Abstract
The combination of transparency and accessible genetics is making zebrafish an increasingly important model in studies of motor control. Much of the work on the model has been done over the past decade. Here we review some of the highlights of this work that serve to reveal both the power of the model and its prospects for providing important future insights into the links between neural networks and behavior.
Introduction
The zebrafish model is a relative newcomer to studies of motor control and neuronal networks in general. Nonetheless, it has a combination of strengths that are allowing studies that are very difficult or impossible in other models. The two major assets of the zebrafish model are its transparency and its genetic accessibility. The transparency allows optical approaches to the study of structure and function in the intact animal. The genetics allows for the production of both mutant animals with motor disruptions and transgenic fish. This combination of transparency and genetics is unavailable in other vertebrate models (although nematodes offer many of the same strengths in an invertebrate) and is what has driven studies of motor control using zebrafish, almost all of which have occurred in the past decade. Here we review some of the major work, with an eye to further questions in motor control that are made accessible by this model.
General aspects of motor behavior
The major motor behaviors and their development have been described in some detail for larval zebrafish (Budick and O’Malley, 2000; Eaton et al., 1977; Saint-Amant and Drapeau, 1998). There is a regular pattern of development with gross body movements followed by the development of rhythmic swimming and, subsequently, slower swimming and pectoral fin movements (Thorsen et al., 2004). Very early movements rely on gap junctional coupling for their production (Saint-Amant and Drapeau, 2001). Later refined movements are used by the larvae during routine swimming, after it has developed a swim bladder to allow it to move from the bottom up into the water column (Borla et al., 2002). Remarkably the larval fish can swim over a very large range of bending frequencies from roughly 15Hz to 100Hz (Budick and O’Malley, 2000; Muller and van Leeuwen, 2004). Escape bends are extremely fast with the head bending to touch the tail in about 10msec, at a rate of about 25 degrees per msec (Liu and Fetcho, 1999).
An early effort to identify the parts of the nervous system responsible for swimming movements indicated that the early spontaneous movements did not require the hindbrain, but that hindbrain was essential for later touch responses and swimming (Saint-Amant and Drapeau, 1998). Recent work indicates that this result was mistaken and that the fish can generate swimming and touch responses without the brain, even at later stages (Downes and Granato, 2006). In this respect zebrafish are like other vertebrates, in which isolated spinal cord maintains some ability to produce reflex responses and rhythmic motor output.
Basic biology of spinal networks
The interpretation of mutants or perturbations often depends upon an understanding of the normal wiring of the networks. Many of the neuronal types in zebrafish spinal cord have been identified and their wiring is the subject of much current work (Bernhardt et al., 1990; Hale et al., 2001; Higashijima et al., 2004b). Several factors have contributed to a reasonably rapid progress in identifying the cells and circuits in zebrafish. In vivo calcium imaging has allowed for the rapid identification of neurons active in a particular behavioral context (Brustein et al., 2003b; Cox and Fetcho, 1996; Fetcho and O’Malley, 1995; O’Malley et al., 1996; Ritter et al., 2001). The ability to do patch recording allows for studies of cellular properties and synaptic connectivity that complements imaging work, in which the activity in groups of neurons can be monitored simultaneously (Drapeau et al., 1999; Higashijima et al., 2004a; Kimura et al., 2006; Masino and Fetcho, 2005). Finally, another major advantage is the similarities to networks in Xenopus tadpoles, which are already reasonably well understood based upon decades of study (Roberts, 2000; Roberts et al., 1998). This has allowed more directed questions about cell types whose structure and transmitter phenotype made them good candidates for roles that had already been suggested for similar neurons in Xenopus. The progress is further accelerated because of the genetics, which have allowed for in situ markers of transmitter phenotype to catalog the transmitters that a neuron probably uses (Higashijima et al., 2004b). This can be combined with GFP labeling to define the structure of the neuron as well as its transmitter properties. Finally, transgenic lines with fluorescent subsets of neurons are leading to animals with color coded nervous systems (Higashijima et al., 2000; Higashijima et al., 2004a; Kimura et al., 2006). This allows for a combination with calcium indicators to look at the pattern of activity in one cell type, or for targeted patch recording directed at neurons of particular phenotypes based upon their fluorescence.
Neuromodulatory systems in zebrafish have also been the subject of considerable attention because of the possibilities of understanding their contributions to behavior and of developing disease models based upon mutations or drugs that disrupt these systems. Dopamine, serotonergic, noradrenergic and histamatergic systems in larvae and/or adults have been described morphologically and some receptors and trasnporters have been cloned (Boehmler et al., 2004; Kaslin and Panula, 2001; Ma, 2003; McLean and Fetcho, 2004a; McLean and Fetcho, 2004b; Wang et al., 2006)}. Pharmacological studies, mutants, and chemical ablations indicate important roles for neuromodulatory systems, but mechanistic studies to reveal the activity patterns of neurodmodulatory neurons and their affects on motor circuits are needed (Anichtchik et al., 2004; Brustein et al., 2003a; Rink and Guo, 2004).
One of the most striking aspects of the zebrafish work is that the genetics have finally allowed the determination of the likely relationships between spinal neuron types in fishes (and amphibians) and those in more complex birds and mammals. Developmental studies in chicks and mice revealed a transcription factor code that directs the development of different neuronal types from different dorsoventral regions in spinal cord (Briscoe et al., 2000). These transcription factors can be used as markers of subsets of neurons. The same transcription factor code operates in fish, so the markers can be used to identify what are probably evolutionarily related cell types in fish and mammals.
This has now been done for two neuronal types. The engrailed-1 positive neurons are ipsilateral, ascending inhibitory neurons that play roles in sensory gating and controlling the duration of motor bursts (Higashijima et al., 2004a; Li et al., 2004) (Fig. 1). The identity of the neurons as engrailed-1 positive was first shown in zebrafish, and was subsequently demonstrated in Xenopus for similar ascending neurons. The engrailed-1 cells in mammals are a heterogeneous population that includes the Renshaw neurons, which provide feedback inhibition of motoneurons (Sapir et al., 2004; Saueressig et al., 1999). The ascending interneurons in fish and frogs have some features akin to Renshaw cells, but they play additional functional roles as well. The evidence suggests that multifunctional neurons like the engrailed cells in fish and frogs gave rise to a series of cell types in mammals, each of which has more restricted functional roles. Primitive ascending inhibitory neurons might have given rise to both Renshaw neurons as well as other engrailed positive cells in mammals. The role of the engrailed cells in frog tadpoles and fishes in shaping motor output to truncate bursts at high frequencies led to the prediction that disrupting this cell type in mammals might slow the frequency of rhythmic movements (Gosgnach et al., 2006; Higashijima et al., 2004a; Li et al., 2004; Sapir et al., 2004; Saueressig et al., 1999). This prediction was recently supported in experiments in which the engrailed neurons were selectively inactivated in mice (Gosgnach et al., 2006).
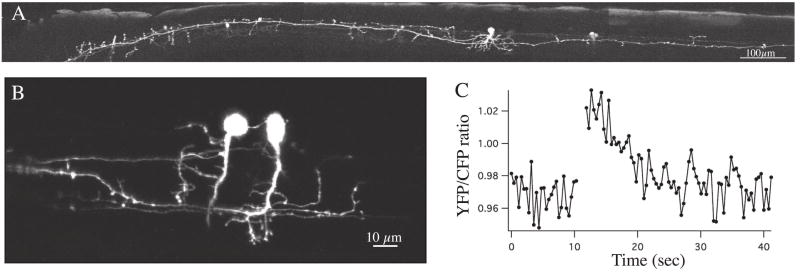
Fig. 1. Imaging structure and function in vivo with genetic tools.
A. Confocal image showing detailed morphology from an intact living fish of an ascending interneuron labeled with GFP under control of the promoter for the engrailed-1 gene, which is expressed in ascending inhibitory interneurons in zebrafish. The view is a lateral view with the head to the left. B. Two ascending interneurons expressing the genetic calcium indicator cameleon 2.1 under control of the engrailed-1 promoter. The calcium response of the neuron marked by the asterisk is shown in C during a swimming episode that begins at the break in the plot. The neurons are active duringswimming, as indicated by the fluorescence ratio increase.
More recently, the neurons in fish that correspond to the Chx-10 positive cells in mammals were identified as ipsilateral descending excitatory neurons, many of which are rhythmically active during swimming and probably help to drive motor output at higher swimming speeds (Kimura et al., 2006). We might predict that Chx-10 positive excitatory neurons in mammals play similar roles in driving motor output and that they would be a good place to search for excitatory elements in the mammalian central pattern generator. The ability to link neurons across species makes the study of zebrafish more broadly relevant, as the results from fish are being used to make predictions about the functional roles of neurons in more complex systems, where it is sometimes difficult to predict what role a class of cell might play in the network. The genetics thus serve as a bridge to unite studies in disparate species and elevates our understanding of both the individual species and their evolution.
Imaging and ablation studies to reveal activity patterns and functional roles of neurons
Although there were pioneering studies of the development of motor behavior and startle response of zebrafish many years ago (Eaton et al., 1977), the utility of the animal as a model expanded as a result of a convergence of new methods to image activity in vivo with the tractability of screening for mutants. The development of an approach to backfill neurons in vivo with calcium indicators offered the possibility to image activity of individual neurons in intact zebrafish (O’Donovan et al., 1993). This allowed the first imaging of neuronal activity with single cell resolution in an intact vertebrate (Fetcho and O’Malley, 1995). The first work was largely technical, both documenting the ability to reliably image groups of motoneurons in vivo during an escape response and showing how massive the recruitment of motoneurons is during the escape bend in fish. Every imaged motoneuron was activated when an escape was triggered by a tap on the head of the fish.
The backfilling was then extended to a broader question centered around the functional organization of vertebrate hindbrains (O’Malley et al., 1996). The hindbrains of all vertebrates are segmentally organized, with segmental domains specified by a homeobox code during development. This segmental organization is evident even in adults, where segmentally repeated cell types are evident (Metcalfe et al., 1986). The presence of segmentally repeated neurons is thought to result from segmental duplication events during evolution. Their presence raised obvious questions about the functional relationships of the segmental neurons. These neurons could be backfilled with calcium indicators in zebrafish, so their activity in relation to behavior could be studied. Early work examined the activity pattern of a repeated set of reticulospinal neurons thought to be involved in the escape response of fish. This work revealed that the pattern of activity in the serially repeated set of neurons (the Mauthner cell, and MiD2cm, and MiD3cm) varied depending on the location of the stimulus used to elicit an escape. This variation is related to variability in the behavior, with stronger escape bends produced in response to stimuli at the head associated with activation of the entire serial set of cells and the weaker escapes produced by stimuli to the tail involving only activation of the Mauthner cell. The implication of this work is that serially repeated hindbrain neurons may form functional groups associated with particular behaviors. The pattern of activity in the group may help to determine the different forms of the behavior. There are many serially repeated groups of hindbrain neurons – whether other sets also form functional groups remains to be established.
The contribution of the hindbrain neurons was later tested by focusing a laser beam into the transparent larval fish to kill particular neurons in the hindbrain array and then examine the resulting behavioral deficits (Liu and Fetcho, 1999). The evidence supported the conclusion that the hindbrain neurons contributed differentially to escapes to head and tail stimuli because deleting the Mauthner cell had a dramatic effect on escapes to tail stimuli and none on escapes produced by a head stimulus. This utility of zebrafish for both imaging activity simultaneously in groups of neurons and for testing their contribution to behavior by optical (or genetic, see below) perturbation is perhaps the major advantage of the model. Other models such as flies and mice allow for one or the other, but both are possible throughout the brain and spinal cord of zebrafish.
Imaging studies subsequently revealed a massive activation of hindbrain neurons in response to taps on the head, suggesting a widespread involvement of the reticulospinal system in response to the stimulus (Gahtan et al., 2002). In this and the earlier work on the hindbrain array, the fish was entirely restrained in agar, so the behavior after the stimulus was difficult to discern. One possible explanation of the large reticulospinal activation is that at least some of the neurons are involved in swimming and or struggling responses following the escape.
As one might expect, descending hindbrain neurons do apparently contribute to behaviors other than escape. A carefully performed single cell laser ablation study revealed that a pair of neurons in the nucleus of the medial longitudinal fasciculus with dendrites in the optic tectum and projections to spinal cord contribute to feeding motor responses (Gahtan et al., 2005). Ablation of the neurons reduced prey capture success and appeared to affect the ability of the fish to properly orient to the prey. This ability to link even a few neurons to different motor behaviors offers the promise of understanding the contribution of many cell types.
In order to link neurons to movements it is important to know which movement is occurring when the neurons are active. This can be difficult with imaging because movement interferes with the image collection, which is why early experiments were done in fish that were entirely embedded in agar. The problem has been resolved to some extent by developing a preparation in which the front of the fish is in agar and the tail is free to move (Ritter et al., 2001). Active neurons can be imaged in the front of the fish while the movements of the tail are filmed to determine which behaviors are being produced in association with the neural activity. This was initially used to study which neurons were active in spinal cord during swimming versus escape responses (Ritter et al., 2001). The work showed that descending commissural neurons (MCoDs), which we now know are excitatory, are active during slow speed movements and another, different descending excitatory interneuron class (CiDs) is active in fast escape responses. This partially moving preparation has allowed studies of the patterns of activation of a class of interneuron during escapes which show that the escape is graded largely by changes in the level of activity in the entire pool of interneurons rather than recruitment of inactive cells (Bhatt et al., 2007). A recent preliminary report indicates that there is actually a switching of spinal interneuron classes as the swimming speed is increased, with excitatory neurons active at slow speeds being inhibited at higher speeds, when another class of excitatory interneurons becomes active (Masino et al., 2005). These studies suggest that we need to be careful about assuming that the circuits that produce slower movements are the same as the ones that produce fast movements, even when both involve the same overall motor pattern (in the case of swimming, alternating bending patterns that propagate from head to tail).
Mutants and genetic perturbations
One of the special strengths of zebrafish is the ability to carry out mutagenesis screens and identify animals with single gene disruptions that affect behavior, including motor behaviors. This was recognized early on in the first large scale mutagenesis study in which mutants were identified with disrupted movements not associated with impaired muscle development (Granato et al., 1996). These fell into many categories, including ones that did not response to a touch at all (eg. macho, space cadet), others that fatigued and so could not sustain swimming (twitch once, shocked), others that contracted both sides at the same time so they looked like an accordion (accordion, bandoneon, diwanka) and still others that moved wildly or inappropriately (space cadet, techno trousers).
Many of these have now been studied in more depth both from genetic and functional viewpoints. In a handful of cases the genes have been identified and there is some understanding of how the gene might contribute to the phenotype. The results can be divided into a few broad categories – those mutants in which synaptic function at the neuromuscular junction or in the CNS is altered leading to a phenotype, and those in which there appear to be axonal pathfinding errors that result is a miswiring of networks.
Synaptic function
Many of the mutants with motor defects have problems with genes associated with the neuromuscular junction, or with processes in the muscle itself. These include mutations of the acetylcholine receptor, such as Nic1 and sofa potato, which cannot move, as well as the immotile mutant relaxed, in which a mutation of the dihydropyridine receptor interferes with the calcium release needed for muscle contraction (Ono et al., 2001; Schredelseker et al., 2005; Sepich et al., 1998; Zhou et al., 2006). These are perhaps less important from a motor control point of view, although they can be used to examine aspects of muscle function and provide a convenient source of immotile fish for physiological experiments.
Some of the more interesting mutations are those that produce fish that can move, but do so in an impaired way. For example, one of these, called twice once, is a mutation in rapsyn, a protein that is involved in localization of acetylcholine receptors at the neuromuscular junction (Ono et al., 2002). These mutants show an inability to swim continuously after a stimulus. Morphological observations with labeled alpha bungarotoxin show that acetylcholine receptors are diffusely distributed rather than localized at the synapses. The mutants also show an abnormally fatigable neuromuscular transmission, consistent with the inability to sustain behavior. This mutation is of some clinical significance because human studies have revealed that the same type of mutation leads to human myasthenic syndrome, also characterized by muscle weakness (Ohno et al., 2002). The accordion class of mutants is a group showing bilateral muscle contractions. Studies of mutants have revealed that there are many ways to make a fish generate accordion-like movements.. One is to disrupt the calcium transporter (SERCA) responsible for removal of the calcium from the cytoplasm after muscle contraction (Gleason et al., 2004; Hirata et al., 2004). When this is not working, as in the mutant named accordion, the muscles do not relax properly and the result is the contraction on one side of the fish overlaps with that on the other, generating the phenotype. The accordion class mutant Zieharmonika is a mutation in acetylchoinesterase, which would elevate acetylcholine at the synapses (Downes and Granato, 2004). Early work proposed that central deficits in glycinergic transmission could also produce an accordion phenotype because glycinergic inhibition is known to play a critical role in the alternation of contractions on opposite side of the fish during bending. One of the mutants in the accordion class, called bandoneon, indeed turns out to be a mutant of a beta subunit of the glycine receptor which disrupts proper inhibitory transmission and leads to co-contraction of opposite sides of the body (Hirata et al., 2005).
Proper glycinergic transmission is also essential to sustain motor behaviors. The mutant shocked shows an inability to sustain movement. The mutation is in the glial, glycine transporter-1, and the disruptions appears to elevate glycine levels which may allow for an inappropriate rise in the level of inhibition during movements, thereby truncating the movements (Cui et al., 2005; Cui et al., 2004). The mutation secondarily leads to a persistent electrical coupling of muscle fibers that may reinforce the inability to sustain swimming by inappropriately prolonging muscle contractions (Luna et al., 2004).
Developmental/pathfinding errors
Some mutants lead to pathfinding errors that result in motor phenotypes. These can be informative about cues used in axonal pathfinding as in the case of the mutants unplugged and diwanka, both of which involve molecules that play a role in guiding motor axons to their targets (Schneider and Granato, 2006; Zhang et al., 2004). More informative from a behavioral viewpoint are those mutants in which central neurons are disrupted in ways that alter circuit function. These also involve changes in genes important for development, that may alter cell number or axonal pathfinding. These are informative when the developmental deficit leads to a wiring change that can offer insight into network function. The mutants deadly seven and space cadet have both offered circuit level insights.
Deadly seven, a mutant of a notch gene, leads to extra Mauthner neurons in the hindbrain (Gray et al., 2001; Liu et al., 2003; van Eeden et al., 1996). Usually there is one on each side of the brain, but there can be as many as 5 or so on each side in mutant fish. Interestingly, this has little impact on the motor output in the escape (Liu et al., 2003). A close look at the morphology of the outputs of individual neurons indicates that the extra neurons divide up the synaptic targets of the neurons normally innervated by a single Mauthner cell. This reveals a good deal of plasticity in the nervous system to accommodate variation in cell number, even when such variations are not seen normally. This makes it easier to imagine how a system such as the escape (startle) built with relatively few neurons in fish could evolve into a mammalian startle circuit with much the same wiring, but many more neurons.
The space cadet mutant involves a wiring deficit (Lorent et al., 2001). The phenotype of this mutant is the generation of multiple, inappropriate escape bends, often repeated ones to the same side. Central commissures in the hindbrain of this animal are disrupted, including one that involves the axons of spiral fibers, which play a role in controlling the activation of the Mauthner neuron which initiates the startle. Thus, it may be that an axon pathfinding deficit leads to a behavioral phenotype because of a miswiring of a neuron critical for the response.
Another developmental avenue to explore function is to inject constructs into single cell embryos that lead to structural and possibly behavioral alterations that can be studied functionally. One compelling example of this approach was to inject a Hox gene, which leads to a conversion of a neuron that does not normally form a Mauthner cell into an extra Mauthner neuron (Hale et al., 2004). The result is fish with two Mauthner cells, one in the normal hindbrain segment #4 and the other in hindbrain segment #2. This mimics a reversion to what may be a primitive evolutionary state because hindbrain segments are thought to have been duplicates of one another that later diverged. Lesion experiments show that the extra Mauthner cell can substitute for the normal one when the normal one is removed, indicating again that such switches in segmental identity can be readily accomplished without dramatic functional impairments. Whatever rules guide the development of wiring, they allow extra neurons to easily slot into the networks to contribute to behavior.
Spinal Injury
Zebrafish larvae and adults have also been used to explore the problem of regeneration of axons in spinal cord after injury (Becker et al., 1998; Becker et al., 1997). Unlike mammals, some central axons in fish regenerate well, while others do not. Studies of adults have revealed differences in the upregulation of growth associated molecules that are correlated with regenerative capacity of neurons projecting from the brain to the spinal cord (Becker et al., 1998). The ability to image the regeneration directly in larvae has revealed that cyclic AMP can convert a neuron that has failed to regenerate across a lesion into one whose axon grows to cross the lesion (Bhatt et al., 2004). That growth is associated with both the recovery of activity of neurons postsynaptic to the regenerating axon and with the recovery of behavior. Once again the combination of direct imaging with genetic approaches offers a set of tools that will allow for exploration of the molecules that promote the regenerative process and that might be clinically relevant in humans.
The future
The advantages of zebrafish are likely to propel the model further as an animal in which motor behaviors (and others) can be understood at the cellular level. Progress on several fronts will make it an even better preparation for revealing and testing features of motor organization. Recent work, for example, indicates that interneurons used to move slowly differ from those used to move quickly and that there is a topographic organization of motoneurons and excitatory interneurons in spinal cord, with increasing more dorsal neurons recruited as the frequency of bending movements increases during swimming (McLean et al., 2007). These features of organization were tested by perturbations in which more ventral excitatory interneurons were removed to affect slow swimming without altering faster movements (McLean et al., 2007). Genetically encoded calcium indicators offer the possibility of transgenic lines of fish in which activity in particular subsets of cells can be monitored with single neuron resolution. The first vertebrate transgenic calcium indicator line was generated in zebrafish, but the signals from the older generation of genetic calcium indicators were not especially strong (Higashijima et al., 2003). Increasingly better genetically encoded indicators are being generated that will make imaging easier in subsets of neurons targeted in transgenic lines (Mank et al., 2006; Miyawaki et al., 1997; Nagai et al., 2004; Pologruto et al., 2004; Reiff et al., 2005; Tallini et al., 2006). Powerful genetic tools for perturbations of various sorts have also been developed. Some of the most promising for zebrafish are those that use light activated channels that can activate or inhibit neurons (Banghart et al., 2004; Boyden et al., 2005; Han and Boyden, 2007; Lechner et al., 2002; Li et al., 2005; Szobota et al., 2007; Zhang et al., 2007). One can easily imagine using light to turn off or to activate particular sets of cells to test their contribution to behavior. This can be done on the population as a whole, perhaps even by playing back patterns of spikes in individual neurons using brief flashes of light that elicit single spikes in particular cells. This will move the work more into the realm of testing contributions of neurons to both motor patterns and behavior. Finally, it is possible to image even subcellular synaptic level events in larval fish, such as the movements of GFP tagged calcium dependent protein kinase II, a molecule implicated in synaptic plasticity (Gleason et al., 2003). This can be done noninvasively anywhere in the brain or spinal cord of animals in which activity patterns and behavior can also be examined. This unique combination of features may make zebrafish a favored model for linking molecular, cellular, network and behavioral levels in studies of the neuronal basis of behavior.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NRSA 44728, DLM; NS 26539, JRF) and the Ministry of Education, Science, Technology, Sports and Culture of Japan.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anichtchik OV, et al. Neurochemical and behavioural changes in zebrafish Danio rerio after systemic administration of 6-hydroxydopamine and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. J Neurochem. 2004;88:443–53. doi: 10.1111/j.1471-4159.2004.02190.x. [DOI] [PubMed] [Google Scholar]
- Banghart M, et al. Light-activated ion channels for remote control of neuronal firing. Nat Neurosci. 2004;7:1381–6. doi: 10.1038/nn1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, et al. Readiness of zebrafish brain neurons to regenerate a spinal axon correlates with differential expression of specific cell recognition molecules. J Neurosci. 1998;18:5789–803. doi: 10.1523/JNEUROSCI.18-15-05789.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker T, et al. Axonal regrowth after spinal cord transection in adult zebrafish. Journal of Comparative Neurology. 1997;377:577–95. doi: 10.1002/(sici)1096-9861(19970127)377:4<577::aid-cne8>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bernhardt RR, et al. Identification of spinal neurons in the embryonic and larval zebrafish. J Comp Neurol. 1990;302:603–616. doi: 10.1002/cne.903020315. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, et al. Grading movement strength by changes in firing intensity versus recruitment of spinal interneurons. Neuron. 2007;53:91–102. doi: 10.1016/j.neuron.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Bhatt DH, et al. Cyclic AMP-induced repair of zebrafish spinal circuits. Science. 2004;305:254–8. doi: 10.1126/science.1098439. [DOI] [PubMed] [Google Scholar]
- Boehmler W, et al. Evolution and expression of D2 and D3 dopamine receptor genes in zebrafish. Dev Dyn. 2004;230:481–93. doi: 10.1002/dvdy.20075. [DOI] [PubMed] [Google Scholar]
- Borla MA, et al. Prey capture by larval zebrafish: evidence for fine axial motor control. Brain Behav Evol. 2002;60:207–29. doi: 10.1159/000066699. [DOI] [PubMed] [Google Scholar]
- Boyden ES, et al. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–8. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- Briscoe J, et al. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–45. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Brustein E, et al. Serotonin patterns locomotor network activity in the developing zebrafish by modulating quiescent periods. J Neurobiol. 2003a;57:303–22. doi: 10.1002/neu.10292. [DOI] [PubMed] [Google Scholar]
- Brustein E, et al. “In vivo” monitoring of neuronal network activity in zebrafish by two-photon Ca(2+) imaging. Pflugers Arch. 2003b;446:766–73. doi: 10.1007/s00424-003-1138-4. [DOI] [PubMed] [Google Scholar]
- Budick SA, O’Malley DM. Locomotor repertoire of the larval zebrafish: swimming, turning and prey capture. J Exp Biol. 2000;203:2565–79. doi: 10.1242/jeb.203.17.2565. [DOI] [PubMed] [Google Scholar]
- Cox KJA, Fetcho JR. Labeling blastomeres with a calcium indicator: a non-invasive method of visualizing neuronal activity in zebrafish. Journal of Neuroscience Methods. 1996;68:185–191. doi: 10.1016/0165-0270(96)00067-2. [DOI] [PubMed] [Google Scholar]
- Cui WW, et al. The zebrafish shocked gene encodes a glycine transporter and is essential for the function of early neural circuits in the CNS. J Neurosci. 2005;25:6610–20. doi: 10.1523/JNEUROSCI.5009-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui WW, et al. shocked Gene is required for the function of a premotor network in the zebrafish CNS. J Neurophysiol. 2004;92:2898–908. doi: 10.1152/jn.00419.2004. [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Acetylcholinesterase function is dispensable for sensory neurite growth but is critical for neuromuscular synapse stability. Dev Biol. 2004;270:232–45. doi: 10.1016/j.ydbio.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Downes GB, Granato M. Supraspinal input is dispensable to generate glycine-mediated locomotive behaviors in the zebrafish embryo. J Neurobiol. 2006;66:437–51. doi: 10.1002/neu.20226. [DOI] [PubMed] [Google Scholar]
- Drapeau P, et al. In vivo recording from identifiable neurons of the locomotor network in the developing zebrafish. J Neurosci Methods. 1999;88:1–13. doi: 10.1016/s0165-0270(99)00008-4. [DOI] [PubMed] [Google Scholar]
- Eaton RC, et al. Functional development in the Mauthner cell system of embryos and larvae of the zebra fish. J Neurobiol. 1977;8:151–172. doi: 10.1002/neu.480080207. [DOI] [PubMed] [Google Scholar]
- Fetcho JR, O’Malley DM. Visualization of Active Neural Circuitry in the Spinal-Cord of Intact Zebrafish. Journal of Neurophysiology. 1995;73:399–406. doi: 10.1152/jn.1995.73.1.399. [DOI] [PubMed] [Google Scholar]
- Gahtan E, et al. Evidence for a widespread brain stem escape network in larval zebrafish. J Neurophysiol. 2002;87:608–14. doi: 10.1152/jn.00596.2001. [DOI] [PubMed] [Google Scholar]
- Gahtan E, et al. Visual prey capture in larval zebrafish is controlled by identified reticulospinal neurons downstream of the tectum. J Neurosci. 2005;25:9294–303. doi: 10.1523/JNEUROSCI.2678-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleason MR, et al. A mutation in serca underlies motility dysfunction in accordion zebrafish. Dev Biol. 2004;276:441–51. doi: 10.1016/j.ydbio.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Gleason MR, et al. Translocation of CaM kinase II to synaptic sites in vivo. Nature Neuroscience. 2003;6:217–218. doi: 10.1038/nn1011. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, et al. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature. 2006;440:215–9. doi: 10.1038/nature04545. [DOI] [PubMed] [Google Scholar]
- Granato M, et al. Genes controlling and mediating locomotion behavior in the zebrafish embryo and larva. Development. 1996;123:399–413. doi: 10.1242/dev.123.1.399. [DOI] [PubMed] [Google Scholar]
- Gray M, et al. Zebrafish deadly seven functions in neurogenesis. Developmental Biology. 2001;237:306–323. doi: 10.1006/dbio.2001.0381. [DOI] [PubMed] [Google Scholar]
- Hale ME, et al. Hox gene misexpression and cell-specific lesions reveal functionality of homeotically transformed neurons. J Neurosci. 2004;24:3070–6. doi: 10.1523/JNEUROSCI.5624-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale ME, et al. A confocal study of spinal interneurons in living larval zebrafish. J Comp Neurol. 2001;437:1–16. doi: 10.1002/cne.1266. [DOI] [PubMed] [Google Scholar]
- Han X, Boyden ES. Multiple-Color Optical Activation, Silencing, and Desynchronization of Neural Activity, with Single-Spike Temporal Resolution. PLOS one. 2007;2:e299. doi: 10.1371/journal.pone.0000299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, et al. Visualization of cranial motor neurons in live transgenic zebrafish expressing green fluorescent protein under the control of the islet-1 promoter/enhancer. J Neurosci. 2000;20:206–18. doi: 10.1523/JNEUROSCI.20-01-00206.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, et al. Engrailed-1 expression marks a primitive class of inhibitory spinal interneuron. J Neurosci. 2004a;24:5827–39. doi: 10.1523/JNEUROSCI.5342-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashijima S, et al. Neurotransmitter properties of spinal interneurons in embryonic and larval zebrafish. J Comp Neurol. 2004b;480:19–37. doi: 10.1002/cne.20279. [DOI] [PubMed] [Google Scholar]
- Higashijima SI, et al. Imaging Neuronal Activity During Zebrafish Behavior With a Genetically Encoded Calcium Indicator. J Neurophysiol. 2003;90:3986–3997. doi: 10.1152/jn.00576.2003. [DOI] [PubMed] [Google Scholar]
- Hirata H, et al. Zebrafish bandoneon mutants display behavioral defects due to a mutation in the glycine receptor beta-subunit. Proc Natl Acad Sci U S A. 2005;102:8345–50. doi: 10.1073/pnas.0500862102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata H, et al. accordion, a zebrafish behavioral mutant, has a muscle relaxation defect due to a mutation in the ATPase Ca2+ pump SERCA1. Development. 2004;131:5457–68. doi: 10.1242/dev.01410. [DOI] [PubMed] [Google Scholar]
- Kaslin J, Panula P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio) J Comp Neurol. 2001;440:342–77. doi: 10.1002/cne.1390. [DOI] [PubMed] [Google Scholar]
- Kimura Y, et al. alx, a zebrafish homolog of Chx10, marks ipsilateral descending excitatory interneurons that participate in the regulation of spinal locomotor circuits. J Neurosci. 2006;26:5684–5697. doi: 10.1523/JNEUROSCI.4993-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner HA, et al. A genetic method for selective and quickly reversible silencing of Mammalian neurons. J Neurosci. 2002;22:5287–90. doi: 10.1523/JNEUROSCI.22-13-05287.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WC, et al. Primitive roles for inhibitory interneurons in developing frog spinal cord. J Neurosci. 2004;24:5840–8. doi: 10.1523/JNEUROSCI.1633-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. Fast noninvasive activation and inhibition of neural and network activity by vertebrate rhodopsin and green algae channelrhodopsin. Proc Natl Acad Sci U S A. 2005;102:17816–21. doi: 10.1073/pnas.0509030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu KS, Fetcho JR. Laser ablations reveal functional relationships of segmental hindbrain neurons in zebrafish. Neuron. 1999;23:325–335. doi: 10.1016/s0896-6273(00)80783-7. [DOI] [PubMed] [Google Scholar]
- Liu KS, et al. Mutations in deadly seven/notch1a reveal developmental plasticity in the escape response circuit. J Neurosci. 2003;23:8159–66. doi: 10.1523/JNEUROSCI.23-22-08159.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorent K, et al. The zebrafish space cadet gene controls axonal pathfinding of neurons that modulate fast turning movements. Development. 2001;128:2131–42. doi: 10.1242/dev.128.11.2131. [DOI] [PubMed] [Google Scholar]
- Luna VM, et al. Persistent electrical coupling and locomotory dysfunction in the zebrafish mutant shocked. J Neurophysiol. 2004;92:2003–9. doi: 10.1152/jn.00454.2004. [DOI] [PubMed] [Google Scholar]
- Ma PM. Catecholaminergic systems in the zebrafish. IV. Organization and projection pattern of dopaminergic neurons in the diencephalon. J Comp Neurol. 2003;460:13–37. doi: 10.1002/cne.10544. [DOI] [PubMed] [Google Scholar]
- Mank M, et al. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys J. 2006;90:1790–6. doi: 10.1529/biophysj.105.073536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masino MA, Fetcho JR. Fictive swimming motor patterns in wild type and mutant larval zebrafish. J Neurophysiol. 2005;93:3177–88. doi: 10.1152/jn.01248.2004. [DOI] [PubMed] [Google Scholar]
- Masino MA, et al. Identification of an intersegmental interneuron that may drive slow swimming movements in larval zebrafish. Soc Neurosci Abst. 2005:31. [Google Scholar]
- McLean DL, et al. A topographic map of recruitment in spinal cord. Nature. 2007;446:71–5. doi: 10.1038/nature05588. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Ontogeny and innervation patterns of dopaminergic, noradrenergic, and serotonergic neurons in larval zebrafish. J Comp Neurol. 2004a;480:38–56. doi: 10.1002/cne.20280. [DOI] [PubMed] [Google Scholar]
- McLean DL, Fetcho JR. Relationship of tyrosine hydroxylase and serotonin immunoreactivity to sensorimotor circuitry in larval zebrafish. J Comp Neurol. 2004b;480:57–71. doi: 10.1002/cne.20281. [DOI] [PubMed] [Google Scholar]
- Metcalfe WK, et al. Segmental homologies among reticulospinal neurons in the hindbrain of the zebrafish larva. J Comp Neurol. 1986;251:147–159. doi: 10.1002/cne.902510202. [DOI] [PubMed] [Google Scholar]
- Miyawaki A, et al. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin [see comments] Nature. 1997;388:882–7. doi: 10.1038/42264. [DOI] [PubMed] [Google Scholar]
- Muller UK, van Leeuwen JL. Swimming of larval zebrafish: ontogeny of body waves and implications for locomotory development. J Exp Biol. 2004;207:853–68. doi: 10.1242/jeb.00821. [DOI] [PubMed] [Google Scholar]
- Nagai T, et al. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci U S A. 2004;101:10554–9. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan MJ, et al. Real-time imaging of neurons retrogradely and anterogradely labelled with calcium-sensitive dyes. Journal of Neuroscience Methods. 1993;46:91–106. doi: 10.1016/0165-0270(93)90145-h. [DOI] [PubMed] [Google Scholar]
- O’Malley DM, et al. Imaging the functional organization of zebrafish hindbrain segments during escape behaviors. Neuron. 1996;17:1145–1155. doi: 10.1016/s0896-6273(00)80246-9. [DOI] [PubMed] [Google Scholar]
- Ohno K, et al. Rapsyn mutations in humans cause endplate acetylcholine-receptor deficiency and myasthenic syndrome. Am J Hum Genet. 2002;70:875–85. doi: 10.1086/339465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, et al. Paralytic zebrafish lacking acetylcholine receptors fail to localize rapsyn clusters to the synapse. Journal of Neuroscience. 2001;21:5439–48. doi: 10.1523/JNEUROSCI.21-15-05439.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono F, et al. The Zebrafish motility mutant twitch once reveals new roles for rapsyn in synaptic function. J Neurosci. 2002;22:6491–8. doi: 10.1523/JNEUROSCI.22-15-06491.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pologruto TA, et al. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci. 2004;24:9572–9. doi: 10.1523/JNEUROSCI.2854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff DF, et al. In vivo performance of genetically encoded indicators of neural activity in flies. J Neurosci. 2005;25:4766–78. doi: 10.1523/JNEUROSCI.4900-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rink E, Guo S. The too few mutant selectively affects subgroups of monoaminergic neurons in the zebrafish forebrain. Neuroscience. 2004;127:147–54. doi: 10.1016/j.neuroscience.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Ritter DA, et al. In vivo imaging of zebrafish reveals differences in the spinal networks for escape and swimming movements. J Neurosci. 2001;21:8956–8965. doi: 10.1523/JNEUROSCI.21-22-08956.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. Early functional organization of spinal neurons in developing lower vertebrates. Brain Res Bull. 2000;53:585–93. doi: 10.1016/s0361-9230(00)00392-0. [DOI] [PubMed] [Google Scholar]
- Roberts A, et al. Central circuits controlling locomotion in young frog tadpoles. Ann N Y Acad Sci. 1998;860:19–34. doi: 10.1111/j.1749-6632.1998.tb09036.x. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Time course of the development of motor behaviors in the zebrafish embryo. J Neurobiol. 1998;37:622–32. doi: 10.1002/(sici)1097-4695(199812)37:4<622::aid-neu10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Saint-Amant L, Drapeau P. Synchronization of an embryonic network of identified spinal interneurons solely by electrical coupling. Neuron. 2001;31:1035–46. doi: 10.1016/s0896-6273(01)00416-0. [DOI] [PubMed] [Google Scholar]
- Sapir T, et al. Pax6 and engrailed 1 regulate two distinct aspects of renshaw cell development. J Neurosci. 2004;24:1255–64. doi: 10.1523/JNEUROSCI.3187-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saueressig H, et al. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126:4201–12. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- Schneider VA, Granato M. The myotomal diwanka (lh3) glycosyltransferase and type XVIII collagen are critical for motor growth cone migration. Neuron. 2006;50:683–95. doi: 10.1016/j.neuron.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Schredelseker J, et al. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102:17219–24. doi: 10.1073/pnas.0508710102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepich DS, et al. An altered intron inhibits synthesis of the acetylcholine receptor alpha-subunit in the paralyzed zebrafish mutant nic1. Genetics. 1998;148:361–72. doi: 10.1093/genetics/148.1.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szobota S, et al. Remote control of neuronal activity with a light-gated glutamate receptor. Neuron. 2007;54:535–45. doi: 10.1016/j.neuron.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Tallini YN, et al. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A. 2006;103:4753–8. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorsen DH, et al. Swimming of larval zebrafish: fin-axis coordination and implications for function and neural control. J Exp Biol. 2004;207:4175–83. doi: 10.1242/jeb.01285. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, et al. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–64. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. Characterization and expression of serotonin transporter genes in zebrafish. Tohoku J Exp Med. 2006;208:267–74. doi: 10.1620/tjem.208.267. [DOI] [PubMed] [Google Scholar]
- Zhang F, et al. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–9. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- Zhang J, et al. Zebrafish unplugged reveals a role for muscle-specific kinase homologs in axonal pathway choice. Nat Neurosci. 2004;7:1303–9. doi: 10.1038/nn1350. [DOI] [PubMed] [Google Scholar]
- Zhou W, et al. Non-sense mutations in the dihydropyridine receptor beta1 gene, CACNB1, paralyze zebrafish relaxed mutants. Cell Calcium. 2006;39:227–36. doi: 10.1016/j.ceca.2005.10.015. [DOI] [PubMed] [Google Scholar]