Abstract
Imprinted genes tend to occur in clusters. We have identified a cluster in distal mouse chromosome (Chr) 2, known from early genetic studies to contain both maternally and paternally imprinted, but unspecified, genes. Subsequently, one was identified as Gnas, which encodes a G protein α subunit, and there is clinical and biochemical evidence that the human homologue GNAS1, mutated in patients with Albright hereditary osteodystrophy, is also imprinted. We have used representational difference analysis, based on parent-of-origin methylation differences, to isolate candidate imprinted genes in distal Chr 2 and found two oppositely imprinted genes, Gnasxl and Nesp. Gnasxl determines a variant G protein α subunit associated with the trans-Golgi network and Nesp encodes a secreted protein of neuroendocrine tissues. Gnasxl is maternally methylated in genomic DNA and encodes a paternal-specific transcript, whereas Nesp is paternally methylated with maternal-specific expression. Their reciprocal imprinting may offer insight into the distal Chr 2 imprinting phenotypes. Remarkably, Gnasxl, Nesp, and Gnas are all part of the same transcription unit; transcripts for Gnasxl and Nesp are alternatively spliced onto exon 2 of Gnas. This demonstrates an imprinting mechanism in which two oppositely imprinted genes share the same downstream exons.
Most imprinted genes in the mouse are located within one of nine imprinting regions distributed across six autosomes [Beechey, C. V. & Cattanach, B. M. (1998) MRC Mammalian Genetics Unit, Harwell, Oxfordshire. World Wide Web Site - Genetic and Physical Imprinting Map of the Mouse (URL: http://www.mgu.har.mrc.ac.uk/anomaly/anomaly.html)]. Uniparental inheritance of any one of these regions results in mice with phenotypic anomalies called imprinting effects, and two of the earliest discovered imprinting effects are associated with distal chromosome (Chr) 2 (1). Both effects have behavioral, morphological, and lethal components: mice with maternal disomy for the region are hypoactive, with long flat-sided bodies, and die within a few hours of birth, whereas mice of the reciprocal type, with paternal disomy have an essentially opposite phenotype for they are hyperactive, with short square bodies, and can survive for several days after birth (1). Genetic studies have shown that the effects must be due to a minimum of two monoallelically expressed genes, one of which is maternally imprinted and the other paternally imprinted (2), although there may be a number of imprinted genes, as there are in other regions (3, 4). To date a single imprinted gene has been identified within the region; this is Gnas, which determines Gsα, the α subunit of the guanine-nucleotide binding protein that stimulates cAMP production after hormone stimulation. There is molecular and biochemical evidence for tissue-specific imprinting of Gnas from studies of mice with uniparental disomy for the region (5) and a Gnas knockout (6), and some evidence from clinical and biochemical studies of patients with Albright hereditary osteodystrophy for tissue-specific imprinting of the homologous human gene GNAS1 (7). The phenotypes of mice heterozygous for a Gnas knockout are intriguing. When the knockout is inherited paternally, the resulting mice have the hypoactive phenotype resembling that found with the maternal disomy, but when maternally inherited, the mice have a hyperactive phenotype similar to that found with the paternal disomy (6). It is not clear how a single gene, Gnas, could be involved in both phenotypes, particularly as genetic data indicate a minimum of two genes must be involved. Therefore, to gain more insight into the imprinted phenotypes, we have set out to identify previously undescribed candidate imprinted genes in the distal Chr 2 imprinting region.
Almost without exception, the two parental alleles of imprinted genes are distinguished by distinct patterns of cytosine methylation. Some of these differences originate in the germ lines and may coincide with elements primarily involved in determining imprinting, whereas others are established after fertilization (8, 9). Allelic methylation differences can be maintained throughout somatic development irrespective of the actual expression status of the gene (10), implying that methylation represents a permanent memory of parental origin. This epigenetic property, therefore, could be exploited as a means to identify imprinted genes. Restriction landmark genome scanning has thus been used to detect parent-of-origin methylation at rare-cutter restriction sites. Although successful in identifying two imprinted genes in the mouse (11–13), the scope of the restriction landmark genome scanning approach was limited. As the basis of a more comprehensive screen, we have adapted a genomic subtractive hybridization method, representational difference analysis (RDA) (14) to identify parent-of-origin methylation differences at sites for frequent cutting methylation sensitive restriction enzymes, such as HpaII. Herein RDA is applied to the detection of sites of imprinted methylation in distal Chr 2 of the mouse to isolate imprinted genes.
METHODS
Mouse Genetics.
Mice with maternal (MatDp.dist2) and paternal duplication (PatDp.dist2) for the distal imprinting region were generated by intercrossing heterozygotes for Chr 2 translocations and identified by using appropriate marker genes (15, 16). Two different translocations were used: T(2;8)26H with a Chr 2 breakpoint in band 2G3 and T(2;19)68H with its breakpoint in band 2A3. Progeny from the T26H intercross were identified prenatally with markers brachypodism, Gdf5bp-H, and D2Mit226, and progeny from the T68H intercross were identified by imprinting phenotype at birth.
Methylation-Sensitive RDA.
RDA was based on the original protocol (14) with modifications appropriate to the use of HpaII. DNAs from five 12.5-dpc MatDp.dist2 and five 12.1-dpc PatDp.dist2 embryos (from translocation T(2;8)26H, in each case 3 Gdf5bp-H/Gdf5bp-H males, one +/+ male, and one +/+ female) were separately digested with HpaII, MatDp.dist2 and PatDp.dist2 pools were made, and 2 μg of DNA from each pool was ligated with 1 nmol of RHpa24 (5′-AGCACTCTCCAGCCTCTCAGCGAC-3′) and RHpa12 (5′-CGGTCGCTGAGA-3′) unphosphorylated oligonucleotides in 60 μl at 16°C overnight. Amplicons were prepared in multiple 200-μl PCR mixtures containing 40 ng of ligated DNA in 50 mM KCl/10 mM Tris⋅HCl (pH 8.8 at 25°C)/1.5 mM MgCl2/0.08% Nonidet P-40/all four dNTPs (each at 200 μM)/1 μM primer RHpa24/4 units of Taq DNA polymerase (MBI Fermentas Helena Biosciences, Sunderland, U.K.). Reaction conditions were 72°C incubation for 5 min and denaturation at 95°C for 90 sec; then 20 cycles of 95°C for 30 sec, 68°C for 30 sec, and 72°C for 90 sec; followed by a final extension at 72°C for 3 min. PCR products were precipitated with sodium acetate and ethanol and taken up in TE (10 mM Tris⋅HCl/1 mM EDTA, pH 8). For preparation of tester (14), 3 μg of amplicon DNA was digested with 30 units of HpaII and resolved on a 2% low-melting-point agarose gel. A gel fragment corresponding to amplicon free of primers was excised and treated with β-agarase (New England Biolabs), and DNA was recovered by precipitation with isopropyl alcohol. Gel-purified amplicons (750 ng) were ligated with MHpa24 (5′-AGCCAACTGTGCTATGCGAGGAAC-3′) and MHpa12 (5′-CGGTTCCTCGCA-3′) oligonucleotides. For the first subtraction, 500 ng of ligated tester was combined with 40 μg of driver (14), extracted with phenol/chloroform/isoamyl alcohol, 25:24:1 (vol/vol), and chloroform/isoamyl alcohol, 24:1 (vol/vol), and precipitated with sodium acetate and ethanol. Pellets were washed in 70% ethanol, resuspended in 4 μl of 2.5× EE [EE = 10 mM N-2-hydroxyethylpiperazine-N′-(3-propanesulfonic acid)/1 mM EDTA, pH 8.25 at 25°C] and transferred to 50 μl of mineral oil equilibrated in a heating block for denaturation at 98°C for 5 min. After the addition of 1 μl of 5 M NaCl, reassociation was allowed to proceed at 67°C. After 20 hr, subtractions were diluted to 100 μl with TE and a recovery PCR was performed with 2.5 μl of diluted subtraction in 100 μl. First, the reactions were incubated with Taq DNA polymerase at 72°C for 5 min before MHpa24 primer was added to 1 μM and 10 cycles of PCR were performed. PCR products were extracted with phenol/chloroform and precipitated with ethanol. Pellets were taken up in 10 μl and digested with 5 units of mung bean nuclease (Promega) at 30°C for 30 min. Reaction products were diluted with 50 μl of 50 mM Tris⋅HCl (pH 8.8) and the enzyme was inactivated at 95°C for 5 min. A second PCR was performed in 200 μl with 20 μl of mung bean nuclease digest for 17–20 cycles to generate first-round difference products (DPs). DPs were digested with HpaII and gel-purified, and then 500 ng was ligated to JHpa24 (5′-ACCGACGTCGACTATCCATGAAGC-3′) and JHpa12 (5′-CGGCTTCATGGA-3′) oligonucleotides. For second-round subtractions, 100 ng of religated first round DPs was subtracted against 40 μg of driver amplicons. Second-round DPs were recovered as before, using JHpa24 as primer and 22 cycles in the second PCR step.
Cloning and Analysis of Difference Products.
For cloning, second-round DPs were digested with HpaII, isolated in low-melting-point agarose, and ligated into the AccI site of pBS+ (Stratagene). Miniprep DNAs (Wizard, Promega) were digested with HpaII, and RNA probes were prepared by transcription with T3 RNA polymerase in the presence of [α-32P]UTP. RNA probes were hybridized to Southern blots containing MatDp.dist2 and PatDp.dist2 DNA pools digested with HindIII alone or in combination with HpaII or MspI. Plasmid DNAs were sequenced on an ABI377 automatic sequencer.
Mapping.
Nesp and Gnasxl were mapped by PCR amplification of yeast artificial chromosomes (YACs) spanning the distal Chr 2 imprinting region. Mouse primers specific for Nesp were designed across the putative intron in mmNesp (Fig. 1d); the forward primer was L3 (5′-GAGAGGATCAGTGGAGGCAC-3′) and the reverse primer was R3 (5′-CTCACCCTCTGGCTCTGCAG-3′), which corresponded to nucleotides 76–95 and 378–359, respectively, of DP clone 317. Primers specific for Gnasxl were designed from the best match between the extra-large exon of the human (17) and rat (18) and corresponded to nucleotides 1081–1098 (5′-CCGCCTACTCCGCGGCCT-3′; Xlf1) and nucleotides 1428–1410 (5′ -GGCTTCTTTGCGCATCTGT 3′; Xlr1) in the rat. PCR was carried out on YAC DNA, prepared by standard methods, using Thermoprime Plus DNA polymerase (Advanced Biotechnologies, Columbia, MD), and PCR products were sequenced to confirm their identity.
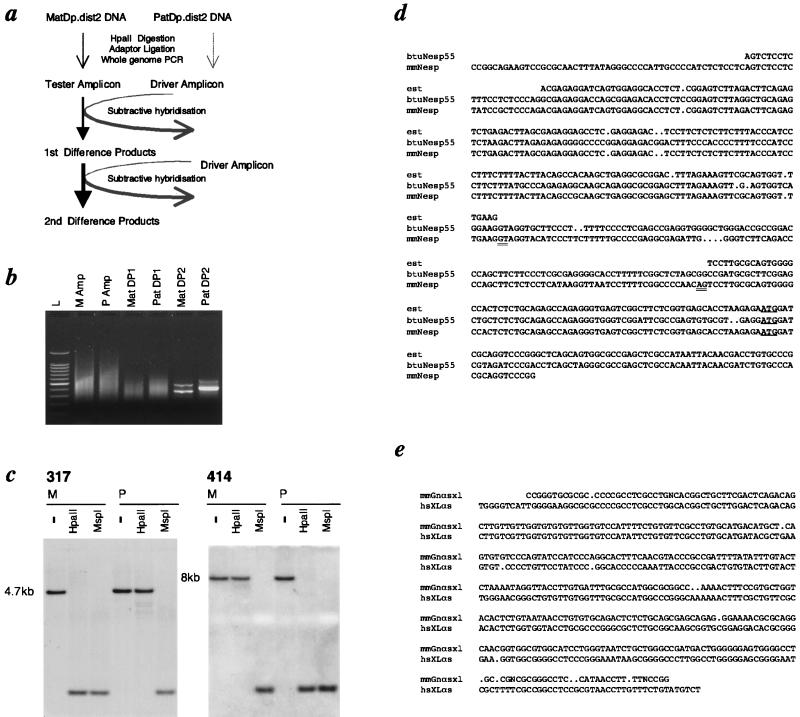
Figure 1.
RDA applied to uniparental methylation. (a) Overview of RDA using MatDp.dist2 DNA as “tester” and PatDp.dist2 DNA as “driver,” so that HpaII fragments specifically unmethylated in MatDp.dist2 DNA are “targets.” MatDp.dist2 and PatDp.dist2 DNAs are digested with HpaII and ligated to adapters, and whole-genome PCR was performed to produce amplicons representing unmethylated HpaII fragments. Subtractive hybridization was performed by denaturing tester amplicon with an excess of driver amplicon and allowing reassociation to take place, thus driving fragments common to tester and driver into heteroduplexes. Postsubtraction PCR with tester-specific primer preferentially amplifies tester–tester duplexes as DPs, thereby enriching target fragments. A second subtraction with first DPs against driver amplicon produces greater enrichment of targets in second DPs (for a full explanation of RDA, see ref. 14). (b) Gel electrophoresis showing HpaII amplicons (Amps) from MatDp.dist2 (M) and PatDp.dist2 (P) embryo DNAs and first-round (MatDP1, PatDP1) and second-round (MatDP2, PatDP2) DPs. L indicates a 100-bp ladder. (c) Probes for MatDP clone 317 and PatDP clone 414 hybridized to Southern blots of MatDp.dist2 (M) and PatDp.dist2 (P) embryo DNAs digested with HindIII alone (−) or with HpaII or MspI. (d) Sequence alignment of MatDP clone 317 (mmNesp) with the bovine cDNA (btuNesp) and a consensus (est) of three mouse ESTs W15839, AA260302, and AA124617. The translational start site for Nesp is underlined; putative splice donor and acceptor sites are double underlined. The maternally unmethylated HpaII sites at either end of clone 317 are in italics. (e) Sequence alignment of PatDP clone 414 (mmGnαsxl) with genomic sequence lying between exons A20 and A21 in human XLαs [hsXLas; GenBank accession no. AJ224868 (17)]. Paternally unmethylated HpaII and AscI sites (in the mouse and human sequences, respectively) are italicized.
Expression Analysis.
For Northern blot analysis, Nesp RNA probe was prepared by linearizing clone 317 with HpaII followed by labeling with the Strip-EZE RNA probe labeling kit (Ambion) using T3 RNA polymerase. For the Gnas RNA probe, cDNA derived from exons 4 and 5 of Gnas was cloned and transcribed to make a RNA probe. Hybridization was done under standard Express Hyb conditions (CLONTECH). RNA was isolated by using standard methods. Approximately 1 μg of total RNA was reverse-transcribed as described (15) with (dT)15. A maternally expressed transcript extending from Nesp to exon 2 of Gnas was detected by using the following primers. The forward primer Nesp.f3 (5′-AGTGGAGGCACCTCTCGGA-3′) was designed from DP clone 317, nucleotides 85–103. The reverse primer ex2r (5′-CTCCGTTAAACCCATTAACATGCA-3′) was designed from exon 2 of Gnas [nucleotides 205–182 (19)]. For Gnasxl expression, the forward primer, Xlf2 (5′-ACAGATGCGCAAAGAAGCC-3′, nucleotides 1410–1428 (18), was designed after comparing the sequences of the extra-large exon from rat and human and the reverse primer ex2r was specific for exon 2 of Gnas as described above. Hprt primers were included as a positive control for the cDNA [5′- GAAATGTCAGTTGCTGCGTC-3′ and 5′-GCCAACACTGCTGAAACATG-3′ (20)].
RESULTS
As a screen for imprinted loci on distal mouse Chr 2, we modified RDA (15) to identify differences in the methylation of HpaII restriction sites (Fig. 1a) in DNAs from embryos with maternal and paternal duplication for distal Chr 2 (hereafter reduced to MatDp.dist2 and PatDp.dist2 to accord generally with mouse nomenclature). After digestion of DNAs with HpaII, MatDp.dist2 and PatDp.dist2 HpaII “amplicons” were generated by PCR. The two amplicons should be identical representations of the unmethylated fraction of the genome, except for HpaII fragments corresponding to sites methylated differently in MatDp.dist2 and PatDp.dist2 DNAs. Unmethylated alleles will be cleaved into HpaII fragments short enough for PCR amplification and will thus be represented in the amplicons. The corresponding regions on the methylated alleles will reside on much larger HpaII fragments that are excluded from the amplicons. These differences constitute the targets for the subtraction. One subtraction was performed with MatDp.dist2 amplicon as “tester” and excess PatDp.dist2 amplicon as “driver”, so that the difference products (MatDPs) recovered should contain HpaII fragments unmethylated in MatDp.dist2 DNA. A reciprocal subtraction was used to clone paternally unmethylated HpaII fragments (as PatDPs). Two rounds of subtraction were performed and second-round DPs were obtained. Second-round MatDPs and PatDPs contained a small number of prominent bands (Fig. 1b), quite distinct from each other, consistent with the existence of a limited number of uniparentally unmethylated HpaII fragments in distal Chr 2. Second-round DPs were cloned and probes were hybridized to genomic Southern blots of HpaII- and MspI-digested MatDp.dist2 and PatDp.dist2 embryo DNAs to examine methylation status. Among cloned MatDPs, five distinct HpaII fragments were identified. Each was unmethylated specifically in MatDp.dist2 DNA, and each detected apparently the same ≈4.7-kb HindIII fragment on Southern blots (Fig. 1c and data not shown). The sequence of MatDP clone 317 was found to be homologous to a bovine cDNA for NESP55 [neuroendocrine secretory protein 55 (21)] and to three mouse expressed sequence tags (ESTs; GenBank accession nos. W15839, AA260302, and AA124617). The match to NESP55 was 77.8% over 380 bp and the match to W15839 was 98.4% over 248 bp, with a 95-bp interruption indicative of the presence of an intron in the DP sequence in comparison to the ESTs (Fig. 1d). The mouse gene symbol is Nesp. MatDP clone 35 was also homologous to bovine NESP55 (79.3% over 284 bp; data not shown), but none of the other three MatDP HpaII fragments detected significant database matches.
Of eight distinct PatDP HpaII fragments found to correspond to uniparental methylation, clone 414 identified homology to the human XLαS gene, an extra-large form of GNAS1 (76.6% over 372 bp; Fig. 1e) (9). The mouse gene symbol is Gnasxl. Clone 414 detected an ≈8-kb HindIII genomic fragment, distinct from Nesp, which was digested by HpaII specifically in PatDp.dist2 DNA (Fig. 1c). From the similar genomic fragments detected by five other PatDP clones, it is probable that they represent additional HpaII fragments at the Gnasxl locus, but none detected significant matches to either the human or rat XLαS sequences. Of the remaining two PatDP clones, one corresponded to the imprinted Peg5/Nnat gene (22, 23) and the identity of the other is presently unassigned.
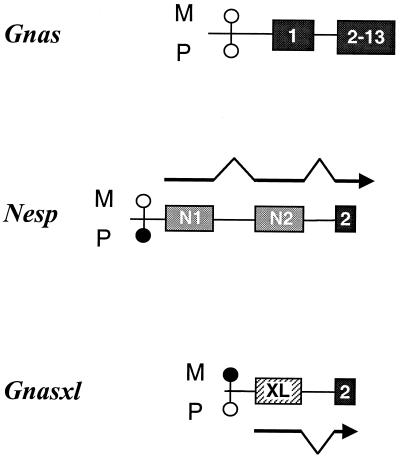
Notably, although they encode distinct proteins, both bovine Nesp and rat Gnasxl transcripts contain Gnas exons 2–13 downstream of their own unique exons (Fig. 4 and refs. 18 and 21). Accordingly, Nesp and Gnasxl were mapped by PCR to a YAC contig spanning the imprinting region on distal mouse Chr 2. They were located in Gnas containing YACs 200–25D1 (MIT Mouse YAC Library, ref. 24, Research Genetics, Huntsville, AL) and B145D8 (St. Mary’s Hospital Library, Genethon Mouse YAC Screening Service, Institut Pasteur, Paris), the smaller being no more than 150 kb (data not shown). However, both genes lie outside the 50-kb BAC 76-N18 (Research Genetics), which is positive for exons 1–13 of Gnas.
Figure 4.
Schematic representation of the cluster of oppositely imprinted transcripts associated with Gnas in distal mouse Chr 2. Dark shaded blocks indicate Gnas exons known in mouse, exon 2 of Gnas being common to the overlapping genes; light shaded blocks show exons exclusive to Nesp (designated N1 and N2), and the hatched block represents the 3′ end of the exon called extra large (XL) that is specific to Gnasxl. M and P refer to maternally and paternally derived alleles, respectively. The approximate position of the methylation-sensitive restriction sites are represented by open circles if unmethylated or solid circles if methylated. Transcripts of Nesp and Gnasxl are represented as arrows and those of Gnas are not shown. Pulse field gel analysis of YACs containing the region indicate the following gene order: Nesp–Gnasxl–Gnas, with Nesp being 16 kb upstream of Gnasxl, which is between 30 and 41 kb upstream of Gnas exon 1 (data not shown). The figure is not to scale.
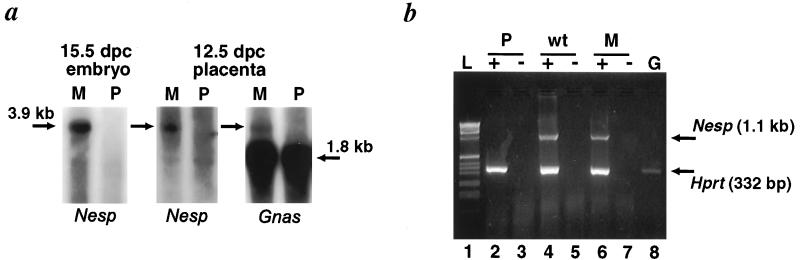
The expression of Nesp and Gnasxl was investigated in MatDp.dist2 and PatDp.dist2 offspring. For Nesp, maternal-specific expression of a 3.9-kb transcript was found in 15.5-day-post-coitum (dpc) embryo and 12.5-dpc placenta by using a Nesp-specific RNA probe (Fig. 2a). Imprinting of this isoform in placenta was also detected by a RNA probe specific to exons 4–5 of Gnas, consistent with the finding that Nesp transcripts include exons 2–13 of Gnas in Bos taurus (21) and providing evidence that the mouse Nesp transcript overlaps Gnas. The exclusive maternal expression of Nesp was consistent with the paternal methylation mark identified above. Reverse transcription-coupled PCR (RT-PCR) analysis was also carried out on wild-type, MatDp.dist2, and PatDp.dist2 whole embryos at 15.5 dpc by using a forward primer specific for Nesp (Nesp.f3) and a reverse primer for Gnas exon 2 (ex2r) for PCR. RT-PCR products of 1.1 kb were present in wild type and MatDp.dist2 but not in PatDp.dist2 samples (Fig. 2b). By sequencing, the 1.1-kb band was found to contain the complete Nesp ORF and exon 2 of Gnas but with the 95-bp putative intron spliced out. In addition to the aforementioned ESTs, it shows homology with a mouse EST (GenBank accession no. AA530580). The similarity of the predicted mouse and bovine proteins is 78.9%.
Figure 2.
Differential expression of Nesp. (a) Northern blot analysis of mRNA extracted from MatDp.dist2 (M) and PatDp.dist2 (P) embryos and placenta derived from T26H. The Nesp-specific RNA probe detected a 3.9-kb transcript in MatDp.dist2 embryo and placenta at 15.5 and 12.5 dpc, respectively. The 3.9-kb transcript detected in placenta also hybridized with a Gnas RNA probe specific for exons 4–5. The 1.8-kb band is the primary Gnas transcript. (b) RT-PCR of oligo(dT)-primed cDNA derived from 15.5-dpc MatDp.dist2 (M), PatDp.dist2 (P), and phenotypically normal (wt) embryos from T26H intercross. Twenty-five cycles of amplification were performed by using the primers Nesp.f3 and ex2r. Hprt primers were included as an amplification control. Lane L is 1-kb ladder and lane G is the product amplified from C3H/HeH genomic DNA. The presence (+) and absence (−) of reverse transcriptase is indicated.
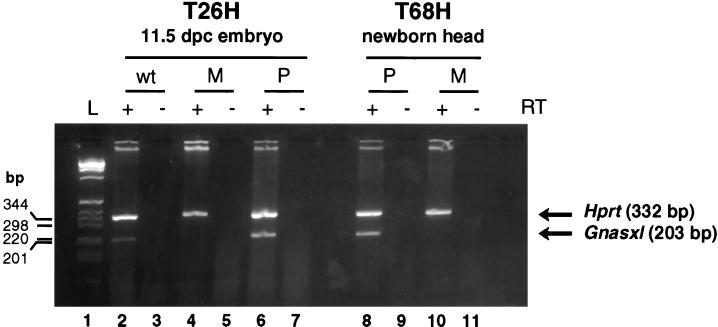
Gnasxl expression in these animals was tested by RT-PCR. The forward primer Xlf2 was specific for the extra-large exon of Gnasxl, and the reverse primer ex2r was specific for exon 2 of Gnas. These primers produced the expected 203-bp band in cDNA derived from wild-type 11.5-dpc embryos (Fig. 3), which corresponded to part of the extra-large exon linked to exon 2 of Gnas as confirmed by sequence analysis (data not shown). Gnasxl was exclusively paternally expressed for the 203-bp band was present in PatDp.dist2 but absent in MatDp.dist2 samples.
Figure 3.
RT-PCR demonstrating differential expression of Gnasxl. Expression analysis was done on MatDp.dist2 (M), PatDp.dist2 (P), and phenotypically normal (wt) tissue derived from the reciprocal translocations T26H and T68H. Oligo(dT)-primed cDNA was amplified for 25 cycles with primers Xlf2 and ex2r; Hprt primers were included as an amplification control. Lane L is 1-kb ladder. The presence (+) and absence (−) of reverse transcriptase is indicated.
DISCUSSION
We have used RDA to detect imprinted methylation in mouse distal Chr 2. The recovery of multiple differentially methylated HpaII fragments at two previously undescribed imprinted genes, Nesp and Gnasxl, demonstrates that the assay has been highly effective. In our adaptation, RDA was successful in that it was able to sample a large number of unmethylated HpaII fragments. As such, it should provide a more comprehensive screen than those based on restriction landmark genome scanning (11, 12), which are restricted because of their limitation to methylation at rare-cutter sites and dependence on restriction fragment length polymorphisms between parental mouse strains. Full details of the RDA screen will be presented elsewhere (G.K., D.B., H.J.M., C.V.B., C. E. Coombes, J.P., and C.M.W., unpublished results).
A remarkable finding is that despite their opposite imprinting, Gnasxl and Nesp are part of the same transcription unit as Gnas, because the Gnasxl and Nesp transcripts are alternatively spliced onto exon 2 of Gnas (Fig. 4 and refs. 17, 18, and 21). Although Gnasxl encodes a G protein α subunit with a long N-terminal extension (17, 18), it is predicted that translation of Gnas exons 2–13 does not occur for Nesp transcripts either in the bovine (21) or mouse (our data). Instead, the Nesp transcript contains a distinct ORF, highly conserved in mouse and bovine, encoding a secretory protein, whose function has yet to be demonstrated. In common with many imprinted genes, Nesp and Gnasxl carry methylation marks with the expressed allele being unmethylated. By contrast, Southern blots showed that BssHII, EagI, and SacII sites within a CpG-rich region at exon 1 of Gnas were unmethylated in both MatDp.dist2 and PatDp.dist2 DNAs (data not shown). Parental-specific methylation would be the simplest explanation for monoallelic expression of these genes. An expression-competition model in which methylation regulates the availability in cis of shared regulatory elements has been postulated for other pairs of closely linked imprinted genes that show reciprocal parental-specific expression (25, 26). Such competition could also account for the opposite imprinting of Nesp and Gnasxl.
The finding that there are Nesp and Gnasxl transcripts that are oppositely imprinted but have exon 2 of Gnas in common, at the very least, calls for reexamination of the molecular evidence for the imprinting of Gnas itself. Thus none of the probes used previously for Northern blot and in situ analysis in the mouse were specific for Gnas (5, 6). So far neither Nesp nor Gnasxl transcripts have been found that contain exon 1 of Gnas, and RT-PCR experiments designed to amplify exon 1 of Gnas indicate biallelic expression in the whole embryo and in the kidney (data not shown). Biallelic expression has also been found in humans by using a similar assay (17). These findings present a conundrum because the biochemical evidence suggests strongly that Gnas is monoallelically expressed in a tissue-specific manner from the maternal allele and thus shows paternal imprinting. Maternal inheritance of a null allele in patients with Albright hereditary osteodystrophy and in mice with a Gnas knockout results in resistance to parathyroid hormone attributable to paternal imprinting of Gnas in proximal kidney tubules. The imprinting of Gnas within tissues, particularly the kidney, merits further investigation.
There is no evidence for differential methylation of Gnas in the mouse from our studies or in human (17) or of imprinted expression from the GNAS1 promoter (17). However, paternal imprinting of Gnas could occur if Gnasxl acts as an “imprintor” (27) of Gnas in a tissue-specific manner. The Igf2 locus offers a precedent, in that imprinted methylation at the closely linked H19 gene controls imprinting of Igf2, without there being profound imprinted methylation at Igf2 (25, 28). Both Gnasxl and Nesp are expressed and imprinted in kidney and transcripts containing exon 1 of Gnas are also present (data not shown). Thus, Gnasxl and Gnas could compete for tissue-specific regulatory elements so that when the paternal allele of Gnasxl is unmethylated and expressed it out competes Gnas, and thus Gnas is silenced and paternally imprinted. On the maternally methylated allele Gnasxl will be silent, perhaps by competition with Nesp, and the regulatory elements will be available for Gnas transcription. This model of paternal silencing would account for the clinical and biochemical observations in Albright hereditary osteodystrophy patients with a maternally inherited GNAS1 null allele.
The findings of opposite imprinting of Nesp and Gnasxl are pertinent to the interpretation of the imprinted phenotypes associated with PatDp.dist2 and MatDp.dist2. Oppositely imprinted genes are clearly responsible for the two phenotypes (2). Recently, a knockout of Gnas exon 2 was reported (6); when paternally inherited a hypoactive phenotype closely resembling that of MatDp.dist2 was detected and when maternally inherited a hyperactive phenotype like that of PatDp.dist2 was found. Therefore, because Nesp and Gnasxl are oppositely imprinted and the imprinted transcripts contain exon 2 of Gnas, it can be predicted that maternal inheritance of the knockout allele will result in mice lacking Nesp transcripts, whereas paternal inheritance will result in mice lacking Gnasxl transcripts. It can therefore be concluded that Gnasxl and Nesp are candidate genes for the distal Chr 2 imprinting effects.
Acknowledgments
We are grateful to Bruce Cattanach and Wolf Reik for useful comments on the manuscript. We thank the Genome Group at the Mouse Genome Centre, Harwell, and the Genethon screening service for the YACs; A. Ford and K. Glover for figures; and E. Shutt for looking after the mice. The animal studies described in this paper were carried out under the guidance issued by the Medical Research Council in “Responsibility in the Use of Animals for Medical Research” (July 1993) and Home Office Project License no. 30/00875. G.K. is a senior fellow of the Medical Research Council. C.A.W. is funded by the National Kidney Research Fund, Grant R12/1/97.
ABBREVIATIONS
- Chr
chromosome
- RDA
representational difference analysis
- DP
difference product
- dpc
day post coitum
- YAC
yeast artificial chromosome
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
References
- 1.Cattanach B M, Kirk M. Nature (London) 1985;315:496–498. doi: 10.1038/315496a0. [DOI] [PubMed] [Google Scholar]
- 2.Beechey C V, Peters J. Mouse Genome. 1994;92:353–354. [Google Scholar]
- 3.Dittrich B, Buiting K, Korn B, Rickard S, Buxton J, Saitoh S, Nicholls R D, Poutska A, Winterpacht A, Zabel B, et al. Nat Genet. 1996;14:163–170. doi: 10.1038/ng1096-163. [DOI] [PubMed] [Google Scholar]
- 4.Paulsen M, Davies K R, Bowden L M, Villar A J, Franck O, Fuermann M, Dean W L, Moore T F, Rodrigues N, Davies K E, et al. Hum Mol Genet. 1998;7:1149–1159. doi: 10.1093/hmg/7.7.1149. [DOI] [PubMed] [Google Scholar]
- 5.Williamson C M, Schofield J, Dutton E R, Seymour A, Beechey C V, Edwards Y H, Peters J. Genomics. 1996;36:280–287. doi: 10.1006/geno.1996.0463. [DOI] [PubMed] [Google Scholar]
- 6.Yu S, Yu D, Lee E, Eckhaus M, Lee R, Corria Z, Accili D, Westphal H, Weinstein L S. Proc Natl Acad Sci USA. 1998;95:8715–8720. doi: 10.1073/pnas.95.15.8715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies S J, Hughes H E. J Med Genet. 1993;3:101–103. doi: 10.1136/jmg.30.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Constância M, Pickard B, Kelsey G, Reik W. Genome Res. 1998;8:881–900. doi: 10.1101/gr.8.9.881. [DOI] [PubMed] [Google Scholar]
- 9.Tremblay K D, Duran K L, Bartolomei M S. Mol Cell Biol. 1997;17:4322–4329. doi: 10.1128/mcb.17.8.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartolomei M S, Webber A L, Brunkow M E, Tilghman S M. Genes Dev. 1993;7:1663–1673. doi: 10.1101/gad.7.9.1663. [DOI] [PubMed] [Google Scholar]
- 11.Hatada I, Sugama T, Mukai T. Nucleic Acids Res. 1993;21:5577–5582. doi: 10.1093/nar/21.24.5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashizaki Y, Shibata H, Hirotsune S, Sugino H, Okazakia Y, Sasaki N, Hirose K, Imoto H, Okuizumi H, Muramatsu M, et al. Nat Genet. 1994;6:33–40. doi: 10.1038/ng0194-33. [DOI] [PubMed] [Google Scholar]
- 13.Plass C, Shibata H, Kalcheva I, Mullins L, Kotelevtseva N, Mullins J, Kato R, Sasaki H, Hirotsune S, Okazaki Y, et al. Nat Genet. 1996;14:106–109. doi: 10.1038/ng0996-106. [DOI] [PubMed] [Google Scholar]
- 14.Lisitsyn N, Lisitsyn N, Wigler M. Science. 1993;259:946–951. doi: 10.1126/science.8438152. [DOI] [PubMed] [Google Scholar]
- 15.Williamson C M, Dutton E R, Beechey C V, Peters J. Genomics. 1994;22:240–242. doi: 10.1006/geno.1994.1373. [DOI] [PubMed] [Google Scholar]
- 16.Williamson C M, Miller H J, Beechey C V, Peters J. Mouse Genome. 1995;93:860. [Google Scholar]
- 17.Hayward B E, Kamiya M, Strain L, Moran V, Campbell R, Hayashizaki Y, Bonthron D T. Proc Natl Acad Sci USA. 1998;95:10038–10043. doi: 10.1073/pnas.95.17.10038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kehlenbach R H, Matthey J, Huttner W B. Nature (London) 1994;372:804–808. doi: 10.1038/372804a0. [DOI] [PubMed] [Google Scholar]
- 19.Sullivan K A, Liao Y-C, Alborzi A, Beiderman B, Chang F-H, Masters S B, Levinson A D, Bourne H R. Proc Natl Acad Sci USA. 1986;83:6687–6691. doi: 10.1073/pnas.83.18.6687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konecki D S, Brennand J, Fuscoe J C, Caskey C T, Chinault A C. Nucleic Acids Res. 1982;10:6763–6775. doi: 10.1093/nar/10.21.6763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ischia R, Lovisetti-Scamihorn P, Hogue-Angeletti R, Wolkersdorfer M, Winkler H, Fischer-Colbrie R. J Biol Chem. 1997;17:11657–11662. doi: 10.1074/jbc.272.17.11657. [DOI] [PubMed] [Google Scholar]
- 22.Kikyo N, Williamson C M, John R M, Barton S C, Beechey C V, Ball S T, Cattanach B M, Surani M A, Peters J. Dev Biol. 1997;190:66–77. doi: 10.1006/dbio.1997.8681. [DOI] [PubMed] [Google Scholar]
- 23.Kagitani F, Kuroiwa Y, Wakana S, Shiroishi T, Myoshi N, Kobayashi S, Nishida M, Kohda T, Kaneko-Ishino T, Ishino F. Nucleic Acids Res. 1997;25:3428–3432. doi: 10.1093/nar/25.17.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kusumi K, Smith J S, Segre J A, Koos D S, Lander E S. Mamm Genome. 1993;4:391–392. doi: 10.1007/BF00360591. [DOI] [PubMed] [Google Scholar]
- 25.Bartolomei M S, Tilghman S M. Semin Dev Biol. 1992;3:107–117. [Google Scholar]
- 26.Wutz A, Smrzka O W, Schweifer N, Schellander K, Wagner E F, Barlow D P. Nature (London) 1997;389:745–749. doi: 10.1038/39631. [DOI] [PubMed] [Google Scholar]
- 27.Barlow D P. EMBO J. 1997;16:6899–6905. doi: 10.1093/emboj/16.23.6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorvaldsen J L, Duran K L, Bartolomei M S. Genes Dev. 1998;12:3693–3702. doi: 10.1101/gad.12.23.3693. [DOI] [PMC free article] [PubMed] [Google Scholar]