Summary
The discovery that dendrites of neurons in the mammalian brain possess the capacity for protein synthesis [1] stimulated interest in the potential role of local, postsynaptic protein synthesis in learning-related synaptic plasticity [2]. But it remains unclear how local, postsynaptic protein synthesis actually mediates learning and memory in mammals. Accordingly, we examined whether learning in an invertebrate, the marine snail Aplysia, involves local, postsynaptic protein synthesis. Previously, we showed that dishabituation and sensitization of the defensive withdrawal reflex in Aplysia [3, 4] require elevated postsynaptic Ca2+, postsynaptic exocytosis, and functional upregulation of postsynaptic α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors [5]. Here, we tested whether the synaptic facilitation that underlies dishabituation and sensitization in Aplysia [6] requires local postsynaptic protein synthesis. We found that the facilitatory transmitter, serotonin (5-HT), enhanced the response of the motor neuron to glutamate, the sensory neuron transmitter, and this enhancement depended on rapid protein synthesis. Using individual motor neurites surgically isolated from their cell bodies, we showed that the 5-HT-dependent protein synthesis occurred locally. Finally, by blocking postsynaptic protein synthesis, we disrupted facilitation of the sensorimotor synapse. By demonstrating its critical role in a synaptic change that underlies learning and memory in a major model invertebrate system, our study suggests that local, postsynaptic protein synthesis is of fundamental importance to the cell biology of learning.
Results
To examine whether the postsynaptic facilitatory processes that mediate, in part, dishabituation and sensitization in Aplysia [5, 7] involve local protein synthesis, we used sensory and motor neurons individually dissociated from the CNS and placed into cell culture [7-9]. This permitted us to specifically isolate the contribution of local, postsynaptic protein synthesis to synaptic facilitation.
Enhancement of the Glutamate Response in Motor Neurons by 5-HT Depends on Rapid Protein Synthesis
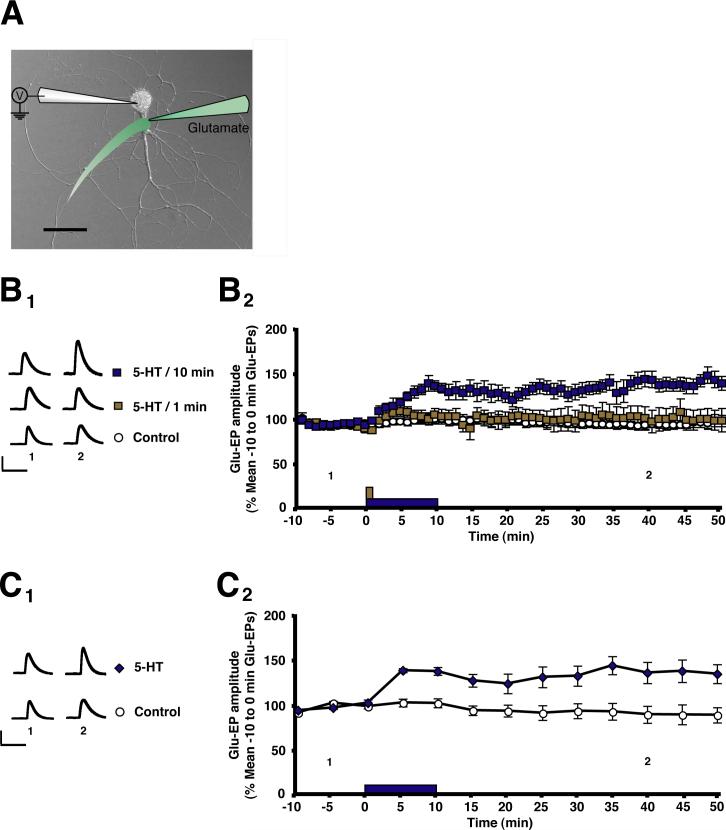
The presynaptic transmitter at the sensorimotor synapse of Aplysia appears to be glutamate [10, 11] (but see [12]). Serotonin (5-HT), an endogenous facilitatory transmitter that mediates dishabituation and sensitization of the withdrawal reflex [13-15], enhances the AMPA receptor-mediated component of the response of isolated LFS [16] siphon motor neurons in cell culture to brief applications (puffs) of glutamate (2 mM in perfusion medium with 0.02% Fast green). The glutamate was ejected from a micropipette onto the initial segment of the motor neurons major neurite via a Picospritzer, and the evoked potential (Glu-EP) was recorded with an intracellular microelectrode (Figure 1A). We first tested whether 5-HT-dependent enhancement of the evoked glutamate response (Glu-EP) in isolated motor neurons requires protein synthesis. In our initial experiments we stimulated the motor neuron with glutamate once every 10 s. A 10-min application of 5-HT produced enhancement of the Glu-EP that persisted for > 40 min (Figure 1B) (see also [7]). By contrast, the enhancement of the Glu-EP produced by a 1-min application of 5-HT was short-lived, lasting < 5 min after 5-HT washout. Furthermore, the persistence of the enhancement of the Glu-EP induced by a 10-min application of 5-HT did not depend on the once per 10-s glutamate stimulation rate. When glutamate was delivered using a much more spaced stimulation protocol (once every 5 min), a 10-min 5-HT treatment also induced prolonged enhancement of the Glu-EP (Figure 1C).
Figure 1. 10 Minutes, But Not 1 Minute, of 5-HT Stimulation Causes Prolonged Enhancement of the Glutamate-Evoked Response in Motor Neurons.
(A) Composite micrograph and cartoon depicting the experimental arrangement. The cell cultures consisted exclusively of isolated small siphon (LFS) motor neurons [16]. Pulses of glutamate were pressure-ejected from a micropipette (right) onto the initial segment of the major neurite of the motor neuron once every 10 s (except for the experiment shown in C). Fast Green was used to visualize the glutamate. The evoked glutamate potentials (Glu-EPs) were recorded from the motor neuron's cell body using a sharp microelectrode (left). See Experimental Procedures for additional information. Scale bar, 50 μm. (B1) Sample Glu-EPs. Each pair of traces shows responses from one experiment. Traces marked “1” represent sample Glu-EPss evoked at the 5-min time point from the experiments summarized in B2; those marked “2” represent Glu-EPs evoked at the 40-min time point. Scale bars, 10 mV and 500 ms. (B2) Comparison of the effects of 10 min (blue bar) and 1 min (brown bar) of 5-HT stimulation. Each symbol in the graph represents the mean normalized amplitude of 6 consecutive Glu-EPs. Motor neurons received 10 min of 5-HT (n = 9), 1 min of 5-HT (n = 10), or perfusion medium alone (n = 10; Control group). The Glu-EP values were log-transformed in order to perform a parametric one-way ANOVA on the data for the 40-min time point. The ANOVA indicated that the group differences were highly significant (F[2,26] = 7.77, p < 0.003). The mean Glu-EP in the 10-min 5-HT treatment group at the 40-min time point (147 ± 8%) was significantly larger than that at the same time point in the 1-min 5-HT treatment group (110 ± 11%), as well as in the group that received the Control treatment (100 ± 4%; post-hoc tests, p < 0.01 for each comparison). The difference between the 1-min 5-HT and the Control groups was not significant (p > 0.05). The numbers below the data indicate the times at which the sample Glu-EPs shown in B1 were recorded. Error bars represent SEM. (C) 5-HT-Dependent Enhancement of the Glutamate Response Can Be Elicited at Low Rates of Test Stimulation (C1) Sample Glu-EPs from experiments in which glutamate was applied to the motor neuron once every 5 min. Otherwise, the procedures were identical to those used in the experiments in which the motor neuron was stimulated with glutamate every 10 s. See the legends for A and B1 for additional details. Scale bars, 10 mV and 500 ms. ( C2) Effect of 5-HT when the motor neuron was stimulated with glutamate at a low rate. Each symbol represents the mean normalized amplitude of 6 Glu-EPs. Values were normalized to the Glu-EP recorded immediately before 5-HT application (t = 0 min). Either 5-HT (n = 7) or normal perfusion medium (n = 6, Control group) was applied for 10 min. The Glu-EP in the 5-HT-treated group at the 40-min time point (140 ± 10%) was significantly larger than the Control Glu-EP at 40 min (88 ± 9%; p < 0.004, unpaired t-test). The numbers below the data indicate the times at which the sample Glu-EPs shown in C1 were recorded. Error bars represent SEM.
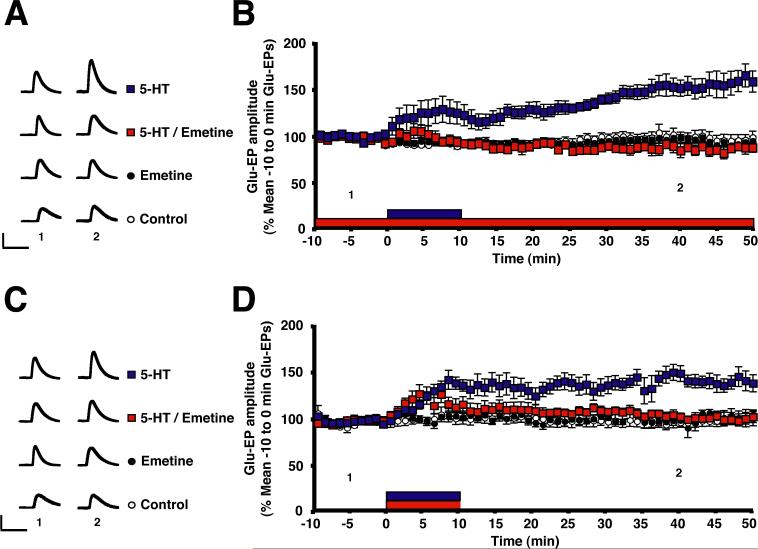
To determine whether the 5-HT-dependent enhancement of the Glu-EP requires protein synthesis, we used the irreversible cell membrane-permeant protein synthesis inhibitor emetine. The inhibitor (1−3 μM) was bath applied 20 min before the onset of 5-HT, and remained in the bath throughout the experiment. Emetine abolished the 5-HT-dependent enhancement (Figures 2A and 2B), but did not depress the baseline Glu-EP in the motor neuron. This result suggests that 5-HT triggers rapid de novo protein synthesis in the isolated motor neuron. But because the protein synthesis inhibitor was present for 20 min before the onset of 5-HT, it is possible that the effect of the inhibitor, rather than being due to disruption of de novo protein synthesis, was due instead to elimination of proteins whose presence is required in the cell for facilitation. The elimination of such essential proteins by emetine might occur prior to the onset of 5-HT if the proteins turn over rapidly. To test this possibility, we performed experiments in which emetine and 5-HT were added coincidentally. In these experiments emetine also blocked 5-HT-dependent enhancement of the Glu-EP, although the disruptive effect of the protein synthesis inhibitor was delayed (Figures 2C and 2D). One-way ANOVAs on the data for each of the time points revealed that the difference between the 5-HT group and 5-HT/Emetine group first became significant at t = 13 min (see Figure 2D for details). These results suggest that enhancement of the Glu-EP depends on protein synthesis within 3 min after washout of 5-HT.
Figure 2. Rapid Protein Synthesis Is Required for Enhancement of the Glutamate Response in Motor Neurons.
(A) Sample Glu-EPs recorded at the 5-min (“1”) and 40-min (“2”) time points in one experiment from each group. The motor neuron was stimulated with glutamate once every 10 s. See the legends, Figures 1A and 1B1, for details. (B) Effect of starting emetine treatment prior to 5-HT treatment. Neurons received 5-HT (blue bar, n = 6), 5-HT in the presence of emetine (red bar, n = 6), emetine alone (n = 6), or neither 5-HT nor emetine (n = 6, Control group). The group differences for the 40-min time point were significant [F[3,20] = 20.41, p < 0.0001). The mean Glu-EP in the 5-HT group (152 ± 9%) was greater than that in the 5-HT/Emetine (87 ± 6%), the Emetine alone (98 ± 4%), and the Control (99 ± 6%) groups (p < 0.001 for each comparison). There were no significant differences among the 5-HT/Emetine, Emetine alone, and Control groups at 40 min (p > 0.05). The numbers below the data indicate the times at which the sample Glu-EPs shown in (A) were recorded. Error bars represent SEM. (C) Sample Glu-EPs for the experiments shown in (D). Refer to (A). (D) Effect of simultaneous 5-HT and emetine treatment. Refer to (B). We wished to perform one-way ANOVAs on each time point in this experiment. Therefore, we first performed a two-way ANOVA on the overall data. The two-way ANOVA indicated that the differences among the experimental groups were highly significant (F[3,23] = 11.61, p < 0.0001. There was also a significant interaction between experimental treatment and time (F[3,23] = 3.44, p < 0.0001). The overall mean Glu-EP for the 5-HT group (131 ± 1%, n = 8) was significantly greater than that in the 5-HT/Emetine (109 ± 1%, n = 7), the Emetine alone (102 ± 1%, n = 6), and Control (101 ± 1%, n = 6) groups (p < 0.05 for each comparison). Furthermore, the overall mean Glu-EP in the 5-HT/Emetine group was significantly greater than that in both the Emetine alone and Control groups (p < 0.05 for each comparison). Overall, the difference between the means in the Emetine alone and Control groups was not significant (p > 0.05). A one-way ANOVA on the 40-min data indicated that the group differences were highly significant (F[3,23] = 14.33, p < 0.0001). Post-hoc tests indicated that the Glu-EP in the 5-HT group (147 ± 8%) at this time was significantly greater than that in the 5-HT/Emetine group (105 ± 4%), as well as the Emetine group (100 ± 6%) and Control group (99 ± 6%; p < 0.0001 for each comparison). The differences among the latter three groups were not significant at 40-min (p > 0.05). To determine the earliest time at which the inhibition of protein synthesis disrupted 5-HT-dependent enhancement of the Glu-EP, we performed one-way ANOVAs on the data for the other time points. The time at which the difference between the amplitude of the Glu-EP in the 5-HT group and that in the 5-HT/Emetine group first became significant was t = 13 min (136 ± 9% vs. 110 ± 9%, p < 0.05).
To test whether emetine's blockade of the enhancement of the glutamate response was due to a nonspecific action of the drug, we also examined the effect of a cell membrane-permeant, but reversible, inhibitor of protein synthesis, cycloheximide. In these experiments the drug (50 μM) was washed into the culture dish 10 min before the start of testing, and then washed out with the 5-HT. Cycloheximide also blocked 5-HT's enhancement of the Glu-EP (Figure S1 in the Supplementary Data available online).
Enhancement of the Glutamate Response in the Surgically Isolated Motor Neurite Also Depends on Protein Synthesis
The speed with which inhibiting protein synthesis disrupted the 5-HT-dependent enhancement of the motor neuron neuron's response to glutamate is consistent with the idea that 5-HT causes rapid synthesis of proteins within the processes of the motor neuron, rather than in the cell body. To further examine this idea we performed experiments on isolated neurites of the identified giant motor neuron L7, which innervates the gill and mantle shelf [17]. The L7 cell was dissociated from the abdominal ganglion and placed into cell culture alone (Figure 3A1). After 24 hr the main neurite of the L7 cell was transected and the cell body removed (Figures 3A1 and 3A2). (Note that neurites of invertebrate neurons can survive the loss of their cell bodies and continue to function normally for days-to-weeks [18, 19].) 24−48 hr after isolating the neurite of the L7 cell from its cell body, we impaled the neurite with a micropipette and stimulated it with glutamate (Figure 3A3). As was the case for the isolated siphon motor neuron (Figure 1), a 10-min application of 5-HT produced prolonged enhancement of the Glu-EP, which was blocked with emetine (2.5 μM) present in the culture dish (Figures 3B and 3C). Therefore, the motor neurite by itself can support persistent enhancement of the Glu-EP; moreover, the enhancement depends on local protein synthesis.
Figure 3. Emetine Blocks Enhancement of the Glutamate-Evoked Response in a Surgically Isolated Motor Neurite.
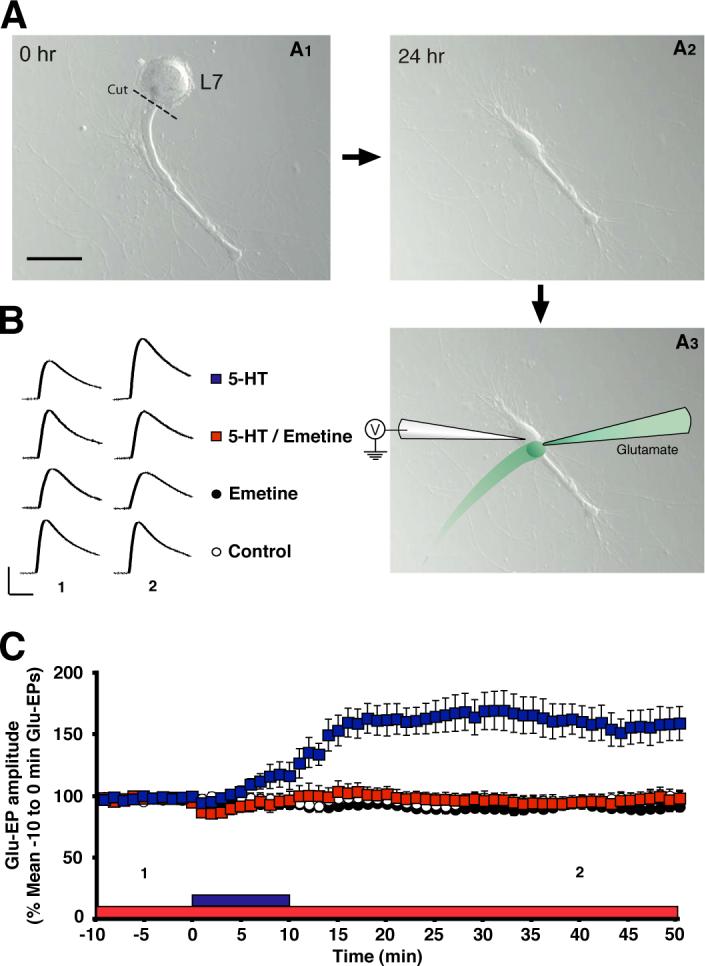
(A1) A gill motor neuron L7 isolated in cell culture prior to soma removal. The dashed line shows where the main neurite was severed. Scale bar, 100 μm. (A2) The neurite was allowed to recover 24−48 hr. after severing. Note the swelling of the neurite proximal to the former site of the cell body. (A3) The neurite was impaled with a sharp electrode. The stimulation and recording methods were like those used in the experiments that used the LFS motor neurons with their cell bodies (Figures 1 and 2). Glutamate was applied to the isolated neurite every 10 s. See Figure 1B1 and the Experimental Procedures for recording and stimulation methods. (B) Sample Glu-EPs recorded at the 5-min (“1”) and 40-min (“2”) time points in one experiment from each group. Scale bars, 10 mV and 500 ms. (C) Effect of inhibiting protein synthesis on enhancement of the glutamate response in the neurite. The Glu-EP values were log-transformed, and one-way ANOVA was performed on data for the 40-min time point. The differences among the four groups at this time were highly significant (F[3,25] = 15.45, p < 0.0001). The mean Glu-EP in the 5-HT group (159 ± 13%, n = 8) was significantly greater than that in the 5-HT/Emetine (98 ± 5%, n = 7), as well as in the Emetine alone (95 ± 2%, n = 7) and Control (99 ± 5%, n = 7) groups (p < 0.001 for each comparison). There were no other significant differences among the groups. The numbers below the data indicate the times at which the sample Glu-EPs shown in (B) were recorded. Error bars represent SEM.
Synaptic Facilitation Requires Postsynaptic Protein Synthesis
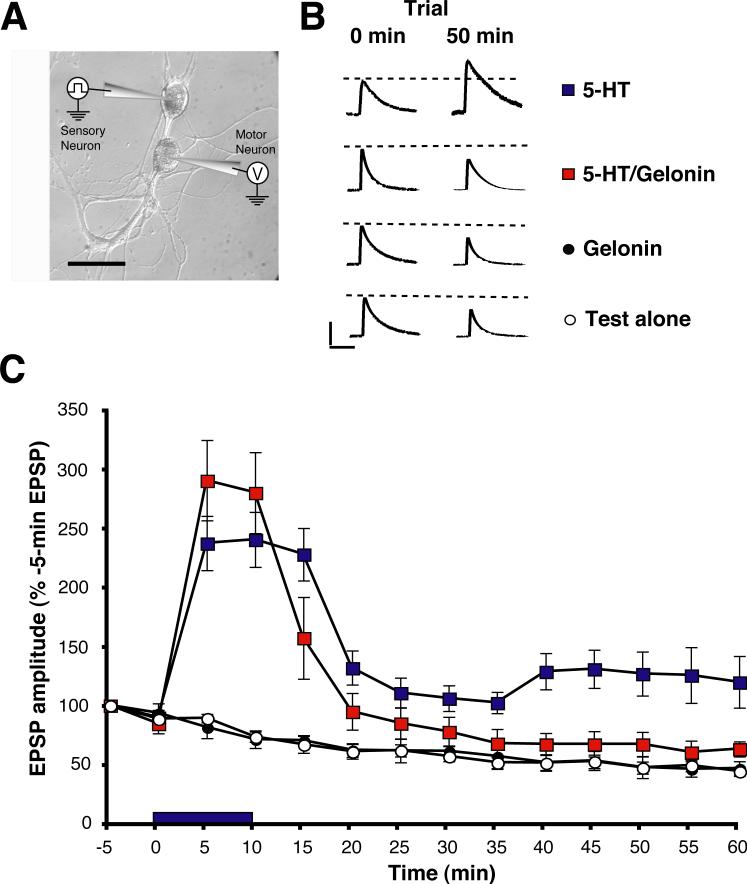
We next asked whether local postsynaptic protein synthesis plays a role in the synaptic facilitation that mediates dishabituation and sensitization of the withdrawal reflex. To address this question, we used cultured synapses between a single sensory neuron and a single LFS siphon motor neuron [5, 20] (Figure 4A). The sensory neuron and motor neuron were each impaled with microelectrodes. The sensory neuron was stimulated every 5 min, and the excitatory postsynaptic potential (EPSP) recorded in the motor neuron. In some experiments 5-HT was added to the perfusion medium after the second test stimulus. In other experiments no 5-HT was added; these experiments (“Test alone”) were included to control for the significant homosynaptic depression exhibited by the sensorimotor synapse in response to low frequency stimulation of the sensory neuron [21]. As reported recently [5], the 5-HT treatment produced facilitation of the EPSP that lasted for ≥ 50 min after washout of the drug. We examined whether this prolonged facilitation requires postsynaptic protein synthesis by injecting the cell membrane-impermeant inhibitor gelonin [22, 23] into the motor neuron prior to testing. Postsynaptic gelonin significantly reduced the facilitation after washout of 5-HT. Interestingly, the protein synthesis inhibitor did not affect facilitation while the drug was present in the bath, consistent with the idea that short-term facilitation does not require postsynaptic protein synthesis [24-26] (but see below). To determine the earliest time at which the postsynaptic gelonin adversely affected facilitation, we performed a one-way ANOVA on each test point in the experiment. The results indicated that the difference between the 5-HT and 5-HT/emetine groups was significant by the 20-min test (see Figure 4 for details). Furthermore, the 20-min test was also the earliest point at which the differences between the 5-HT/Emetine and the Emetine alone groups, as well as between the 5-HT/Emetine and Test alone groups, were insignificant. Therefore, inhibition of protein synthesis impaired synaptic facilitation by 10 min after 5-HT washout.
Figure 4. Postsynaptic Inhibition of Protein Synthesis Blocks Persistent Synaptic Facilitation.
(A) Experimental arrangement. Scale bar, 50 μm. (B) Representative EPSPs. Trial times correspond to those shown in the graph in (C). (C) Mean normalized amplitude of the EPSPs. The EPSP values were long-transformed prior to performing a two-way ANOVA on the overall data. The two-way ANOVA indicated that the differences among the experimental groups (F[3,22] = 24.76, p < 0.0001). There was also a significant interaction between experimental treatment and time (F[3,22] = 11.22, p < 0.0001). SNK post-hoc tests showed that the overall mean EPSP in the 5-HT group (144 ± 7%, n = 7) was significantly greater than that in the 5-HT/Gelonin (112 ± 10%, n = 6), the Gelonin alone (62 ± 2%, and the Test alone (61 ± 2%, n = 6) groups (p < 0.05 for each comparison). There was also a significant difference between the 5-HT/Gelonin and the Gelonin alone groups, and between the 5-HT/Gelonin and the Test alone groups (p < 0.05 for each comparison). There was no significant overall difference between the Gelonin alone and Test alone groups (p > 0.05). One-way ANOVAs were performed on every time point for the experiment. These revealed that the difference between the 5-HT and 5-HT/Gelonin EPSPs became significant at t = 20 (132 ± 15% vs. 95 ± 16%, p < 0.05). Moreover, the differences between the 5-HT/Gelonin EPSP and Gelonin alone EPSP (61 ± 6%), as well as between 5-HT/Gelonin EPSP and the Test alone EPSP (62 ± 4%), were not significant at t = 20 (p > 0.05 for each comparison). The numbers below the data indicate the times at which the sample EPSPs in (B) were recorded. Error bars represent SEM.
Discussion
Clusters of ribosomes have been found in the postsynaptic region of the giant synapse of a cephalopod mollusk, the squid [27]; this finding indicates that local, postsynaptic translation can occur in at least some invertebrate synapses, and provides additional support for the present results. Our data agree with those from studies in mammals, which point to a critical role for local postsynaptic protein synthesis in learning-related synaptic plasticity, including long-term potentiation (LTP) and long-term depression (LTD) (reviewed in [28]), as well as in certain forms of learning [29]. Particularly intriguing in light of our results are recent data showing that 15 min of stimulation with the monamine dopamine triggers synthesis of the GluR1 subunit of AMPA receptors in dendrites of hippocampal neurons in culture [30]. We have found that 5-HT produces upregulation of the functional expression of AMPA receptors in Aplysia motor neurons [5, 7]. The idea that 5-HT treatment triggers rapid synthesis of AMPA receptors in motor neurites is therefore attractive [31]. At present, however, we do not know the identity of the local postsynaptic protein(s) whose synthesis is stimulated by 5-HT.
The persistent in vitro synaptic facilitation produced in the present study by 10 min of 5-HT treatment (Figure 4) may represent a type of intermediate-term facilitation (ITF), which has been previously described for the sensorimotor synapse [24, 26]. This form of synaptic plasticity lasts more than 30 min, but less than 3 hr, and requires protein synthesis, but not gene transcription [24, 25]. A role for postsynaptic protein synthesis in ITF has not been shown previously. Our results suggest that at least some forms of ITF involve local protein synthesis in the motor neuron.
The rapidity of the requirement for postsynaptic protein synthesis in the enhancement of the glutamate response (Figures 2 and 3) and in synaptic facilitation (Figure 4) in the present study is unprecedented in studies of learning-related plasticity in Aplysia. Our results indicate that the onset of de novo local protein synthesis recruited in the motor neuron by a 10-min application of 5-HT is not later than 10 min after washout of the drug. Because this estimate includes the time required for the protein synthesis inhibitor to cross the cell membrane and reach critical sites for protein synthesis within the neurites of the motor neuron, 5-HT may actually trigger de novo synthesis of proteins in motor neurites significantly earlier. Previous results indicate that anisomycin, a cell membrane-permeant inhibitor of protein synthesis, can produce ∼ 90% inhibition of leucine incorporation in the abdominal ganglion of Aplysia within 15 min [32]. Furthermore, injection of emetine into intact Aplysia inhibits protein synthesis, as measured by leucine incorporation, within central ganglia by > 90% within 30 min [33]. These results provide support for the present study by indicating that inhibitors of protein synthesis can significantly reduce amino acid incorporation in the CNS of Aplysia within minutes. Although unprecedented in studies of Aplysia, a requirement for rapid protein synthesis has been documented in studies of synaptic plasticity in the mammalian brain [e.g., 34, 35, 36].
Short-term facilitation (STF) in Aplysia is generally thought to last for < 30 min and not to require protein synthesis [37-40] [but see 24]. The present results challenge this common view. We observed that, although inhibition of postsynaptic protein synthesis did not affect synaptic facilitation while 5-HT was present in the bath, it quickly (within 10 min) impaired facilitation after washout of 5-HT (Figure 4C). Interestingly, Ghirardi et al. [24] found that treatment with 5 spaced pulses of very low concentrations of 5-HT (1−10 nM, compared to the 10−20 μM concentration used in the present experiments) produced facilitation of sensorimotor synapses in culture that persisted for 0.5 hr after the 5-HT treatment and did not depend on protein synthesis. Because the 5-HT treatment period lasted 1.5 hr, this means that very low doses of 5-HT can yield persistent facilitation in the absence of protein synthesis. Due to its lack of dependence on protein synthesis, Ghirardi et al. referred to such facilitation as STF, rather than ITF, despite its duration. If a requirement for protein synthesis and the lack of a requirement for gene transcription are considered to be the defining properties of ITF, then our results indicate that 10 min of 10−20 μM 5-HT can produce ITF whose onset is ≤ 10 min after washout of 5-HT.
In summary, this study, together with our earlier study [5], present compelling evidence that dishabituation and sensitization of the withdrawal reflex in Aplysia depend on rapid, local synthesis of proteins in the postsynaptic motor neuron. Our data therefore provide strong support for the idea that local, postsynaptic protein synthesis is an important general mechanism in learning and memory.
Experimental Procedures
Cell Cultures
Small siphon (LFS) [16] motor neurons were individually dissociated from abdominal ganglia of adult Aplysia californica (80−120 g) and isolated in cell culture [7]. The cell cultures used in the glutamate puff experiments consisted exclusively of isolated motor neurons or motor neurites. Experiments were performed on the neurons 3−5 d after they were placed into culture. In experiments on isolated motor neurites, the large identified motor neuron, L7, was dissociated from juvenile animals (1−4 g) and placed into cell culture. After 24 h the cell body of the L7 neuron was removed. The L7 neurites were maintained in culture for 24−48 h, after which the experiments were performed. Sensorimotor cocultures, each comprising a single pleural sensory neuron and a single LFS motor neuron, were made and experiments performed on them as described previously [5].
Electrophysiology
The electrophysiological methods used in the experiments on isolated motor neurons and sensorimotor cocultures were identical to those used in earlier studies [5, 7]. Briefly, cultures were perfused with a solution consisting of 50% L-15/50% artificial seawater (ASW) during electrophysiological recording, which was performed at room temperature. For experiments on isolated motor neurons, the cell body—or, in the case of the experiments on isolated neurites, the stump of the severed major neurite—was impaled with a sharp microelectrode (20−30 MΩ) and held at ∼ −85 mV (−0.2 to −0.6 nA holding current) throughout the experiment. Glutamate (2 mM in perfusion medium with 0.02% Fast green) was pressure-ejected from a micropipette onto the initial segment of the motor neuron's major neurite or the proximal stump of the L7 neurite. The duration and pressure of the ejection pulses used to deliver glutamate were adjusted at the start of each experiment to evoke an initial response of (5−30 mV) in isolated neurites, and 5−15 mV in isolated motor neurons; thereafter, the ejection pulses were held constant throughout the experiment. The glutamate was washed out immediately after application via a rapid perfusion system.
For experiments on in vitro sensorimotor synapses, the presence of a chemical synaptic connection was first ascertained by injecting a brief pulse of positive current (40 ms, 0.2−0.8 nA) into the sensory neuron, and recording the resulting EPSP, if any. Given that the initial test pulse elicited an EPSP, gelonin was injected into some of the motor neurons via the recording electrode using air pressure (10 ms pulses, 1−5 psi; Pico-Spritzer II, Parker Hannifin, Fairfield, NJ). The injection solution consisted of 25 μM gelonin, 0.5 M potassium acetate, 10 mM Tris-HCl (pH = 7.5), and 0.2% fast green to visualize the injection. In additional control experiments, the vehicle solution was pressure injected into the motor neuron. The synapses were then rested for 30−45 min. Afterwards the synapses were stimulated once every 5 min throughout the experiments.
5-HT was prepared fresh daily as a 2 mM stock solution dissolved in ASW. The 5-HT was diluted to a final concentration of 10−20 μM just before an experiment in the perfusion medium and applied to the cultures for 10 min, after which it was rapidly washed out with normal perfusion medium. Cycloheximide was dissolved in 0.1% DMSO. Emetine was simply dissolved in perfusion medium. Emetine or cycloheximide was added to the bath 20 min before the start of testing, unless otherwise indicated. All drugs were from Sigma (St. Louis, MO), except for gelonin (Aczon, Bologna, Italy). l
Statistical Analyses
For the experiments on isolated motor neurons/neurites, the peak amplitudes of the evoked glutamate potentials (Glu-EPs) were normalized to the mean amplitude of the 60 Glu-EPs immediately before application of 5-HT, 5-HT/drug, or vehicle—or to the amplitude of the Glu-EP on the 0-min trial in the experiments presented in Figure 1C—and expressed as percent mean ± SEM. Parametric tests were used for all statistical analyses. For the experiments involving either the isolated motor neuron or the isolated neurite group differences were assessed using the 40-min time point. A one-way analysis of variance (ANOVA) was performed on the data for the 40-min time point, followed by Student-Newman-Keuls (SNK) post-hoc tests for pairwise comparisons. (Because there were only two groups in the experiment presented in Figure 1C, the differences at the 40-min time point were assessed with an unpaired t-test.) We wished to determine the earliest time point at which the group differences became significant in the data presented in Figures 2D and 4C. Accordingly, a two-way ANOVA, with time as one factor and treatment as the other, was performed on all of the data; one-way ANOVAs were then performed on the data for each of the time points.
Acknowledgments
We thank P. Legendre, K. Martin, T. O'Dell, S. Otani, and F. Schweizer for their helpful comments on the manuscript; and L. Olson for assistance with the figures. This research was supported by US National Institutes of Health grants (R37 NS029563 and K02 MH067062) to D.L.G., and (F31 NS04556401) to G.V.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material
References
- 1.Steward O, Levy WB. Preferential localization of polyribosomes under the base of dendritic spines in granule cells of the dentate gyrus. J Neurosci. 1982;2:284–291. doi: 10.1523/JNEUROSCI.02-03-00284.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Pinsker H, Kupfermann I, Castellucci V, Kandel E. Habituation and dishabituation of the gill-withdrawal reflex in Aplysia. Science. 1970;167:1740–1742. doi: 10.1126/science.167.3926.1740. [DOI] [PubMed] [Google Scholar]
- 4.Carew TJ, Castellucci VF, Kandel ER. An analysis of dishabituation and sensitization of the gill-withdrawal reflex in Aplysia. Int. J. Neurosci. 1971;2:79–98. doi: 10.3109/00207457109146995. [DOI] [PubMed] [Google Scholar]
- 5.Li Q, Roberts AC, Glanzman DL. Synaptic facilitation and behavioral dishabituation in Aplysia: dependence upon release of Ca2+ from postsynaptic intracellular stores, postsynaptic exocytosis and modulation of postsynaptic AMPA receptor efficacy. J. Neurosci. 2005;25:5623–5637. doi: 10.1523/JNEUROSCI.5305-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Antonov I, Kandel ER, Hawkins RD. The contribution of facilitation of monosynaptic PSPs to dishabituation and sensitization of the Aplysia siphon withdrawal reflex. J. Neurosci. 1999;19:10438–10450. doi: 10.1523/JNEUROSCI.19-23-10438.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chitwood RA, Li Q, Glanzman DL. Serotonin facilitates AMPA-type responses in isolated siphon motor neurons of Aplysia in culture. J. Physiol. 2001;534:501–510. doi: 10.1111/j.1469-7793.2001.00501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayport SG, Schacher S. Synaptic plasticity in vitro: cell culture of identified Aplysia neurons mediating short-term habituation and sensitization. J. Neurosci. 1986;6:759–763. doi: 10.1523/JNEUROSCI.06-03-00759.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glanzman DL, Kandel ER, Schacher S. Identified target motor neuron regulates neurite outgrowth and synapse formation of Aplysia sensory neurons in vitro. Neuron. 1989;3:441–450. doi: 10.1016/0896-6273(89)90203-1. [DOI] [PubMed] [Google Scholar]
- 10.Dale N, Kandel ER. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proc. Natl. Acad. Sci. USA. 1993;90:7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levenson J, Sherry DM, Dryer L, Chin J, Byrne JH, Eskin A. Localization of glutamate and glutamate transporters in the sensory neurons of Aplysia. J Comp Neurol. 2000;423:121–131. doi: 10.1002/1096-9861(20000717)423:1<121::aid-cne10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 12.Trudeau LE, Castellucci VF. Excitatory amino acid neurotransmission at sensory-motor and interneuronal synapses of Aplysia californica. J. Neurophysiol. 1993;70:1221–1230. doi: 10.1152/jn.1993.70.3.1221. [DOI] [PubMed] [Google Scholar]
- 13.Brunelli M, Castellucci V, Kandel ER. Synaptic facilitation and behavioral sensitization in Aplysia: possible role of serotonin and cyclic AMP. Science. 1976;194:1178–1181. doi: 10.1126/science.186870. [DOI] [PubMed] [Google Scholar]
- 14.Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. J Neurosci. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of Aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002;22:2299–2312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. J. Neurobiol. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- 17.Koester J, Kandel ER. Further identification of neurons in the abdominal ganglion of Aplysia using behavioral criteria. Brain Res. 1977;121:1–20. doi: 10.1016/0006-8993(77)90435-8. [DOI] [PubMed] [Google Scholar]
- 18.Bittner GD. Long-term survival of anucleate neurons and its implications for nerve regeneration. Trends Neurosci. 1991;14:188–193. doi: 10.1016/0166-2236(91)90104-3. [DOI] [PubMed] [Google Scholar]
- 19.Schacher S, Wu F. Synapse formation in the absence of cell bodies requires protein synthesis. J Neurosci. 2002;22:1831–1839. doi: 10.1523/JNEUROSCI.22-05-01831.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proc. Biol. Sci. 1994;255:113–118. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- 21.Castellucci VF, Kandel ER. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc. Natl. Acad. Sci. USA. 1974;71:5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stirpe F, Olsnes S, Pihl A. Gelonin, a new inhibitor of protein synthesis, nontoxic to intact cells. Isolation, characterization, and preparation of cytotoxic complexes with concanavalin A. J Biol Chem. 1980;255:6947–6953. [PubMed] [Google Scholar]
- 23.Martin KC, Casadio A, Zhu H, E Y, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 24.Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of Aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 25.Montarolo PG, Goelet P, Castellucci VF, Morgan J, Kandel ER, Schacher S. A critical period for macromolecular synthesis in long-term heterosynaptic facilitation in Aplysia. Science. 1986;234:1249–1254. doi: 10.1126/science.3775383. [DOI] [PubMed] [Google Scholar]
- 26.Sutton MA, Carew TJ. Parallel molecular pathways mediate expression of distinct forms of intermediate-term facilitation at tail sensory-motor synapses in Aplysia. Neuron. 2000;26:219–231. doi: 10.1016/s0896-6273(00)81152-6. [DOI] [PubMed] [Google Scholar]
- 27.Bleher R, Martin R. Ribosomes in the squid giant axon. Neuroscience. 2001;107:527–534. doi: 10.1016/s0306-4522(01)00366-9. [DOI] [PubMed] [Google Scholar]
- 28.Pfeiffer BE, Huber KM. Current advances in local protein synthesis and synaptic plasticity. J Neurosci. 2006;26:7147–7150. doi: 10.1523/JNEUROSCI.1797-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- 30.Smith WB, Starck SR, Roberts RW, Schuman EM. Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron. 2005;45:765–779. doi: 10.1016/j.neuron.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 31.Glanzman DL. Simple minds: the neurobiology of invertebrate learning and memory. In: North G, Greenspan RJ, editors. Invertebrate Neurobiology. Cold Spring Harbor Laboratory Press; New York: 2007. pp. 347–380. [Google Scholar]
- 32.Schwartz JH, Castellucci VF, Kandel ER. Functioning of identified neurons and synapses in abdominal ganglion of Aplysia in absence of protein synthesis. J Neurophysiol. 1971;34:939–953. doi: 10.1152/jn.1971.34.6.939. [DOI] [PubMed] [Google Scholar]
- 33.Levenson J, Byrne JH, Eskin A. Levels of serotonin in the hemolymph of Aplysia are modulated by light/dark cycles and sensitization training. J. Neurosci. 1999;19:8094–8103. doi: 10.1523/JNEUROSCI.19-18-08094.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- 35.Calixto E, Thiels E, Klann E, Barrionuevo G. Early maintenance of hippocampal mossy fiber--long-term potentiation depends on protein and RNA synthesis and presynaptic granule cell integrity. J Neurosci. 2003;23:4842–4849. doi: 10.1523/JNEUROSCI.23-12-04842.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsokas P, Grace EA, Chan P, Ma T, Sealfon SC, Iyengar R, Landau EM, Blitzer RD. Local protein synthesis mediates a rapid increase in dendritic elongation factor 1A after induction of late long-term potentiation. J Neurosci. 2005;25:5833–5843. doi: 10.1523/JNEUROSCI.0599-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory—a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 38.Mauelshagen J, Parker GR, Carew TJ. Dynamics of induction and expression of long-term synaptic facilitation in Aplysia. J. Neurosci. 1996;16:7099–7108. doi: 10.1523/JNEUROSCI.16-22-07099.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kandel ER. The molecular biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–1038. doi: 10.1126/science.1067020. [DOI] [PubMed] [Google Scholar]
- 40.Sutton MA, Masters SE, Bagnall MW, Carew TJ. Molecular mechanisms underlying a unique intermediate phase of memory in Aplysia. Neuron. 2001;31:143–154. doi: 10.1016/s0896-6273(01)00342-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.