Abstract
In addition to DNA polymerase complexes, DNA replication requires the coordinate action of a series of proteins, including regulators Cdc28/Clb and Dbf4/Cdc7 kinases, Orcs, Mcms, Cdc6, Cdc45, and Dpb11. Of these, Dpb11, an essential BRCT repeat protein, has remained particularly enigmatic. The Schizosaccharomyces pombe homolog of DPB11, cut5, has been implicated in the DNA replication checkpoint as has the POL2 gene with which DPB11 genetically interacts. Here we describe a gene, DRC1, isolated as a dosage suppressor of dpb11–1. DRC1 is an essential cell cycle-regulated gene required for DNA replication. We show that both Dpb11 and Drc1 are required for the S-phase checkpoint, including the proper activation of the Rad53 kinase in response to DNA damage and replication blocks. Dpb11 is the second BRCT-repeat protein shown to control Rad53 function, possibly indicating a general function for this class of proteins. DRC1 and DPB11 show synthetic lethality and reciprocal dosage suppression. The Drc1 and Dpb11 proteins physically associate and function together to coordinate DNA replication and the cell cycle.
In response to DNA damage or DNA replication blocks, cells arrest the cell cycle and induce the transcription of genes that facilitate DNA damage repair and DNA replication. The portion of this surveillance pathway that orchestrates cell cycle control is called a checkpoint. Checkpoint pathways ensure that cell cycle events occur at the proper time and in the appropriate order (1). Like other signal transduction pathways, this stress response pathway consists of sensors, transducers, and effectors. In Saccharomyces cerevisiae two kinases, Mec1, a member of the phosphoinositide kinase (PIK) family, and Rad53, a serine/threonine protein kinase, appear to form the central transducers of DNA damage and DNA replication block response and control both cell cycle arrest and transcriptional induction pathways. Rad53 is phosphorylated in a MEC1-dependent manner and leads to further activation of the downstream effectors (2, 3). Less is known about the pathway upstream of Rad53 activation. There appear to be groups of genes involved in transducing particular subsets of signals to Rad53. Those implicated in sensing and transducing the DNA damage signal are RAD9, RAD17, RAD24, MEC3, and DDC1 (1, 4). Rad9 and Ddc1 are phosphorylated in response to DNA damage (5–7). POL2 (8), DPB11 (9), and RFC5 (10) act specifically to prevent mitotic entry when replication is blocked. POL2 encodes the catalytic subunit of the DNA polymerase II (Pol ɛ) complex and is essential for DNA replication (11, 12). A C-terminal “checkpoint” domain unique to the Pol ɛ family is required for checkpoint signaling (8). This C terminus is required for association with three proteins, Dpb2, Dpb3, and Dpb4 (13). DPB11 was isolated as a multicopy suppressor of pol2 and dpb2 mutants, both of which are essential (9). DPB11 is essential for cell proliferation. Thermosensitive dpb11–1 mutants are defective for DNA replication at the restrictive temperature, and cells display inappropriate mitotic entry, resulting in uneven chromosome separation and rapid cell death. These defects mirror the phenotypes of pol2 mutants. This phenotypic similarity, together with their genetic interactions, suggests that DPB11 and POL2 work together in the same pathway to regulate DNA replication and cell cycle progression. RFC5 is part of the replication factor C complex that recruits PCNA and polymerases to primed templates and may be responsible for bringing Pol2 to replication forks. These observations are consistent with the idea that the DNA replication machinery sends signals to prevent mitotic entry.
To further elucidate the pathway involved in initiating DNA replication and coordinating cell cycle phases, we have initiated a study of the DPB11 gene. We further established DPB11’s role in the S-phase checkpoint and discovered that it is a regulator of Rad53. We carried out a dosage-suppressor screen of dpb11–1 mutant and identified a gene, DRC1 (DNA replication and checkpoint protein 1). Through a variety of biochemical and genetic analyses, we have discovered that DRC1 functions together with DBP11 to control DNA synthesis and S-phase checkpoint function.
MATERIALS AND METHODS
Yeast Strains and Plasmids Construction.
Yeast strains used in this study are listed in Table 1. pHW21 and pHW58 were constructed by subcloning the MluI(−109 bp)/BamHI(1,730 bp) fragment of DRC1 into pRS326 and pRS415 (14), respectively. The coding region of DRC1 was amplified by PCR, and a NdeI site was engineered at the start codon and a SalI site was engineered just after the stop codon. This PCR product was cloned into pBAD98 (CEN GAL URA3), resulting in pHW55.
Table 1.
Strains used in this study
| Strain | Genotype | Source |
|---|---|---|
| YHA401 | MATa, ade5-1, leu2-3,112, ura3-52, trp1-289, dpb11-1 | (9) |
| Y791 | YHA401 + YEp195DPB11 | (9) |
| Y792 | YHA401 + pRS326 | This study |
| Y793 | YHA401 + pHW21(pRS326-DRC1) | This study |
| Y300 | MATa, trp1-1, ura3-1, his3-11,15, leu2-3,112, ade2-1, can1-100 | (27) |
| Y794 | MATa, ade2-1, ura3-1, his3-11,15, trp1-1, leu2-3,112, can1-100, ρ° (W303 ρ°) | Ron Butow |
| Y795 | MATa, ade2-1, ura3-1, his3-11,15, trp1-1, leu2-3,112, can1-100, ρ°, drc1-1 | This study |
| Y796 | MATa, trp1-1, ura3-1, his3-11,15, leu2-3,112, ade2-1, can1-100, Δdrc1∷HIS3 + pHW58 (pRS415-DRC1) | This study |
| Y797 | MATa, trp1-1, ura3-1, his3-11,15, leu2-3,112, ade2-1, can1-100, Δdrc1∷HIS3 + pHWW55 (pBAD98-DRC1) | This study |
| Y798 | MATa, trp1-1, ura3-1, his3-11,15, leu2-3,112, ade2-1, can1-100, Δdrc1∷HIS3 + pHW132 (pRS415-drc1-8) | This study |
| Y799 | MATa, trp1-1, ura3-1, his3-11,15, leu2-3,112, ade2-1, can1-100, drc1-1 | This study |
Construction of the Mutagenized drc1 Library.
pHW58 (pRS415-DRC1) was used as template to carry out the random PCR mutagenesis. Primers used in PCR were: Drc1–9, 5′-GTGAGTTACCTCACTCATTAGGC-3′ (located in the vector sequence), and Drc1–10, 5′-CCCCATCGTCCATTAGAGAATC-3′ (located on DRC1). Low-fidelity PCR was carried out by using 10 ng of plasmid DNA, 1 μM of each primer, 3 mM MgCl2, 5 mM MnCl2, 10× Taq buffer, and 2.5 units of Taq polymerase (Fisher) (15). Four reactions were performed in parallel, and in each reaction one of the four nucleotides was used at a concentration five times less than the other three (0.2 mM). The PCR products were combined, purified, and digested by SacII and BamHI, and then cloned into the SacII and BamHI sites of pHW58. Approximately 36,000 Escherichia coli transformants were obtained, of which 99% were estimated to contain the insert.
This library was used to transform Y797, and Leu+ transformants grown at 24°C were replica-plated to 0.1% 5-fluoroorotic acid (5-FOA) plates at 24°C and 37°C to isolate temperature-sensitive (Ts) mutants and to 5-FOA/0.1 M hydroxyurea (HU) plates at 24°C to isolate HU-sensitive mutants. From those Ts and HU-sensitive clones, plasmid DNA were recovered and transformed back to Y797 to confirm the phenotype. The plasmids recovered were: pHW79(drc1–1), pHW81(drc1–2), pHW133(drc1–3), pHW134(drc1–4), pHW135(drc1–5), pHW136(drc1–6), pHW137(drc1–7), and pHW138(drc1–8). The SacII–XhoI fragment from pHW79 was subcloned into pRS406 to make pHW80, which was used to integrate drc1–1 into the chromosome.
Gene Disruption and Integration.
The DRC1 coding region was precisely deleted from the chromosome by transplacement with a PCR product that contains the HIS3 gene surrounded by 50 nt of sequence homologous to the target DRC1 gene on each end (16). Y799 and Y795 were created by transforming Y300 or Y794 with BamHI-cleaved pHW80 (pRS406-drc1–1). Ura+ transformants were grown in yeast extract/peptone/dextrose (YPD) overnight at 24°C and plated on 5-FOA at 24°C. 5-FOAR colonies were replica-plated to 37°C. Those colonies that could grow at 24°C, but not 37°C, were selected and transformed with pHW21(pRS326-DRC1). Clones whose Ts phenotype could be rescued by pHW21 were selected and used for further study.
Fluorescence-Activated Cell Sorter (FACS) Analysis and Microtubule Staining.
All FACS analysis and microtubule staining were performed as described (17).
RESULTS
Examination of the Role of DPB11 in the S-Phase Checkpoint.
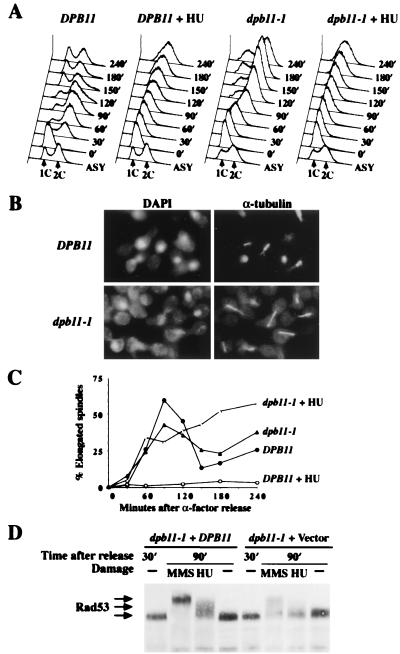
To further elucidate how the DNA replication interference signal is transduced to the central transducers Rad53 and Mec1, we initiated a study of the DNA replication regulator Dpb11. dpb11–1 mutant cells are sensitive to HU, defective in DNA synthesis at the restrictive temperature, and display uneven distribution of genetic material (9). These phenotypes suggest that dpb11–1 mutant cells are defective in the DNA replication checkpoint like mutants in their fission yeast homolog cut5 (18). Although consistent with a checkpoint defect, spindle elongation studies in the absence of completed DNA replication and other indicators of checkpoint function were not analyzed (9) and are required to definitively demonstrate a checkpoint role for DPB11. Therefore, we examined the kinetics of spindle elongation in wild-type and dpb11–1 cells when released from an α-factor-induced G1 arrest into media containing 0.2 M HU. dpb11–1 mutant cells released into 0.2 M HU have less than 2C DNA content (Fig. 1A). However, dpb11–1 cells, but not wild-type cells, showed spindle elongation under these conditions (Fig. 1B). In addition, the kinetics of spindle elongation is the same for dpb11–1 in the presence or absence of HU, indicating dpb11–1 mutant cells are totally defective in cell cycle arrest in response to incomplete DNA replication (Fig. 1C).
Figure 1.
The S-phase checkpoint is deficient in dpb11–1 mutant. (A–C) Wild-type (Y791) and dpb11–1 (Y792) cells were synchronized by α-factor at 24°C. After G1 arrest, cultures were shifted to 36°C for 45 min and divided into two, and equal portions were released from the block into SC-Ura or SC-Ura containing 0.2 M HU. At regular intervals after release, aliquots were withdrawn to examine DNA content, nuclear and spindle morphology. (A) FACS profile shows the DNA content of wild-type and dpb11–1 cells after release from G1 into medium with or without 0.2 M HU. Time indicates minutes after release. (Lower) Asynchronous cells untreated with HU at 24°C, which are included as a reference. (B) Photomicrographs of wild-type and dpb11–1 cells at 120 min after release from α-factor into SC-Ura with 0.2 M HU at 36°C. Nuclear morphology was visualized with 4′,6-diamidino-2-phenylindole, and microtubule morphology was visualized by indirect immunofluorescence using anti-α-tubulin antibody. (C) Kinetics of spindle elongation in the presence or absence of HU. (D) Y791 and Y792 were grown in SC-Ura, synchronized in G1 by α-factor, then prewarmed at 34°C for 1 hr before release at 34°C into media lacking α-factor. At 30 min after release, when most of the cells enter S phase, HU (0.2 M final) or MMS (0.1% final) was added to block DNA replication or damage DNA. An hour later (90 min after release), protein extracts were prepared, fractionated by SDS/PAGE, and immunoblotted with antibodies to Rad53 (2).
We also examined Rad53 regulation in response to replication blocks and damage. To eliminate the effect of cell cycle stage, wild-type and dpb11–1 mutant cells were synchronized in G1 by α-factor, released into synthetic complete (SC)-Ura medium for 30 min, and then treated or untreated with HU or methyl methanesulfonate (MMS) for 60 min before protein extract were prepared for analysis. Rad53 modification in response to MMS and HU treatment was significantly reduced in dpb11–1 mutant cells relative to wild-type cells (Fig. 1D). This result further confirmed that DPB11 is required for the S-phase checkpoint and functions upstream of RAD53 in the DNA replication checkpoint pathway.
Isolation of DRC1.
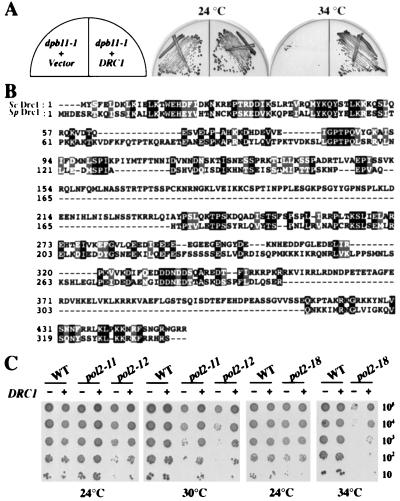
To identify additional proteins in the DPB11 pathway we screened for high-copy suppressors of the temperature sensitivity of dpb11–1. A 2-μ URA3 S. cerevisiae genomic library was transformed into dpb11–1 mutants, and transformants were plated on SC-Ura plates and incubated for 3 days at 34°C, the lowest restrictive temperature for dpb11–1 (Fig. 2A). Of 160,000 transformants, 81 clones were isolated after testing on 5-FOA plates for plasmid dependency. Twenty-eight were found to carry DPB11 by Southern blot analysis, and the remaining 53 clones represented a single ORF, YKL108w, that we named DRC1. By using a frame-shift drc1 mutant, we confirmed that DRC1 is an authentic suppressor. We also observed that DRC1 also suppresses the growth defect of dpb11–1 at 37°C even when present on a CEN plasmid, indicating strong suppression with little overproduction (data not shown).
Figure 2.
(A) High copy of DRC1 rescues the growth defect of dpb11–1. dpb11–1 containing either pRS326 (2 μ URA3) or pHW21(2 μ URA3 DRC1) were struck out on SC-Ura plates and incubated at 24°C and 34°C. (B) Conservation between Drc1 and a related protein from S. pombe. Identities are shown as black boxes and similarities as shaded boxes. (C) High-copy DRC1 suppresses the lethality of pol2 mutants. TC102–2D (POL2), TC102–2–11 (pol2–11), TC102–12 (pol2–12) (19), YHA300 (POL2), and YHA301 (pol2–18) (20) strains were transformed with either pRS326 (−) or pHW21(DRC1) (+). Cultures of those transformants were grown in SC-Ura medium at 24°, then serial dilutions of 105, 104, 103, 102, and 10 cells were spotted onto SC-Ura plates and incubated at the indicated temperatures.
The protein encoded by DRC1 has similarity to a hypothetical protein in Schizosaccharomyces pombe, which we are referring to as S. pombe Drc1. The S. pombe protein shows 25% identity and 40% similarity with Drc1, although Drc1 has two large insertions not present in the S. pombe protein (Fig. 2B).
DRC1 Can Suppress the Lethality of DNA Polymerase II Ts Mutants.
High-copy DPB11 can suppress the temperature sensitivity of pol2–11 but not pol2–18 mutants. We tested whether overexpression of DRC1 has any effect on four Ts pol2 mutant alleles. pHW21 (2 μ URA3 DRC1) was transformed into these mutants and examined for growth at the restrictive temperature. pol2–11 and pol2–12 mutants are defective in both DNA replication and checkpoint control, whereas pol2–9 and pol2–18 are defective only in DNA replication (8, 19, 20). As shown in Fig. 2C, high-copy DRC1 can partially suppress the growth defect of all pol2 alleles, except pol2–9 (data not shown), demonstrating a strong genetic interaction between Drc1 and DNA polymerase II.
DRC1 Is an Essential Cell Cycle-Regulated Gene.
To investigate the phenotype of a DRC1 null mutant, one copy of DRC1 was replaced with HIS3 in a diploid strain. Three independent DRC1/drc1∷HIS3 heterozygous disruptants were sporulated at 24°C. Forty-one tetrads were dissected and germinated at 24°C on rich medium (YPD). Only two spore clones were viable from each ascus, and all were His−. Inviable spores germinated and divided 1–2 times, indicating that DRC1 is essential for mitotic growth.
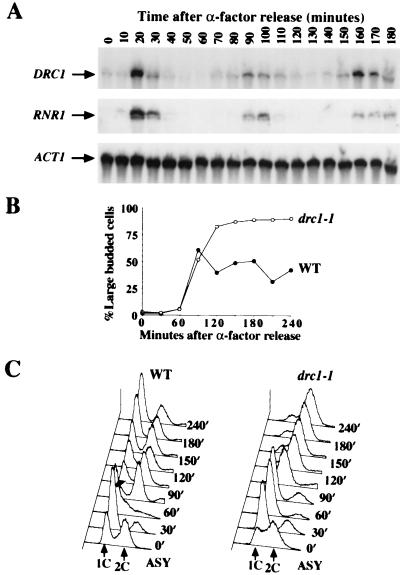
DRC1 genetically interacts with DPB11, a gene required for proper DNA replication, suggesting that DRC1 also might function in S phase. In S. cerevisiae, many DNA replication genes, such as those involved in the initiation or enzymology of DNA replication in S phase like RNR1, the regulatory subunit of ribonucleotide reductase, are periodically expressed in late G1. The ACGCGT sequence (MCB box) in the promoter region of these genes is responsible for their transcriptional regulation (21). The promoter region of DRC1 also contains an MCB box. This observation prompted us to examine DRC1’s mRNA levels throughout the cell cycle. Northern blot analysis shows that DRC1 is coordinately cell cycle regulated with RNR1, suggesting that DRC1 might function in S phase (Fig. 3A).
Figure 3.
(A) Cell cycle regulation of DRC1. Cultures of wild-type (Y300) cells were synchronized with α-factor and released into YPD at 30°C. Samples for RNA isolation were collected at 10 min, and Northern blot analysis was performed by using DRC1, RNR1, and ACT1 as probes. (B and C) drc1–1 mutants are defective in DNA synthesis at the restrictive temperature. Cells from both W303 ρ0 (wild type) (Y794) and an isogenic drc1–1 derivative (Y795) were arrested with α-factor at 24°C, then shifted to 37°C for 1 hr before releasing from the α-factor block into YPD at 37°C. Aliquots were removed at 30 min intervals for analysis of the budding profile (B) and DNA content by FACS (C).
DRC1 Is Required for DNA Replication.
To explore the physiological role of DRC1, we generated conditional DRC1 alleles. A mutagenized DRC1 gene library was created by random PCR mutagenesis and was screened for Ts alleles by using a plasmid shuffle strategy as described in Materials and Methods. Five Ts alleles of drc1 were isolated, and all arrested as large budded cells with a single nucleus at 37°C. FACS analysis showed mutant cells at 37°C have DNA contents distributed between 1 N and 2 N, indicating a defect in DNA replication (not shown).
To more carefully analyze the defect of drc1 mutants in DNA replication, we sought to eliminate the contribution of mitochondrial DNA to the FACS profile by introducing the drc1–1 allele into its chromosome locus in a W303 ρ0 background. drc1–1 ρ0 cells can grow at 24°C, grow poorly at 30°C, and fail to grow at 37°C. Asynchronous drc1–1 cells at 24°C have greater portion of S-phase cells than wild-type (Fig. 3C), consistent with an abnormal S-phase progression. After release from G1 arrest at 24°C, FACS analysis revealed that drc1–1 cells progress though S phase much more slowly than wild-type cells (data not shown). When released at 37°C, drc1–1 mutant cells display a very severe S-phase entry defect, and DNA replication was not completed even after 3 hr, while the control cells already have entered another cell cycle (Fig. 3C). Cell morphology analysis shows 90% of drc1–1 cells arrest as large budded cells 4 hr after release at 37°C (Fig. 3B). DAPI (4′,6-diamidino-2-phenylindole) and tubulin staining shows they have a single nucleus and short preanaphase spindle (data not shown).
The FACS profile and the terminal phenotype of drc1–1 at 37°C are similar to many DNA replication mutants, such as pol2–9 (20), cdc17–1(pol1) (22), and cdc2–1(pol3) (23). The DNA replication defect in those mutants activates a checkpoint pathway, resulting in preanaphase arrest. To examine whether the drc1–1 arrest depends on the checkpoint pathway, we attempted to generate drc1–1 mec1–21 and drc-1rad53–21 double mutants by standard genetic crosses. We were unable to isolate any double mutants whereas single mutants appeared at the expected frequencies (data not shown). This result is consistent with the idea that a functional checkpoint pathway is required for survival of drc1–1 mutants.
DRC1 Is Required for the S-Phase Checkpoint.
Because POL2 and DPB11 function in the same pathway for DNA replication and the checkpoint response to replication blocks, we examined whether DRC1 was also essential for this checkpoint. The five Ts alleles were examined for sensitivity to HU. Although all five alleles show sensitivity to the drug (very slow growth on 0.1 M HU plates compared with wild type), none displayed a checkpoint defect as measured by spindle elongation in the presence of HU (data not shown).
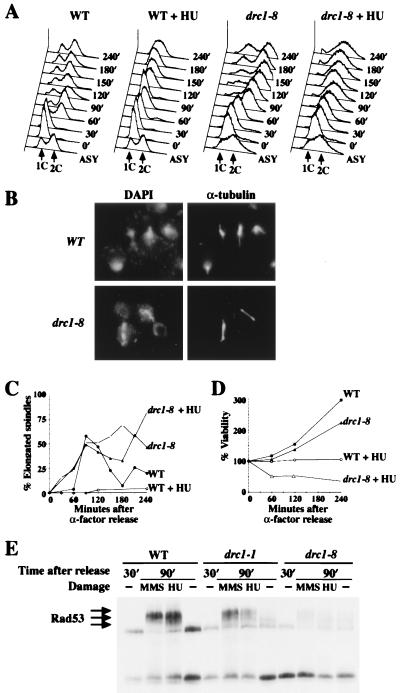
The failure to observe a checkpoint defect in the drc1 Ts mutants might indicate that DRC1 does not function in this pathway. However, only a subset of pol2 Ts alleles have a checkpoint defect, indicating that it is possible to miss such a defect in a small sample of mutants selected for an unrelated phenotype. To provide a more thorough examination we performed a second genetic screen for drc1 mutants, this time searching specifically for HU-sensitive mutants and focusing on mutants that were more HU sensitive than the five Ts mutants. This screen yielded three additional HU-sensitive alleles, one, drc1–6, that was Ts and grew very poorly on 0.1 M HU, and two, drc1–7 and drc1–8, that were completely dead on 0.1 M HU but grew, albeit poorly, at 37°C. We examined these mutants for checkpoint defects by using the standard G1 release into HU experiment. drc1–6 (data not shown) and drc1–8 mutants showed checkpoint defects. drc1–8 appeared to be the most severely affected. When drc1–8 cells were released into HU from G1 arrest, they entered anaphase at the same rate as a population without HU (50% at 90 min) as judged by the presence of elongated spindles (Fig. 4 B and C). In contrast, only 5% of wild-type cells in HU enter anaphase. Anaphase entry in drc1–8 mutants occurs when DNA has not been fully replicated (Fig. 4A) and is accompanied by a 60% drop in viability (Fig. 4D). This finding indicates that drc1–8 mutants are defective in arresting the cell cycle in response to DNA replication blocks and that premature onset of anaphase may contribute to cell death.
Figure 4.
drc1–8 mutants are defective for the S-phase checkpoint. (A–D) Wild-type (Y796) and drc1–8 (Y798) cells were synchronized by α-factor at 24°C. After G1 arrest, cultures were divided into two and released from the block into either YPD or YPD containing 0.2 M HU. At regular intervals after release, aliquots were withdrawn to examine DNA content, nuclear and spindle morphology, and cell viability. (A) FACS profile showing the DNA content of wild-type and drc1–8 cells after release from G1 into medium with or without 0.2 M HU. Time indicates minutes after release. (Lower) Untreated asynchronous cells at 24°C and are included as a reference. (B) Photomicrographs of wild-type and drc1–8 cells at 150 min after release from α-factor into YPD with 0.2 M HU. Nuclear morphology was visualized with 4′,6-diamidino-2-phenylindole, and microtubule morphology was visualized by indirect immunofluorescence using anti-α-tubulin antibodies. (C) Kinetics of spindle elongation in the presence or absence of HU. (D) Cell viability was analyzed by scoring colony-forming units on YPD plates at 24°C. (E) Wild-type (Y300), drc1–1 (Y799), and drc1–8 (Y798) cells were synchronized in G1 by α-factor at 24°C. After release for 30 min, HU or MMS was added to block DNA replication block or damage DNA for 1 hr. Protein extracts were prepared, separated by SDS/PAGE, and immunoblotted with antibodies to Rad53.
Rad53 phosphorylation also was examined in synchronized drc1–8 mutants. After released from G1 for 30 min, cells were treated with HU or MMS for 1 hr. Protein extracts were prepared and analyzed by Western blotting. Rad53 phosphorylation was reduced in drc1–8 compared with wild-type and drc1–1 mutants (Fig. 4E). These data indicate that Drc1, like Pol2 and Dpb11, functions upstream of Rad53 in both DNA damage and replication interference pathways. Furthermore, it is possible to separate the DNA replication and S-phase checkpoint functions of Drc1 by mutation. Two additional observations were apparent in this study. First, both drc1–1 and drc1–8 mutants show modification of Rad53 at the 90-min time point without the presence of damaging agents, unlike wild-type cells. It is likely that their replication defects are generating damage signals. Although both dpb11–1 and drc1–8 mutants are defective in the S-phase checkpoint, they are not completely defective in Rad53 regulation, consistent with the observation that RAD9 and POL2 control parallel signaling pathways (24). Second, the levels of Rad53 protein are severely reduced in drc1–8 mutants and this defect is likely to contribute to the checkpoint defect.
Drc1 Physically Interacts with Dpb11.
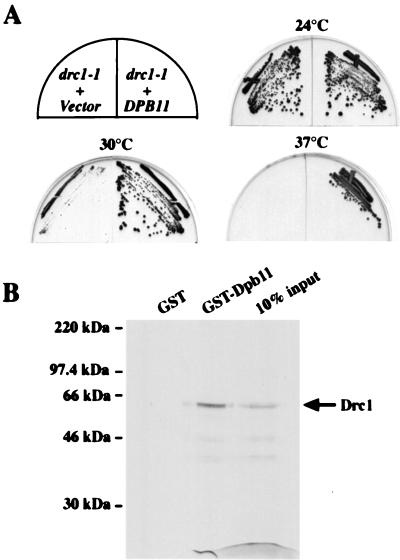
Because DRC1 can suppress the growth defect of dpb11–1, we tested whether DPB11 overexpression could rescue the Ts phenotype of drc1–1 mutants. DPB11 overproduction suppresses the Ts phenotype of drc1–1 mutants at all temperatures tested (Fig. 5A). This reciprocal suppression suggests these two proteins might physically interact with each other and depend on each other for their activity. Under these circumstances, the phenotype of one mutant generally is exacerbated by mutations in its interacting protein. In the case of essential genes, this relationship can result in synthetic lethality. We crossed dpb11–1 mutant to drc1–1 mutant covered by DRC1 on a URA3 plasmid. Double mutant haploid from this cross survive only in the presence of DRC1, and these strains are 5-FOA sensitive, indicating synthetic lethality (data not shown).
Figure 5.
DPB11 and DRC1 show reciprocal suppression and their proteins physically associate. (A) The Ts phenotype of drc1–1 is suppressed by the overproduction of DPB11. Y799 (drc1–1) cells harboring either an empty vector, pRS326, or DPB11 on a 2-μ plasmid, YEp195DPB11, were struck upon SC-Ura plates and incubated at 24°C, 30°C, and 37°C respectively. (B) Physical interaction between Dpb11 and Drc1 in vitro. GST or GST-Dpb11 bound to glutathione beads purified from baculovirus-infected insect cells were incubated with 35S-methionine-labeled Drc1 made by in vitro translation. Proteins bound to the beads after several washes were resolved by SDS/PAGE (10%) and visualized by autoradiography.
To determine whether the interaction between Drc1 and Dpb11 was direct, in vitro-translated Drc1 was incubated with baculovirus-produced glutathione S-transferase (GST)-Dpb11 or GST bound to glutathione beads. As shown in Fig. 5B, GST-Dpb11 showed very strong binding to Drc1, retaining more than 20% of the input Drc1 protein, but GST alone binds less than 1% of input protein. We have performed the same experiment by using GST-Rad53 and observed less than 2% retention of input Dcr1 (data not shown), indicating that the binding to Dpb11 is specific and very likely to be direct. The reciprocal genetic suppression, synthetic lethality, common loss of function phenotypes, and in vitro binding of Drc1 and Dpb11 indicate that these two proteins work together to coordinate DNA replication and cell cycle progression during S phase.
DISCUSSION
In this study we describe the identification of a gene, DRC1, that coordinates DNA replication and cell cycle checkpoint control together with a previously identified gene, DPB11, the S. cerevisiae homolog of S. pombe cut5. The cut5 gene in S. pombe is required for initiation of DNA replication and for the prevention of mitosis until DNA replication is completed (25). This phenotype is similar to the cdc18 mutant phenotype in which the failure to initiate DNA replication leads to a reductional mitosis (26). However, subsequent studies revealed that unlike cdc18, cut5 also was required to prevent mitosis when DNA replication is blocked with HU after initiation of DNA synthesis (18). DPB11 also was shown to be required for DNA replication, but the role in control of the S-phase checkpoint had not been fully demonstrated. We have shown that DPB11 is required for the S-phase checkpoint based on the observation that dpb11–1 cells fail to delay mitosis when treated with HU and that cells with less than 2C DNA content are undergoing mitosis. Further support for this conclusion stems from the fact that dpb11–1 cells show a profound defect in Rad53 modification in response to HU and MMS treatment. Thus, we conclude that Dpb11 is the structural and functional homolog of Cut5 and functions upstream of Rad53.
DRC1 is an essential gene that is primarily expressed in S phase. DRC1 has a specific role in DNA synthesis as evidenced by the fact that drc1 mutants are defective in DNA replication but do not appear to delay other START-associated events such as budding. After an initial delay, Ts drc1 mutants appear to slowly enter S phase, eventually arresting with a Cdc− phenotype of large budded cells with an approximate 2C DNA content. Furthermore, DRC1 overproduction can suppress DNA replication defects in pol2 and dpb11 mutants, both of which are defective in DNA replication. Whether it is required for initiation, elongation, or both is not yet clear from the current studies.
DRC1 also is required for the S-phase checkpoint that restrains mitosis in the presence of DNA replication blocks. The evidence supporting this conclusion comes from the fact that the drc1–8 mutant, an allele that maintains its essential function for DNA replication, fails to prevent mitotic entry when DNA replication is blocked by HU. Furthermore, drc1–8 cells are also defective in Rad53 regulation in response to DNA replication blocks, much like dpb11–1 mutants. These phenotypes are similar to those observed for pol2–11, pol2–12, and dpb11–1 mutants.
DRC1 is a member of the DPB11 pathway and is likely to function together with DPB11 to coordinate DNA replication and cell cycle progression. The evidence that it functions together with DPB11 is the following: (i) drc1 and dpb11 mutants have very similar phenotypes being defective for DNA replication, Rad53 regulation, and cell cycle checkpoint functions; (ii) drc1 and dpb11 mutants are synthetically lethal; (iii) DPB11 and DRC1 display reciprocal dosage suppression of their Ts mutant alleles; and (iv) Dpb11 and Drc1 can physically associate in vitro. These data suggest that a Drc1-Dpb11 complex coordinately controls DNA synthesis and the S-phase checkpoint pathway. During the course of this study, the DRC1 gene was independently identified as a mutant, sld2, that was synthetically lethal with dpb11 mutants (27). That study also demonstrated a role for Drc1 in DNA replication and showed physical association of these proteins in vivo, consistent with our studies. How the Drc1/Dpb11 complex coordinates these processes is not currently clear. However, there are some interesting differences between the phenotypes of the mutants. Although both dpb11–1 and drc1–8 mutants are defective for the checkpoint, drc1–8 mutants are unique in that they appear to display reduced Rad53 protein levels. Why Rad53 levels are reduced is not known; however, this reduction may help explain its severe checkpoint defect. Furthermore, this reduction may indicate that Drc1 functions closer to Rad53 than is Dpb11, possibly even directly stabilizing Rad53, although that remains to be determined.
We previously have shown that a BRCT domain protein, Rad9, is required for the regulation of the Rad53 kinase in response to DNA damage (3, 24). Recently Rad9 was observed to be in the same complex with Rad53 in the presence of DNA damage (7). We now have shown that a second BRCT domain-containing protein, Dpb11, functions upstream of Rad53 in response to DNA replication blocks. This finding allows us to make the more general observation that multiple BRCT proteins control signaling to Rad53 through distinct pathways, Rad9 sensing DNA damage, and Dpb11 sensing replication interference. This observation opens the possibility that multiple BRCT proteins in mammals, such as Brca1, Bard1, p53BP1, and the hypothetical human Dbp11 homolog, will be controlling the response of Chk2, the human Rad53 homolog (28), to DNA damage and replication blocks. Therefore, analysis of DRC1 should help further resolve how these BRCT repeat proteins transduce signals to control cell cycle and repair processes and may shed light on the genomic instability responsible for cancers in patients containing lesions in the BRCA1 gene.
Acknowledgments
We thank R. Butow, H. Araki, and A. Sugino for strains and W. Shoeber for FACS analysis. We also thank J. Diller and members of the Elledge lab for comments, helpful discussions, and/or reagents. This work was supported by National Institutes of Health Grant GM44664 (to S.J.E.). S.J.E. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- DRC1
DNA replication and checkpoint protein 1
- 5-FOA
5-fluoroorotic acid
- HU
hydroxyurea
- Ts
temperature sensitive
- YPD
yeast extract/peptone/dextrose
- FACS
fluorescence-activated cell sorter
- SC
synthetic complete
- MMS
methyl methanesulfonate
- GST
glutathione S-transferase
References
- 1.Elledge S J. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 2.Sanchez Y, Desany B A, Jones W J, Liu Q, Wang B, Elledge S J. Science. 1996;271:357–360. doi: 10.1126/science.271.5247.357. [DOI] [PubMed] [Google Scholar]
- 3.Sun Z, Fay D S, Marini F, Foiani M, Stern D F. Genes Dev. 1996;10:395–406. doi: 10.1101/gad.10.4.395. [DOI] [PubMed] [Google Scholar]
- 4.Weinert T. Cell. 1998;94:555–558. doi: 10.1016/s0092-8674(00)81597-4. [DOI] [PubMed] [Google Scholar]
- 5.Longhese M P, Paciotti V, Fraschini R, Zaccarini R, Plevani P, Lucchini G. EMBO J. 1997;16:5216–5226. doi: 10.1093/emboj/16.17.5216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paciotti V, Lucchini G, Plevani P, Longhese M P. EMBO J. 1998;17:4199–4209. doi: 10.1093/emboj/17.14.4199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun Z, Hsiao J, Fay D S, Stern D F. Science. 1998;281:272–274. doi: 10.1126/science.281.5374.272. [DOI] [PubMed] [Google Scholar]
- 8.Navas T A, Zhou Z, Elledge S J. Cell. 1995;80:29–39. doi: 10.1016/0092-8674(95)90448-4. [DOI] [PubMed] [Google Scholar]
- 9.Araki H, Leem S H, Phongdara A, Sugino A. Proc Natl Acad Sci USA. 1995;92:11791–11795. doi: 10.1073/pnas.92.25.11791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sugimoto K, Ando S, Shimomura T, Matsumoto K. Mol Cell Biol. 1997;17:5905–5914. doi: 10.1128/mcb.17.10.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugino A. Trends Biochem Sci. 1995;20:319–323. doi: 10.1016/s0968-0004(00)89059-3. [DOI] [PubMed] [Google Scholar]
- 12.Aparicio O M, Weinstein D M, Bell S P. Cell. 1997;91:59–69. doi: 10.1016/s0092-8674(01)80009-x. [DOI] [PubMed] [Google Scholar]
- 13.Morrison A, Araki H, Clark A B, Hamatake R K, Sugino A. Cell. 1990;62:1143–1151. doi: 10.1016/0092-8674(90)90391-q. [DOI] [PubMed] [Google Scholar]
- 14.Sikorski R S, Hieter P. Genetics. 1989;122:19–27. doi: 10.1093/genetics/122.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Muhlrad D, Parker R. Genes Dev. 1992;6:2100–2111. doi: 10.1101/gad.6.11.2100. [DOI] [PubMed] [Google Scholar]
- 16.Lorenz M C, Muir R S, Lim E, McElver J, Weber S C, Heitman J. Gene. 1995;158:113–117. doi: 10.1016/0378-1119(95)00144-u. [DOI] [PubMed] [Google Scholar]
- 17.Desany B A, Alcasabas A A, Bachant J B, Elledge S J. Genes Dev. 1998;12:2956–2970. doi: 10.1101/gad.12.18.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saka, Y., Fantes, P. & Yanagida, M. (1994) J. Cell Sci.18, Suppl., 57–61. [DOI] [PubMed]
- 19.Budd M E, Campbell J L. Mol Cell Biol. 1993;13:496–505. doi: 10.1128/mcb.13.1.496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araki H, Ropp P A, Johnson A L, Johnston L H, Morrison A, Sugino A. EMBO J. 1992;11:733–740. doi: 10.1002/j.1460-2075.1992.tb05106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McIntosh E M. Curr Genet. 1993;24:185–192. doi: 10.1007/BF00351790. [DOI] [PubMed] [Google Scholar]
- 22.Lucchini G, Falconi M M, Pizzagalli A, Aguilera A, Klein H L, Plevani P. Gene. 1990;90:99–104. doi: 10.1016/0378-1119(90)90444-v. [DOI] [PubMed] [Google Scholar]
- 23.Suszek W, Baranowska H, Zuk J, Jachymczyk W J. Curr Genet. 1993;24:200–204. doi: 10.1007/BF00351792. [DOI] [PubMed] [Google Scholar]
- 24.Navas T A, Sanchez Y, Elledge S J. Genes Dev. 1996;10:2632–2643. doi: 10.1101/gad.10.20.2632. [DOI] [PubMed] [Google Scholar]
- 25.Saka Y, Yanagida M. Cell. 1993;74:383–393. doi: 10.1016/0092-8674(93)90428-s. [DOI] [PubMed] [Google Scholar]
- 26.Kelly T J, Martin G S, Forsburg S L, Stephen R J, Russo A, Nurse P. Cell. 1993;74:371–382. doi: 10.1016/0092-8674(93)90427-r. [DOI] [PubMed] [Google Scholar]
- 27.Kamimura Y, Masumoto H, Sugino A, Araki H. Mol Cell Biol. 1998;18:6102–6109. doi: 10.1128/mcb.18.10.6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matsuoka S, Huang M, Elledge S J. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]