its contribution to signaling from distinct tyrosine kinases, we have previously introduced a mutation into the murine gene encoding the Shp2 protein (5). This mutation deletes the third exon, which removes a critical portion of the amino-terminal SH2 domain. Shp2 is required for early postimplantation development, as embryos homozygous for the mutant allele die in utero because of defects in gastrulation.
Gastrulation is a complex process that involves the coordination of cell division, differentiation, and migration and culminates in the formation and patterning of the three distinct embryonic germ layers (11). It is also at this time that the anterior–posterior and dorsal–ventral axes, and indeed the entire basic body plan of the embryo, are defined. Gastrulation of the mouse embryo begins as cells at the primitive streak of the egg cylinder undergo an epithelial-to-mesenchymal transition. Subsequently, these nascent mesodermal cells migrate between the epiblast and the endoderm layers laterally around the circumference of the embryo, leading to the formation of the mesodermal wings.
Signal transduction initiated by fibroblast growth factors (FGFs) is essential for gastrulation and mesoderm patterning (12, 13). Relatively little is known about the downstream signaling events initiated by FGF receptors; however, one event that is likely to be important is the recruitment and tyrosine phosphorylation of the myristylated docking protein p90-FRS2 to the activated FGF receptor 1 (FGFR1) tyrosine kinase (14). Once phosphorylated, FRS2 recruits the SH2 domain-containing proteins Grb2 and Shp2, both of which are required for the activation of the Ras–mitogen-activated protein kinase pathway by FGFs (3, 5–7, 14). Of interest, embryos homozygous for the Shp2 exon 3 deletion have a remarkable resemblance to embryos with mutations within the FGFR1 gene.
Shp2 mutant embryos characteristically possess defects in the node and notochord, exhibit a reduced number of somites that appear disorganized, and show retardation in posterior elongation, among other deficiencies. Because of the multifaceted nature of the phenotype observed for the Shp2 mutant embryos, it has been difficult to define the primary cellular defect that results in the failure of these embryos to gastrulate normally.
In this report we used embryonic stem (ES) cells deficient for the Shp2 tyrosine phosphatase in a chimeric analysis to determine the primary functional requirement for Shp2 during gastrulation. We show that, rather than migrating through the primitive streak, Shp2 mutant cells accumulate in the posterior epiblast of the gastrulating embryo and thus have a reduced contribution to the mesoderm lineage. This accumulation of Shp2 mutant cells in the primitive streak of embryonic day (E)7.5 embryos is reflected in the contribution pattern observed in E10.5 chimeric embryos, where Shp2 mutant cells are predominantly observed in the posterior ectoderm-derived tissues and within the tail bud. Boyden chamber migration assays indicate that Shp2 mutant cells have an impaired chemotactic response to FGFs, potentially explaining the inability of Shp2 mutant cells to complete morphogenetic movements during gastrulation. These results indicate that Shp2 plays a key role in transducing tyrosine kinase signals that are used to regulate cell movement in the developing embryo.
MATERIALS AND METHODS
Generation of ES Cells.
Blastocysts were plated onto feeder cells in DMEM containing 1,000 units/ml lymphocyte inhibiting factor until embryos hatched and the inner cell mass outgrew (15). Inner cell mass cells were then dispersed, and individual ES cell-like colonies were picked, expanded, and genotyped. All cell lines derived were able to contribute to chimeras, and the phenotypes observed for both the mutant cell lines were identical. Genotyping and Western blot analysis were performed as described (5).
Generation and Analysis of Chimeric Embryos.
A 4- to 6-cell ES clump was aggregated with an ICR strain morula, developed in vitro to the blastula stage, and implanted into the uteri of pseudopregnant females. Chimeric embryos were dissected, stained in whole mount for β-galactosidase activity, and examined histologically as described (5, 16); chromogenic reagent 5-bromo-4-chloro-3-indolyl β-d-galactoside (X-Gal) was sometimes substituted by 6-chloro-3-indolyl β-d-galactopyranoside (Salmon-Gal; Biosynth International, Naperville, IL).
Migration Assays.
Boyden chambers (Costar; 8 μm pore) were prepared by coating the membranes with 0.1% gelatin and placing in the bottom chamber DMEM/0.1% BSA/10 mM Hepes plus either platelet-derived growth factor (PDGF) or acidic FGF (aFGF) at 25 ng/ml. FGF assays also contained 0.5 μg/ml heparin salt. Embryo fibroblast cells were harvested with cell-dissociation buffer (Sigma), spun, and resuspended to 500,000 cells per ml in DMEM/BSA/Hepes medium, and 100 μl of cells was placed on the top of the membrane. Chambers were incubated for 6 hr at 37°C in 5% CO2. Cells were then removed from the top of the membrane, fixed with MeOH, stained with hematoxylin (VWR Scientific), and mounted on glass. Photos were taken at five random sections of the membrane, and representative photos are shown. Alternately, numbers of cells per field of view were counted, averaged, and depicted in graph form.
Scanning Electron Microscopy.
Chimeric embryos were fixed in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer, post-fixed in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer, dehydrated in a graded ethanol series, and critical-point dried with a Ladd CPD. The specimens were then mounted on aluminum studs by using carbon adhesive and coated with gold with a Denton Desk II sputter coater. Microscopy was performed on a Hitachi S2500 scanning electron microscope by using digital image collection.
RESULTS
Generation of ES Cells Containing the Rosa-26 LacZ Marker.
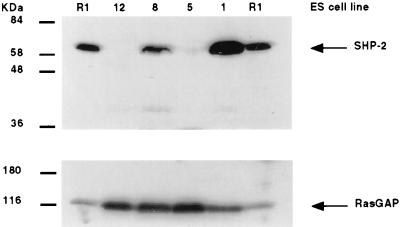
To create Shp2 homozygous mutant ES cells, mice doubly heterozygous for the Shp2 exon 3 deletion (5) and the Rosa-26 LacZ transgene (17) were intercrossed, and blastocysts were collected and used to establish ES cell lines (15, 18). The Rosa-26 transgene is ubiquitously expressed throughout the developing embryo. This makes it an ideal marker, as the β-galactosidase enzyme provides a fast, simple assay to determine ES cell contribution to the chimeric embryos. Four LacZ-positive lines were established. Genotyping at the Shp2 locus identified one wild-type (wt; no. 1), one heterozygous (no. 8) and two homozygous mutant (nos. 5 and 12; data not shown) lines. Western blot analysis confirmed the absence of wt Shp2 protein in the homozygous mutant ES cell lines (Fig. 1).
Figure 1.
Western blot analysis of ES cell lines. Upper, αShp2; Lower, αp120RasGAP. αShp2 antibody (UBI) does not recognize the mutant Shp2 protein. ES cell lines no. 12 and no. 5 were derived from Shp2 mutant blastocysts. R1 ES cell line lysates are shown for comparison (15).
Contribution of ES Cells to E10.5 Chimeric Embryos.
Embryos that are entirely composed of Shp2 mutant cells are resorbed by E10.0 of development (5, 19), precluding the analysis of functions for Shp2 at this stage by using standard genetic methods. However, by generating embryos composed of mixtures of both wt and Shp2 mutant cells, embryos can be analyzed beyond this lethal stage. A chimera analysis can potentially yield new information regarding the functions of Shp2 in two different ways. First, in chimeric embryos that appear morphologically normal, the exclusion of Shp2 mutant cells from a particular tissue or cell lineage indicates that Shp2 may be required for proper development of that tissue; conversely, tissues that allow a high proportion of Shp2 mutant cells presumably do not require Shp2 for their proper development. Second, in high-contribution chimeras, the accumulation of Shp2 mutant cells within tissues from which they are normally excluded may result in abnormal phenotypes that would provide important information concerning the roles of this phosphatase during development.
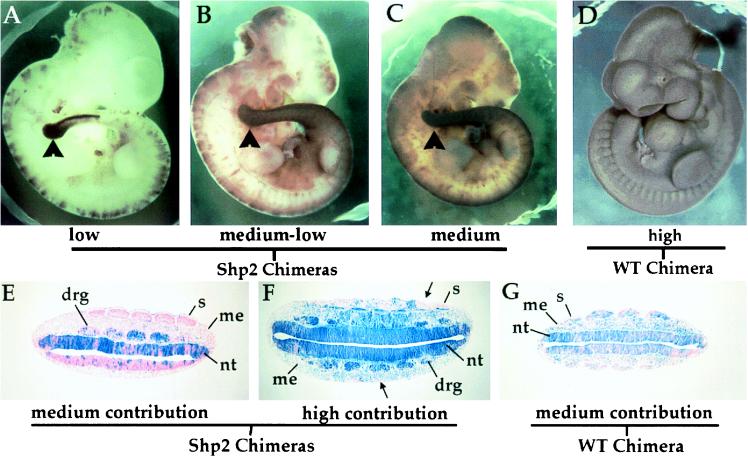
Chimeric embryos were generated by aggregating wt or Shp2 mutant ES cells with morula-stage embryos that were then reimplanted into pseudopregnant females (15). Chimeras were dissected at E10.5 and stained with Salmon-Gal (a chromogenic substrate for β-galactosidase similar to X-Gal) to determine ES cell contribution. We found that the degree to which Shp2 mutant cells contributed to chimeric embryos could vary over a wide range while still yielding embryos that looked phenotypically wt (Fig. 2 and data not shown), indicating that wt cells could rescue the early embryonic lethality and gastrulation defects exhibited by germ-line Shp2 mutant embryos. Strikingly, at low (5–25%) contribution (Fig. 2A; Table 1) and medium (25–50%) contribution (Fig. 2 B and C; Table 1) contributions, Shp2 mutant cells accumulated within the tail bud and posterior region of the embryo. Chimeras generated with wt ES cells showed a random distribution of LacZ-positive cells, with no bias toward contribution to a particular region (Fig. 2 D and G and data not shown; n = 21). When the contribution of mutant ES cells was high (50–75% contribution), abnormal phenotypes were observed (see below and Table 1). Indeed, chimeric embryos that were almost entirely ES-cell derived were indistinguishable from germ-line Shp2 mutant embryos (data not shown).
Figure 2.
Shp2 mutant cells accumulate in the tail bud and neural tissue of developing chimeric embryos. Chimeras generated with Shp2 (A–C) or wt (D) ES cells were dissected at E10.5 of development and stained for β-galactosidase activity with Salmon-Gal to determine the extent of ES cell contribution. Arrowheads in A–C point to the tail bud, which exhibits a strong accumulation of mutant cells. wt cells were able to contribute to the entire developing embryo (D). Histological sections through the trunk region of X-Gal-stained E10.0 chimeric embryos. Chimeric embryos were generated with Shp2 (E and F) or wt (G) ES cells. In medium-contribution Shp2 chimeric embryos (E), almost all of the mutant cells (blue) were found within the neural tissues of the embryo, which is ectoderm-derived. Ectoderm is the only tissue that is not generated by the progression of cells through the primitive streak at gastrulation. In high-contribution Shp2 chimeras (F), there was a strong deficiency of mutant cell contribution to the somitic mesoderm (arrows). In medium-contribution wt chimeras (G), the ES cells can contribute to all tissues of the developing embryo. drg, dorsal root ganglia; me, mesenchyme; s, somites; nt, neural tube.
Table 1.
Shp2 mutant cell contribution to E10.5 chimeric embryos
Histological sections through the trunk region of chimeric embryos highlight the skewed distribution of Shp2 mutant cells observed in the developing embryos (Fig. 2 E–G). In medium-contribution Shp2 chimeric embryos (Fig. 2E), LacZ-positive Shp2 mutant cells resided primarily in neural tissues, such as the neural tube and the dorsal root ganglia. Shp2 mutant cells were notably absent from mesodermal lineages, including the somites and the trunk mesenchyme.
In higher contribution Shp2 chimeras (Fig. 2F), the neuroepithelium and the dorsal root ganglia were almost entirely composed of mutant cells. Whereas the trunk mesenchyme was a mosaic of both wt and mutant cells, there was a striking deficiency of Shp2 mutant cells from the somites (Fig. 2F, arrows), indicating that Shp2 function is required for the proper development of this tissue. Nascent mesoderm cells that exit at the mid-streak level of the gastrulating embryo are fated to become paraxial mesoderm or somites. The diminution of Shp2 mutant cells from this tissue correlates well with the phenotype of germ-line Shp2 embryos, which exhibit a reduced number of highly disorganized somites. In contrast to the pattern observed for the Shp2 chimeras, chimeras generated with wt ES cells were a mosaic of ES- and morula-derived cells in all lineages of the embryo (Fig. 2G). This result is in keeping with studies showing a substantial intermingling of cells within the epiblast before and during gastrulation (20), such that ES cells incorporated into the inner cell mass of the blastula do not grow together as coherent clones but are dispersed throughout the epiblast and thus are found in all tissues of the resulting embryo. Therefore, in chimeras generated with Shp2 ES cells, the reduced number of mutant cells in mesoderm tissues indicates that Shp2 function is required for their proper development.
Abnormal Phenotypes Observed in E10.5 Shp2 Mutant Chimeras.
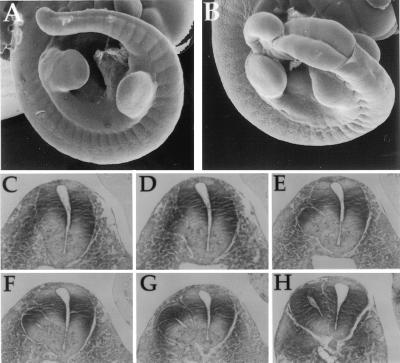
Shp2 mutant cells, which tended to accumulate within the caudal region of the chimeric embryos, were predominantly of the neuroectoderm lineage. When the contribution of Shp2 mutant ES cells to the developing embryo was high, mutant phenotypes became apparent, and neuroectoderm tissue was severely affected (Table 1). Embryos with a high degree of chimerism but that were generally wt in size and appearance (50–75% contribution; n = 22) were examined for defects. Any phenotypes observed are likely to be primary in nature and not secondary effects caused by the improper development of other tissues that would affect neuroectoderm development. First, scanning electron microscopy of E10.5 embryos shows the open neural tube, or spina bifida, that was frequently observed (Fig. 3 A and B; n = 13, 60%). Second, transverse histological sections through the posterior region of an E10.5 Shp2 chimera revealed the formation of a secondary neural tube (Fig. 3 C–H; n = 8, 36%). Of the sections shown, Fig. 3C is the most rostral and Fig. 3H is the most caudal, indicating that the phenotype is more severe in the posterior region of the developing tail. This correlates with the whole-mount β-galactosidase staining, which illustrated that the strongest contribution of Shp2 mutant cells was to the caudal region of the chimeric embryos (Figs. 2 A–C and 4B; data not shown).
Figure 3.
Accumulation of Shp2 mutant cells within the caudal neuroectoderm leads to multiple defects, including spina bifida and secondary neural tubes. (A and B) Scanning electron micrograph images of E10.5 wt (A) and Shp2 mutant (B) chimeric embryos, highlighting the spina bifida, or open neural tube. (C–H) Transverse histological sections through the tail of an Shp2 mutant chimera depict the formation of a secondary neural tube. Rostral (C) to caudal (H) sections are shown.
Contribution of ES Cells to E7.5 Chimeric Embryos.
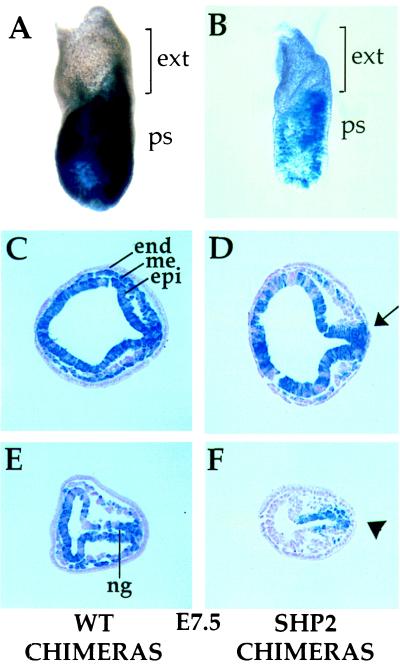
To determine when the posterior accumulation of Shp2 mutant cells first occurs, chimeras were generated with wt and mutant ES cells and analyzed at E7.5 of development (Fig. 4). Whole-mount staining with X-Gal showed that wt ES cells could essentially contribute to the entire embryo (Fig. 4A). However, in Shp2 mutant chimeras, the mutant cells accumulated within the primitive streak, the future posterior end of the embryo (Fig. 4B; n = 12). Tissue sections showed that in the E7.5 Shp2 chimeras, the mesodermal wings exhibited a greater proportion of wt cells than Shp2 mutant cells, even when the epiblast was mostly Shp2 mutant cell-derived (Fig. 4D). wt chimeras dissected at this stage showed equal contribution of ES cells to the epiblast and mesoderm lineages, indicating no bias toward residing in a particular tissue (Fig. 4 C and E; n = 22).
Figure 4.
Shp2 mutant cells show reduced contribution to the mesodermal wings of gastrulating chimeric embryos. wt (A, C, and E) and Shp2 mutant (B, D, and F) chimeras in whole-mount (A and B) or tissue sections (C–F). Anterior is to the left. ES cells do not contribute to the extraembryonic region and primitive endoderm of chimeric embryos and are therefore unstained. Arrow in D points to hyperaccumulation at the posterior epiblast (leading to a misshapen epiblast at this region fated to become neuroectoderm); similarly, arrowhead in F points to buildup of mutant cells within the posterior epiblast. end, endoderm; me, mesoderm; epi, epiblast; ng, neural groove; ext, extraembryonic region; ps, primitive streak.
When the contribution of Shp2 mutant cells was high, these cells seemed to accumulate at the posterior epiblast (Fig. 4D, arrow), in the region of the epiblast fated to become the neuroectoderm (21, 22). This potentially explains the buildup of mutant cells in the trunk region and in the neuroectoderm lineage of E10.5 chimeric embryos. At medium contributions, the mesodermal wings were almost entirely composed of wt cells, and the Shp2 mutant cells collected at the primitive streak (Fig. 4F, arrowhead). This tendancy for mutant cells to remain within the epiblast while wt cells populate the mesodermal wings indicates that mutant cells are either unable to contribute to this lineage or are outcompeted by wt cells. Thus, Shp2 mutant cells are ineffective at undergoing the morphogenetic movements at gastrulation that would result in their contribution to the mesoderm lineage.
Shp2 Is Required for a Positive Chemotactic Response to aFGF.
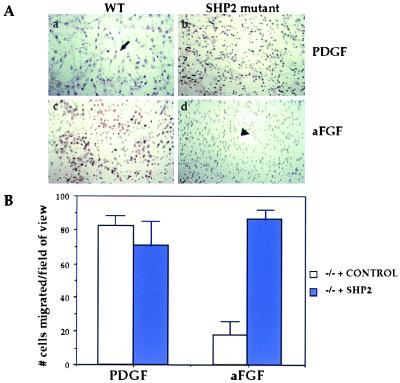
As cell migration events are important during gastrulation (11), Boyden-chamber migration assays (23) were employed to determine whether Shp2 mutant fibroblast cells respond normally to growth factors as chemoattractants. Fibroblasts established from wt and Shp2 mutant embryos were tested for their ability to respond chemotactically to PDGF and aFGF (Fig. 5A). Both wt and Shp2 embryo fibroblasts migrated toward the chamber containing PDGF as a chemotactic agent (Fig. 5A, a and b). However, when these cells were challenged with aFGF as a chemotactic agent, only wt cells could migrate through the chamber membrane efficiently (Fig. 5A, c and d). This result indicates that the Shp2 mutant cells were unable to respond to aFGF as a chemoattractant signal. FGFs are known to participate in the morphogenetic events that occur at gastrulation (24, 25), suggesting that this defect in the ability of Shp2 mutant cells to respond to signaling initiated by aFGF is biologically relevant.
Figure 5.
Shp2 is required for cell migration. (A) Boyden chamber migration assays with wt (a and c) or Shp2 mutant (b and d) cells challenged with PDGF (a and b) or aFGF (c and d) as chemoattractive agents. Photos were taken with brightfield optics with the ×20 objective. Hematoxylin stains cell nuclei purple (arrow, a); the pores on the membrane through which the cells migrate are clearly visible (arrowhead, d). Similar results were obtained in three separate experiments by using two different Shp2 mutant cell lines. (B) Boyden chamber migration assays comparing Shp2 mutant cells infected with a control retrovirus (white bars) or a retrovirus containing the Shp2 gene (blue bars). Reexpressing Shp2 within the mutant fibroblast cells rescues their ability to respond to aFGF as a chemotactic agent. Shown is the average number of cells from five random fields of view within one membrane. Similar results were obtained in three separate experiments using two different rescued cell lines.
One could argue that there may be inherent differences between the fibroblast cell lines derived from wt and Shp2 mutant embryos that could account for the differential response to aFGF. To address this issue, Shp2 mutant fibroblasts were infected with replication-defective control retrovirus (26) or a retrovirus containing an Shp2 cDNA to restore expression of Shp2 within these mutant cell lines. The empty vector control or Shp2-expressing cell lines were then challenged with the same chemotactic assays (Fig. 5B). Once again, Shp2 mutant cells or mutant cells reexpressing Shp2 both responded to PDGF as a chemoattractant. Shp2 mutant control cells did not migrate toward the chamber containing aFGF, whereas mutant cells reexpressing Shp2 were now able to respond to this growth factor as a chemoattractant. These results indicate that the defective migration of mutant cells in response to FGF was caused solely by the loss of Shp2 function.
DISCUSSION
We have previously shown that mouse embryos homozygous for a mutation within the Shp2 gene display defects in gastrulation (5). Here, we have investigated the basis for Shp2 function during mammalian gastrulation at the cellular level, by using a chimeric analysis. By using this approach, we have followed the fate of Shp2 mutant cells marked with β-galactosidase in chimeric embryos and have explored the defects resulting from increasing levels of mutant cells within the chimeric embryos. Specifically, Shp2 mutant cells accumulate within the primitive streak of gastrulating embryos, potentially because of the inability of Shp2-deficient cells to undergo changes in cell shape, adhesion, or migration that are required for cells to exit the streak and thus contribute to the mesoderm lineage. Furthermore, the retention of mutant cells within the streak presages accumulation of these cells at the posterior end of the embryo, particularly within the neuroectoderm lineage. In high-contribution chimeras, the posterior neuroectoderm exhibited many defects, including spina bifida and secondary neural tubes.
Shp2 Mutant Cells Accumulate at the Primitive Streak of Gastrulating Embryos.
Epiblast cells delaminate from the epithelial sheet at the primitive streak, differentiate into mesoderm, and subsequently move laterally around the embryo between the endoderm and the epiblast cell layers (11). As Shp2 mutant cells are underrepresented within the mesodermal lineage, this phosphatase is apparently required for epiblast cells to undergo the epithelial-to-mesenchymal transition and migrate away from the primitive streak. These morphogenetic events involve changes in cell shape, adhesion, and migration. Indeed, recent experiments have shown that Shp2 binds to multiple proteins involved in adhesion, including the transmembrane glycoprotein SHPS-1 (or SIRP-1) (27–29) and the platelet/endothelial cell-adhesion molecule PECAM-1 (30, 31). Furthermore, Shp2 mutant cells are deficient in signaling initiated by engagement of integrin receptors (8, 32). Therefore, Shp2 is required for cell shape (ref. 8; T.M.S., unpublished observations), adhesion, and migration responses initiated by FGF (this study) to occur normally, indicating that Shp2 may have multiple roles in controlling morphogenetic events during gastrulation.
Although gastrulation is a highly dynamic process, fate-mapping studies indicate that cells progress through the primitive streak in a precise and orderly fashion (21, 22). For example, cells closest to the site of streak formation are the first to exit from it; those that were initially more lateral to it progress through the primitive streak later, and epiblast cells farthest away never enter the streak and ultimately give rise to the ectoderm of the embryo. In low- and medium-contribution chimeras, Shp2 mutant cells exit the streak in reduced numbers and therefore remain within the epiblast and eventually populate ectoderm-derived tissues of the embryo, such as the neuroepithelium and dorsal root ganglia. The dorsal root ganglia are derived from neural crest cells, which originate in the ectoderm; it is therefore not surprising that because Shp2 mutant cells predominantly populate the ectoderm of early chimeric embryos, the neural crest cells would consist of Shp2 mutant cells. The migration of the nascent neural crest cells to their proper embryonic location seems unaffected by the Shp2 mutation, indicating that the defect observed here in response to aFGF does not represent a global defect in cell migration but a particular deficiency in response to this particular chemoattractant.
Cells destined to become paraxial mesoderm are recruited from a progenitor population found first within the epiblast, but these cells then move through the primitive streak and emerge at mid-streak level before ultimately forming the somites. This tissue, along with the trunk mesenchyme, was one of the last to be populated by the mutant cells, reflecting the difficulty experienced by Shp2 mutant cells in performing differentiation and/or migration events during gastrulation that result in the specification of mesoderm lineages. Furthermore, as the site of the primitive streak of the egg cylinder demarcates the future posterior end of the embryo, the Shp2 mutant cells that remain within the streak tend to accumulate in the tail of E10.5 embryos.
Accumulation of Shp2 Mutant Cells in the Neuroectoderm Causes Neural Defects.
The failure of Shp2 mutant cells to properly exit the primitive streak and their consequent retention in the region of the epiblast fated to become neuroectoderm might be expected to result in abnormalities in developing neural tissue. Indeed, high-contribution chimeras frequently displayed an open neural tube (spina bifida). Hyperaccumulation of cells within the neuroectoderm lineage at the posterior of the Shp2 chimeric embryos caused the process of neural fold closure to occur aberrantly. This is potentially caused by the excessive numbers of cells within the neural folds, which could lead to enhanced ventral curvature of the body axis and likely inhibits fusion of the neural folds (33, 34), thus resulting in spina bifida. Secondary neural tubes are another possible result of too many cells adopting the neural cell fate (18, 35). We speculate that these defects arise as a secondary consequence of the overpopulation of the neuroepithelium with mutant cells that were originally impaired in their ability to progress through the primitive streak in response to FGF signals.
Shp2 Is Required for Migration Events Initiated by FGFRs.
FGFs have firmly established roles in chemotactic migration events in invertebrate species. For example, in Caenorhabditis elegans, FGF (egl-17) acts in conjunction with its corresponding receptor (FGFR, egl-15) to direct an anterior migration of the sex myoblast precursor cells for the proper localization of the egg-laying muscles within the developing worm (36, 37). In Drosophila, the phenotypes caused by the breathless and heartless mutations result from a failure of precursor cells to migrate to the correct region of the developing embryo. These phenotypes result from mutations within the Drosophila FGFR1 and FGFR2 genes, respectively (38–40). Of interest, the Drosophila homolog of Shp2, corkscrew (csw), has been shown to be required for signaling downstream of the Breathless receptor (41). In vertebrates, the role of FGFs as chemoattractants is less clear, but they have been implicated, mainly in in vitro assays, in migration events of the developing limb (42), glial cells (43), endothelial cells (44), and smooth muscle cells (45), as well as in the gastrulating embryo (18). Here, we show that chemotactic response to aFGF is abrogated in Shp2 mutant fibroblasts, and implicate FGF-dependent signaling through Shp2 as being important for morphogenetic movements during gastrulation.
Although the biochemical mechanism through which Shp2 might augment signaling initiated by FGF receptors during gastrulation is not known, recent studies in PC12 cells lend insight into a potential signaling pathway. FGF receptors do not engage Shp2 directly; rather they phosphorylate a membrane-associated docking protein FRS2 at a tyrosine site that binds the amino-terminal SH2 domain of Shp2. Transfection experiments suggest that the interaction between Shp2 and FRS2 is essential for FGF-induced signaling to the mitogen-activated protein kinase pathway which is crucial for the induction of neurites by these cells in response to FGFs (6, 7).
Parallel Requirements for FGFR1 and Shp2 During Murine Development.
It is interesting to note that FGFR1 chimeric embryos also show both caudal accumulations and the appearance of secondary neural tubes (18). Consistent with this finding, Shp2-deficient cells have impaired responses to FGFs, including the failure to properly activate mitogen-activated protein kinase (3, 5) or migrate (this study) in response to exogenous FGFs. Indeed, not only do the germ-line phenotypes of the Shp2 exon 3 deletion and mutations within the FGFR1 gene look strikingly similar (5, 12, 13), the overall contribution pattern of both Shp2 and FGFR1 cells to developing chimeric embryos is also remarkably similar (refs. 18 and 46, this study, and unpublished results). It is interesting that although many tyrosine kinases are required for development to proceed normally (i.e., Flk-1, epidermal growth factor receptor, PDGFαR), there does not seem to be a clear correlation between the described loss-of-function phenotypes for these tyrosine kinases and the Shp2 chimeric phenotype. The FGFR1 pathway seems to be predominately affected over other tyrosine kinases by the perturbation of Shp2 function. Taken together, the behavior of Shp2 mutant cells in vitro and in vivo strongly implicates this SH2-containing tyrosine phosphatase as playing a critical role in signaling downstream of receptors for the FGF family of growth factors.
Acknowledgments
We thank J. MacRae, D. Jones, B. Ciruna, J. Rossant, and M. Henkemeyer for invaluable discussions, F. Kiefer for the retroviral vectors, D. Holmyard for scanning electron microscopy, K. Harpal for histology, and S. Kulkarni for transgenic work. Predoctoral support for T.M.S. was from the Medical Research Council of Canada (MRC). This work was supported by a grant from Bristol-Myers-Squibb, a Terry Fox Program grant from the National Cancer Institute of Canada (NCIC), and a Howard Hughes International Scholar award to T.P.; T.P. is a Distinguished Scientist of the MRC.
ABBREVIATIONS
- FGF
fibroblast growth factor
- FGFR1
FGF receptor 1
- ES
embryonic stem
- E
embryonic day
- PDGF
platelet-derived growth factor
- aFGF
acidic FGF
- wt
wild-type
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Cohen G B, Ren R, Baltimore D. Cell. 1995;80:237–248. doi: 10.1016/0092-8674(95)90406-9. [DOI] [PubMed] [Google Scholar]
- 2.Pawson T. Nature (London) 1995;373:573–580. doi: 10.1038/373573a0. [DOI] [PubMed] [Google Scholar]
- 3.Tang T L, Freeman R J, O’Reilly A M, Neel B G, Sokol S Y. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 4.Bennett A M, Hausdorff S F, O’Reilly A M, Freeman R M, Neel B G. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saxton T M, Henkemeyer M, Gasca S, Shen R, Rossi D J, Shalaby F, Feng G S, Pawson T. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright J H, Drueckes P, Bartoe J, Zhao Z, Shen S H, Krebs E G. Mol Biol Cell. 1997;8:1575–1585. doi: 10.1091/mbc.8.8.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hadari Y R, Kouhara H, Lax I, Schlessinger J. Mol Cell Biol. 1998;18:3966–3973. doi: 10.1128/mcb.18.7.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yu D H, Qu C K, Henegariu O, Lu X, Feng G S. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 9.Pluskey S, Wandless T J, Walsh C T, Shoelson S E. J Biol Chem. 1995;270:2897–2900. doi: 10.1074/jbc.270.7.2897. [DOI] [PubMed] [Google Scholar]
- 10.Hof P, Pluskey S, Dhe P S, Eck M J, Shoelson S E. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 11.Tam P P, Behringer R R. Mech Dev. 1997;68:3–25. doi: 10.1016/s0925-4773(97)00123-8. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi T P, Harpal K, Henkemeyer M, Rossant J. Genes Dev. 1994;8:3032–3044. doi: 10.1101/gad.8.24.3032. [DOI] [PubMed] [Google Scholar]
- 13.Deng C X, Wynshaw B A, Shen M M, Daugherty C, Ornitz D M, Leder P. Genes Dev. 1994;8:3045–3057. doi: 10.1101/gad.8.24.3045. [DOI] [PubMed] [Google Scholar]
- 14.Kouhara H, Hadari Y R, Spivak K T, Schilling J, Bar S D, Lax I, Schlessinger J. Cell. 1997;89:693–702. doi: 10.1016/s0092-8674(00)80252-4. [DOI] [PubMed] [Google Scholar]
- 15.Nagy A, Rossant J, Nagy R, Abramow N W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henkemeyer M, Orioli D, Henderson J T, Saxton T M, Roder J, Pawson T, Klein R. Cell. 1996;86:35–46. doi: 10.1016/s0092-8674(00)80075-6. [DOI] [PubMed] [Google Scholar]
- 17.Zambrowicz B P, Imamoto A, Fiering S, Herzenberg L A, Kerr W G, Soriano P. Proc Natl Acad Sci USA. 1997;94:3789–3794. doi: 10.1073/pnas.94.8.3789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ciruna B G, Schwartz L, Harpal K, Yamaguchi T P, Rossant J. Development. 1997;124:2829–2841. doi: 10.1242/dev.124.14.2829. [DOI] [PubMed] [Google Scholar]
- 19.Arrandale J M, Gore W A, Rocks S, Ren J M, Zhu J, Davis A, Livingston J N, Rabin D U. J Biol Chem. 1996;271:21353–21358. doi: 10.1074/jbc.271.35.21353. [DOI] [PubMed] [Google Scholar]
- 20.Gardner R L, Cockroft D L. Development. 1998;125:2397–2402. doi: 10.1242/dev.125.13.2397. [DOI] [PubMed] [Google Scholar]
- 21.Lawson K A, Pedersen R A. Development. 1987;101:627–652. doi: 10.1242/dev.101.3.627. [DOI] [PubMed] [Google Scholar]
- 22.Lawson K A, Meneses J J, Pedersen R A. Development. 1991;113:891–911. doi: 10.1242/dev.113.3.891. [DOI] [PubMed] [Google Scholar]
- 23.Doerr M E, Jones J I. J Biol Chem. 1996;271:2443–2447. doi: 10.1074/jbc.271.5.2443. [DOI] [PubMed] [Google Scholar]
- 24.Slack J M, Isaacs H V, Song J, Durbin L, Pownall M E. Biochem Soc Symp. 1996;62:1–12. [PubMed] [Google Scholar]
- 25.Goldfarb M. Cytokine Growth Factor Rev. 1996;7:311–325. doi: 10.1016/s1359-6101(96)00039-1. [DOI] [PubMed] [Google Scholar]
- 26.Markowitz D, Goff S, Bank A. J Virol. 1988;62:1120–1124. doi: 10.1128/jvi.62.4.1120-1124.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fujioka Y, Matozaki T, Noguchi T, Iwamatsu A, Yamao T, Takahashi N, Tsuda M, Takada T, Kasuga M. Mol Cell Biol. 1996;16:6887–6899. doi: 10.1128/mcb.16.12.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kharitonenkov A, Chen Z, Sures I, Wang H, Schilling J, Ullrich A. Nature (London) 1997;386:181–186. doi: 10.1038/386181a0. [DOI] [PubMed] [Google Scholar]
- 29.Stofega M R, Wang H, Ullrich A, Carter S C. J Biol Chem. 1998;273:7112–7117. doi: 10.1074/jbc.273.12.7112. [DOI] [PubMed] [Google Scholar]
- 30.Jackson D E, Ward C M, Wang R, Newman P J. J Biol Chem. 1997;272:6986–6993. doi: 10.1074/jbc.272.11.6986. [DOI] [PubMed] [Google Scholar]
- 31.Sagawa K, Kimura T, Swieter M, Siraganian R P. J Biol Chem. 1997;272:31086–31091. doi: 10.1074/jbc.272.49.31086. [DOI] [PubMed] [Google Scholar]
- 32.Oh, E.-S., Gu, H., Saxton, T. M., Timms, J. F., Hausdorff, S., Kamatkar, S., Frevert, E. U., Kahn, B. B., Pawson, T., Neel, B. G. & Thomas, S. M. (1999) Mol. Cell. Biol., in press. [DOI] [PMC free article] [PubMed]
- 33.Copp A J. Ciba Found Symp. 1994;181:118–134. doi: 10.1002/9780470514559.ch8. [DOI] [PubMed] [Google Scholar]
- 34.Peeters M C, Schutte B, Lenders M H, Hekking J W, Drukker J, Van S H. Dev Dyn. 1998;211:382–389. doi: 10.1002/(SICI)1097-0177(199804)211:4<382::AID-AJA9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 35.Yoshikawa Y, Fujimori T, McMahon A P, Takada S. Dev Biol. 1997;183:232–242. doi: 10.1006/dbio.1997.8502. [DOI] [PubMed] [Google Scholar]
- 36.DeVore D L, Horvitz H R, Stern M J. Cell. 1995;83:611–620. doi: 10.1016/0092-8674(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 37.Burdine R D, Chen E B, Kwok S F, Stern M J. Proc Natl Acad Sci USA. 1997;94:2433–2437. doi: 10.1073/pnas.94.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reichman F M, Shilo B Z. Mech Dev. 1995;52:265–273. doi: 10.1016/0925-4773(95)00407-r. [DOI] [PubMed] [Google Scholar]
- 39.Beiman M, Shilo B Z, Volk T. Genes Dev. 1996;10:2993–3002. doi: 10.1101/gad.10.23.2993. [DOI] [PubMed] [Google Scholar]
- 40.Gisselbrecht S, Skeath J B, Doe C Q, Michelson A M. Genes Dev. 1996;10:3003–3017. doi: 10.1101/gad.10.23.3003. [DOI] [PubMed] [Google Scholar]
- 41.Perkins L A, Johnson M R, Melnick M B, Perrimon N. Dev Biol. 1996;180:63–81. doi: 10.1006/dbio.1996.0285. [DOI] [PubMed] [Google Scholar]
- 42.Webb S E, Lee K K, Tang M K, Ede D A. Dev Dyn. 1997;209:206–216. doi: 10.1002/(SICI)1097-0177(199706)209:2<206::AID-AJA6>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 43.Holland E C, Varmus H E. Proc Natl Acad Sci USA. 1998;95:1218–1223. doi: 10.1073/pnas.95.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ratajska A, Torry R J, Kitten G T, Kolker S J, Tomanek R J. Dev Dyn. 1995;203:399–407. doi: 10.1002/aja.1002030403. [DOI] [PubMed] [Google Scholar]
- 45.Herbert J M, Lamarche I, Carmeliet P. J Biol Chem. 1997;272:23585–23591. doi: 10.1074/jbc.272.38.23585. [DOI] [PubMed] [Google Scholar]
- 46.Deng C, Bedford M, Li C, Xu X, Yang X, Dunmore J, Leder P. Dev Biol. 1997;185:42–54. doi: 10.1006/dbio.1997.8553. [DOI] [PubMed] [Google Scholar]