Summary
Intact endogenous cannabinoid signaling is involved in several aspects of drug addiction. Most importantly, endocannabinoids exert pronounced influence on primary rewarding effects of abused drugs, including exogenous cannabis itself, through the regulation of drug-induced increase in bursting activity of dopaminergic neurons in the ventral tegmental area (VTA). Previous electrophysiological studies have proposed that these dopaminergic neurons may release endocannabinoids in an activity-dependent manner to regulate their various synaptic inputs; however the underlying molecular and anatomical substrates have so far been elusive. To facilitate understanding of the neurobiological mechanisms involving endocannabinoid signaling in drug addiction, we carried out detailed analysis of the molecular architecture of the endocannabinoid system in the VTA. In situ hybridization for sn-1-diacylglycerol lipase-alpha (DGL-α), the biosynthetic enzyme of the most abundant endocannabinoid, 2-arachidonoylglycerol (2-AG), revealed that DGL-α was expressed at moderate to high levels by most neurons of the VTA. Immunostaining for DGL-α resulted in a widespread punctate pattern at the light microscopic level, whereas high-resolution electron microscopic analysis demonstrated that this pattern is due to accumulation of the enzyme adjacent to postsynaptic specializations of several distinct morphological types of glutamatergic and GABAergic synapses. These axon terminal types carried presynaptic CB1 cannabinoid receptors on the opposite side of DGL-α-containing synapses and double immunostaining confirmed that DGL-α is present on the plasma membrane of both tyrosine hydroxylase (TH)-positive (dopaminergic) and TH-negative dendrites. These findings indicate that retrograde synaptic signaling mediated by 2-AG via CB1 may influence the drug-reward circuitry at multiple types of synapses in the VTA.
Keywords: cannabinoid, DGL-α, CB1, dopamine, synaptic plasticity, addiction, DAGL
Introduction
Cannabis is the most commonly used illicit drug worldwide. In parallel with a steady increase in concentration of Δ9-tetrahydrocannabinol (Δ9-THC), its main psychoactive ingredient in the various cannabis products, prevalence of cannabis abuse is growing and the rate of cannabis dependence has doubled in the last decade (Anthony et al., 1994; Substance Abuse and Mental Health Services Administration, 2003). It is generally accepted that its addictive potential can be explained by a complex modification of the brain’s reward circuitry (Lupica et al., 2004; Gardner, 2005). One brain area particularly involved in exogenous cannabis effects is the posterior part of the ventral tegmental area (VTA) in the mesencephalon (Zangen et al., 2006), where release of the corresponding endogenous cannabinoids has also been shown to be indispensable for primary rewarding effects of several other drugs of abuse (Lupica and Riegel, 2005; Maldonado et al., 2006; Cheer et al., 2007). Both Δ9-THC and other potent synthetic agonists of the neuronal cannabinoid receptor (CB1) increase the frequency of phasic dopamine transients in the nucleus accumbens shell in vivo (Chen et al., 1990; Tanda et al. 1997; Cheer et al., 2004) by evoking intense burst firing of mesolimbic dopaminergic neurons (French, 1997; French et al., 1997; Gessa et al., 1998; Wu and French, 2000). Since electrophysiological experiments performed in acute brain slices containing the VTA recapitulated this finding (Cheer et al., 2000), the current notion is that activation of CB1 receptors on local neuronal elements within the VTA is responsible for some of the reward-relevant aspects of cannabinoid exposure (Lupica et al., 2004). Initially, a disinhibitory mechanism was suggested to underlie this observation (Cheer et al., 2000; Lupica et al., 2004) and indeed, perisomatic GABAergic synaptic inputs deriving from sources intrinsic to the VTA can be blocked by CB1 receptor agonists (Szabó et al., 2002). However, further investigations uncovered a more complex response pattern in the firing activity of VTA neurons upon cannabinoid administration (Cheer et al., 2003), which can be explained by endocannabinoid signaling at other synapses formed by glutamatergic and GABAergic afferents deriving from extrinsic sources (Melis et al., 2004a, 2004b; Riegel and Lupica, 2004). This widespread distribution of cannabinoid signaling is intriguing, however, because previous radioligand binding and immunocytochemical studies have consistently reported either a sparse density or a lack of cannabinoid binding sites and CB1 receptor distribution in the VTA (Herkenham et al., 1991b; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998).
To fill this apparent gap between the anatomical and physiological findings of cannabinoid signaling in this core reward center of the brain, we carried out high-resolution anatomical experiments, which revealed that sn-1-diacylglycerol lipase-alpha (DGL-α), a synthetic enzyme of the endocannabinoid 2-arachidonoylglycerol (2-AG) (Bisogno et al., 2003), is widely expressed in the VTA and is positioned postsynaptically on the plasma membrane around glutamatergic and GABAergic synapses. Furthermore, CB1 cannabinoid receptors are localized presynaptically on both types of axon terminals. Thus, most synapses are equipped with the key molecular players required for endocannabinoid-mediated synaptic signaling in the VTA supporting a central role of the endocannabinoid system in diverse addictive processes.
Methods
Animal handling
Experiments were carried out according to the guidelines of the institutional ethical code and the Hungarian Act of Animal Care and Experimentation (1998. XXVIII. Section 243/1998.), which are in accordance with the National Institutes of Health Guide for Care and Use of Laboratory Animals (2302/003). Adult male C57BL/6H mice (eight wild type, 50±13 days old) and C57BL/6J mice (littermates; three wild type and three CB1 knockout, 186±15 days old; described in Zimmer et al., 1999) were used in the present study.
Perfusion and preparation of tissue sections
All mice were perfused transcardially under deep Equithesin anesthesia (including 4.2 g chloral hydrate, 2.12 g MgSO4*7H2O, 9.97 g Pentobarbital, 39.6 ml concentrated propylene glycol, and 10 ml abs. ethanol completed to a final volume of 100 ml with distilled H2O; 0.3 ml/100 g, i.p.). Animals were first perfused with 0.9% saline solution for 2 min and then, with Zamboni’s fixative containing 4% paraformaldehyde (Sigma Aldrich) and 0.05% glutaraldehyde (EMS) in 0.1 M phosphate buffer (PB; pH = 7.4) for 30 min. After perfusion, the brain was removed from the skull and coronal sections (40 μm thick for in situ hybridization and 50 μm thick for immunocytochemistry) containing the VTA were cut with a Leica VTS-1000 vibratome.
In situ hybridization
The synthesis of riboprobe for mouse DGL-α used in the present study was previously described by Katona et al. (2006). The length of the open reading frame on the DGL-α mRNA is 3135 bp long; the total length of the probe was 1169 bp long, from bases 1967 to 3135 (base numbering starts from the “A” of the open reading frame). For incubation of the sections, all solutions used were first treated with 0.1% DEPC for 1 h and then autoclaved. Incubation of the 40-μm-thick brain slices was performed in a free-floating manner in RNase-free sterile culture wells throughout the entire procedure. First, the sections were washed in PBST (containing 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KH2PO4, and 0.1% Tween 20, pH = 7.4) three times for 20 min. Hybridization was then performed overnight at 65°C in 1 ml of hybridization buffer containing the digoxigenin-labeled riboprobe (2.5 μg/ml). Hybridization buffer consisted of 50% formamide, 5×SSC, 1% SDS, 50 μg/ml yeast tRNA, and 50 μg/ml heparin in DEPC-treated distilled H2O. During the overnight incubation and the following three washing steps, the sections were continuously incubated on a shaker within a humid chamber. After incubation, the sections were first washed for 30 min at 65°C in wash solution 1 (containing 50% formamide, 5× SSC, and 1% SDS in DEPC-treated H2O) and then twice for 45 min at 65°C in wash solution 2 (containing 50% formamide and 2× SSC in DEPC-treated H2O). The sections were next washed for 5 min in 0.05 M Tris-buffered saline (TBS) containing 0.1% Tween-20 (TBST; pH = 7.6), and then blocked in TBST containing 10% normal goat serum (TBSTN) for 1 h, both at room temperature. Next, sections were incubated overnight at 4°C with sheep anti-digoxigenin Fab fragment conjugated to alkaline phosphatase (Roche Molecular Diagnostics) diluted at 1:1,000 in TBSTN. The next day, the sections were washed three times for 20 min in TBST and then developed with freshly prepared chromogen solution in a total volume of 10 ml, containing 3.5 μl of 5-bromo-4-chloro-3-indolylphosphate and 3.5 μl of nitroblue-tetrazolium-chloride dissolved in chromogen buffer (containing 100 mM NaCl, 100 mM Tris-Cl, pH = 9.5, 50 mM MgCl2, 2 mM (−)tetramisole hydrochloride, and 0.1% Tween 20). The sections were gently rinsed in 1 ml of the above developing solution in the dark for 4–6 h, and the reaction was stopped using PBST. Finally, the sections were washed in 0.1 M PB three times for 10 min and mounted in Vectashield (Vector Laboratories) onto glass slides, and the coverslips were sealed with nail polish. All chemicals were purchased from Sigma, unless otherwise stated.
Immunocytochemistry
After slicing and extensive washing in 0.1 M PB (5 times for 10 min), the 50-μm-thick sections were incubated in 30% sucrose overnight, followed by freeze thawing over liquid nitrogen four times. Afterwards, the sections were processed either for immunoperoxidase, immunogold, or preembedding immunogold labeling combined with a second immunoperoxidase staining. All washing steps and dilutions of the antibodies were done in 0.05 M TBS buffer (pH = 7.4). After extensive washing in TBS (5 times for 10 min), the sections were blocked in 5% normal goat serum for 45 min and then incubated in the primary antibody for a minimum of 48 h at 4°C. The following primary antibodies were used in the present study: affinity-purified rabbit anti-DGL-α polyclonal antibody called “ab-INT” recognizing a 119 amino acids long segment of the large intracellular loop of human DGL-α protein (residues: 790–908; dilution: 0.3 μg/ml; Katona et al., 2006); affinity-purified rabbit anti-DGL-α polyclonal antibody called “ab-C42” recognizing the C-terminal 42 amino acids of the mouse DGL-α protein (residues: 1003–1044; dilution: 1 μg/ml; Yoshida et al., 2006); affinity-purified guinea pig anti-CB1 polyclonal antibody recognizing the C-terminal 31 amino acids of the mouse CB1 protein (residues: 443–473; dilution: 1 μg/ml; Fukudome et al., 2004); ascites fluid of mouse anti-tyrosine hydroxylase (TH) monoclonal antibody recognizing an epitope in the mid-portion of the rat TH protein (dilution: 1:8000; Product No: 22941; ImmunoStar). Specificity of the antibodies was confirmed by the laboratories of origin (see the corresponding papers). Furthermore, the immunostaining with two DGL-α antibodies revealed identical staining pattern both at the light and the electron microscopic level, in spite of being raised against different epitopes. The specificity of the CB1 antibody was also confirmed by the lack of immunostaining in the CB1 knockout mice (Zimmer et al., 1999). Immunostaining with the TH antibody visualized the well characterized catecholaminergic neurons in the brain.
In the immunoperoxidase staining procedure, after primary antibody incubations, the sections were treated with either biotinylated goat anti-rabbit IgG or biotinylated anti-guinea pig IgG or biotinylated horse anti-mouse IgG (all three antibodies were diluted at 1:300; Vector Laboratories) for 2 h. Afterwards, the sections were incubated with avidin biotinylated–horseradish peroxidase complex (1:500; Elite ABC; Vector Laboratories) for 1.5 h. The immunoperoxidase reactions were finally developed using 3,3′-diaminobenzidine 4HCl (DAB) as the chromogen. In the immunogold staining procedure, after the 48 hours incubation in one of the above mentioned primary antibodies (except the TH antibody), the sections were further processed in two ways. One method utilized incubation with the corresponding biotinylated secondary antibodies, followed by incubation with 0.8 nm gold-conjugated streptavidin overnight at 4°C. Alternatively, sections were incubated with the corresponding 0.8 nm gold-conjugated goat anti-rabbit or 0.8 nm gold-conjugated goat anti-guinea pig antibody for CB1 or DGL-α, respectively (1:50 dilution; Aurion), overnight at 4°C. The two methods revealed similar staining quality. Finally, all sections were silver intensified using the silver enhancement system R-GENT SE-EM according to the kit protocol (Aurion). In the combined immunogold-immunoperoxidase double-immunostaining experiments, the sections were first developed according to the immunogold protocol and then a similar procedure was carried out as described above for the immunoperoxidase staining. Lack of cross-reactivity of the secondary antibodies in the sequential detection scheme was verified by omission of either primary antibody, which eliminated labeling by the irrelevant secondary antibody. After development of the immunocytochemical reaction, all sections regardless of the staining protocol were treated for electron microscopy with 1% OsO4 in 0.1 M PB for 20 min, then dehydrated in an ascending series of ethanol and propylene oxide, and embedded in Durcupan (ACM; Fluka). During dehydration, the sections were treated with 1% uranyl acetate in 70% ethanol for 20 min. From sections embedded in Durcupan, areas of interest were reembedded and resectioned for electron microscopy. Sections were collected on Formvar-coated single-slot grids, stained with lead citrate, and examined with a Hitachi 7100 electron microscope.
Results
DGL-α, the biosynthetic enzyme of 2-arachidonoylglycerol is expressed by most neurons in the ventral tegmental area
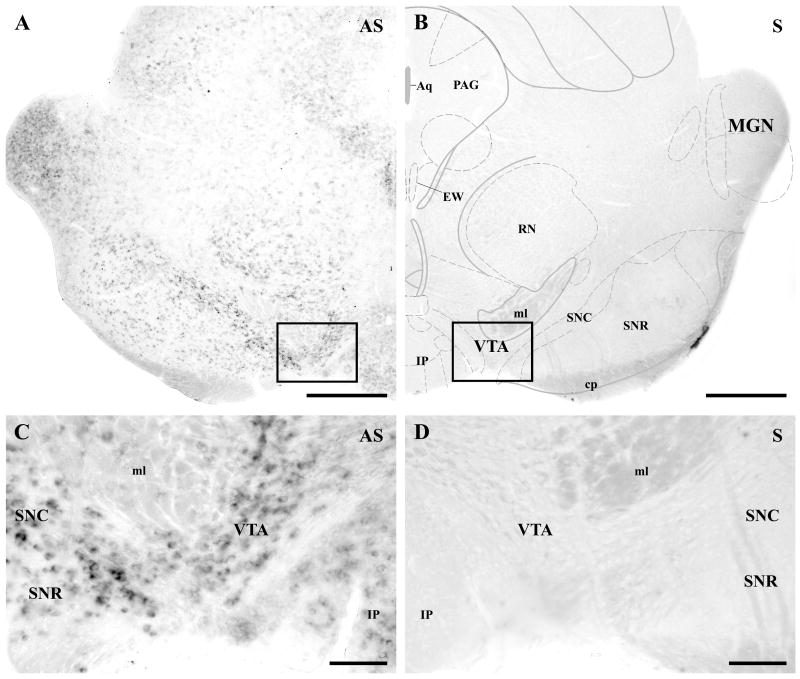
Previous biochemical measurements have shown that 2-arachidonoylglycerol (2-AG) is the most abundant endocannabinoid in the midbrain; its concentration is at least two orders of magnitude higher than that of anandamide (Bisogno et al., 1999). Moreover, recent electrophysiological studies have further proposed that 2-AG, but not anandamide may contribute to short-term plasticity of glutamatergic neurotransmission in the ventral tegmental area (VTA) (Melis et al., 2004a and 2006). These observations prompted us to examine whether sn-1-diacylglycerol lipase-alpha (DGL-α), a key enzyme in the biosynthesis of 2-AG in the brain (Bisogno et al., 2003), is expressed in the VTA. Non-radioactive free-floating in situ hybridization using a digoxigenin-tagged riboprobe under stringent conditions revealed a moderate, but widespread expression of DGL-α mRNA in the mouse midbrain (Fig. 1). Remarkably, the relative highest density of labeling was observed in the VTA and in the pars compacta of substantia nigra, two midbrain regions, where most of the dopaminergic cell bodies were located. The vast majority of VTA neurons showed a moderate or high intensity of DGL-α labeling (Fig. 1C) suggesting that dopaminergic cells, which represent about 55–80% of neurons in the VTA (Swanson, 1982; Margolis et al., 2006), express the key enzyme for 2-AG biosynthesis. In addition, DGL-α expressing cells were also found in the pars reticulata of substantia nigra and in other midbrain nuclei, although with a lower level of DGL-α expression (Fig. 1A and C).
Fig. 1.
DGL-α mRNA is expressed by most neurons at moderate to high levels in the ventral tegmental area. A–B) In situ hybridization using an antisense riboprobe (AS) against the mouse DGL-α mRNA sequence visualizes numerous neurons in the mouse mesencephalon. Note that a relatively high expression level is found in the ventral midbrain, i.e. in the substantia nigra pars compacta (SNC) and in the ventral tegmental area (VTA), where most dopaminergic neuron cell bodies are located. Neurons in the substantia nigra pars reticulata (SNR) express lower levels of DGL-α mRNA. In contrast, in situ hybridization with the sense riboprobes (S) of the corresponding DGL-α sequence did not result in any labeling, confirming the specificity of the reaction in A. C–D) Higher magnification view of the framed area in A demonstrates that nearly all cells show moderate to high expression levels of DGL-α mRNA. The presence of labeling in most neurons indicates that both dopaminergic and non-dopaminergic neurons express the 2-AG synthesizing enzyme. In the corresponding control staining, cells are completely negative in D (higher magnification of the framed area in B). Abbreviations: Aq, aqueduct (Sylvius); cp, cerebral peduncle, basal part; EW, Edinger-Westphal nucleus; IP, interpeduncular nuclei; MGN, medial geniculate nucleus; ml, lemniscus medialis; PAG, periaqueductal gray nucleus; RN, red nucleus. Scale bars: A–B, 500 μm; C–D, 100 μm.
DGL-α is localized postsynaptically in the proximity of synaptic specializations formed by putative glutamatergic and GABAergic axon terminals
To reveal the precise cellular and subcellular distribution of DGL-α protein in the VTA, we used two polyclonal antibodies raised against two non-overlapping epitopes, the large intracellular loop (INT) or the C-terminal 42 residues (C42) of the DGL-α protein (Fig. 2) (Katona et al., 2006; Yoshida et al., 2006). To aid the precise identification of the territory of A10 dopaminergic cells for further analysis of DGL-α immunoreactivity, immunoperoxidase staining for tyrosine hydroxylase (TH) was also carried out in neighboring sections (Fig. 2C).
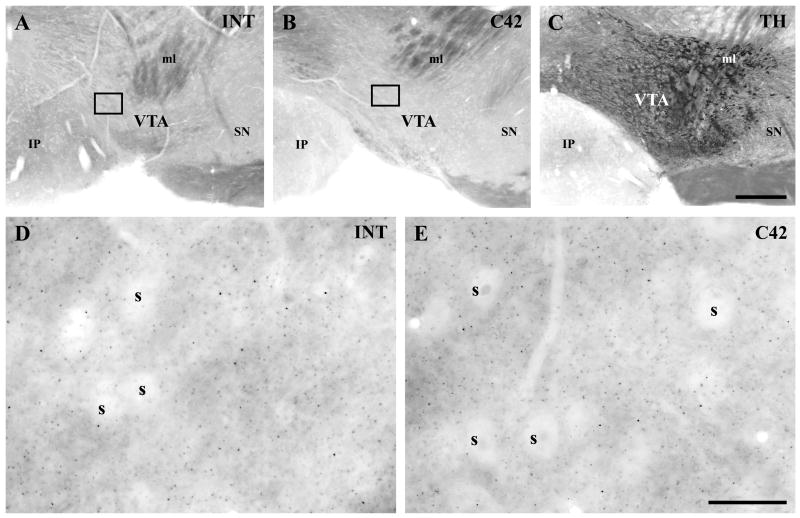
Fig. 2.
Localization of DGL-α protein in the mouse ventral tegmental area. A–B) Two antibodies were raised against two, non-overlapping segments of DGL-α. The abbreviation ‘INT’ refers to an antibody recognizing a large intracellular loop (A and D), while the ‘C42’ antibody was raised against the last 42 amino acids of the C-terminal tail of DGL-α (B and E). Both antibodies revealed a similar faint labeling in the ventral tegmental area, where A10 dopaminergic neurons are located as outlined by the tyrosine hydroxylase (TH)-staining in C. Remarkably, the characteristic punctate staining pattern appears only at higher magnification in D–E). Note that cell bodies (s) are largely devoid of DGL-α immunostaining, in contrast to the neuropil of the framed area in A–B. The identical labeling pattern of the two antibodies supports the specificity of the antibodies. Abbreviations: IP, interpeduncular nuclei; ml, lemniscus medialis; SN, substantia nigra; VTA, ventral tegmental area. Scale bars: A–C, 200 μm; D–E, 20 μm.
In accordance with the moderate DGL-α mRNA level, immunostaining for the DGL-α protein resulted in very faint labeling at low magnification (Fig 2A–B), whereas a punctate immunostaining pattern was clearly visible throughout the neuropil at higher magnification (Fig. 2D–E). Importantly, the pattern of immunostaining obtained by both antibodies was similar both at low and high magnifications (Fig. 2A–B and D–E). This granular staining pattern showed striking similarities with previous findings obtained in the hippocampus, the dorsal striatum and cerebellum, where the staining was restricted to dendritic spine heads or to the bases of spine necks that received excitatory synaptic inputs (Katona et al., 2006; Yoshida et al., 2006, Uchigashima et al., 2007). This is somewhat surprising as predominantly aspiny dopaminergic neurons receive most of their excitatory synaptic inputs onto dendritic shafts (Sesack and Pickel, 1992), in contrast to hippocampal pyramidal, striatal medium spiny and cerebellar Purkinje neurons. To clarify this issue, we carried out a detailed high-resolution distribution analysis of DGL-α immunostaining at the electron microscopic level.
Notably, although the DAB precipitate, the end product of the immunoperoxidase staining procedure is diffusible, in case of the DGL-α immunolabeling it clearly accumulated in intermediate-sized patches (size range is about 10–100 nm) in a large number of thick (Fig. 3B) and thin dendrites (Fig. 3A and C), instead of filling the entire dendrite as other markers often do (see for example TH-immunostaining below). Moreover, in most cases, it was unevenly distributed along the plasma membrane, preferentially found in the neighborhood of both asymmetric and symmetric synapses indicating that both types of synapses may contain DGL-α in the VTA (Fig. 3A–C).
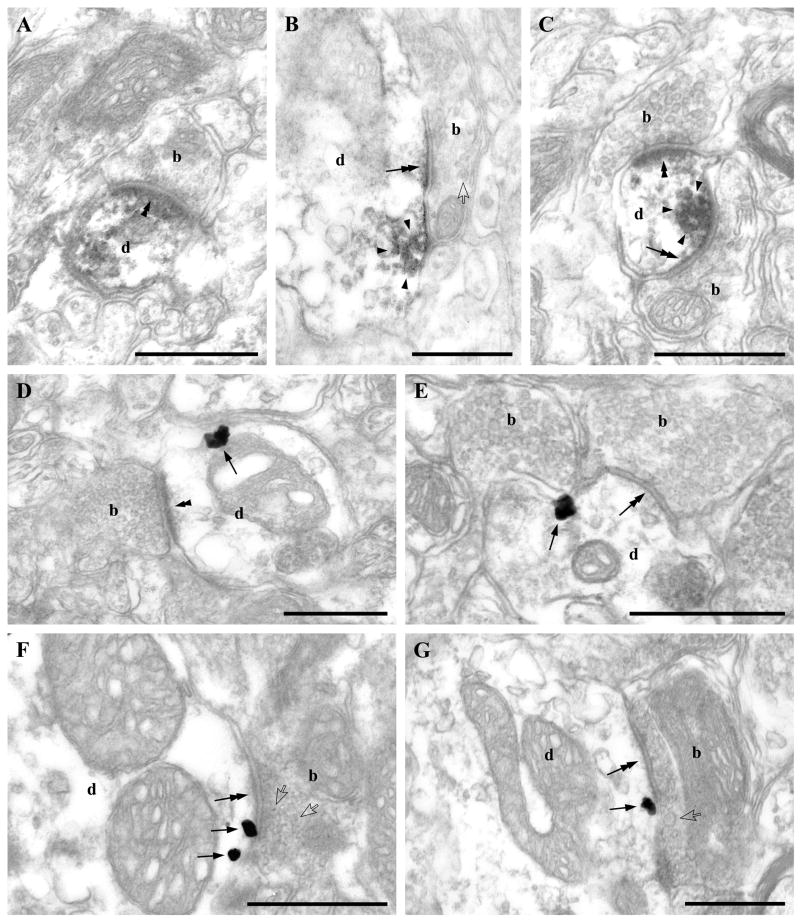
Fig. 3.
DGL-α is localized on the plasma membrane adjacent to asymmetric and symmetric synapses. A–C) The electron dense DAB end product of the immunoperoxidase reaction (encircled by arrowheads) depicts the concentrated presence of DGL-α in thin (A and C) as well as in thick (B) dendrites (d) of neurons in the ventral tegmental area. Note that despite the diffusible nature of the DAB end product, in most cases it does not fill the entire dendritic profile, but is concentrated adjacent to the plasma membrane and in close proximity to asymmetric (double arrowheads in A and C) and symmetric synapses (double arrows in B and C). Boutons (b) forming these synapses are immunonegative and represent several morphological types (for details on morphological criteria and corresponding literature see Results). In A, a small, possibly glutamatergic axon terminal (b) containing only a few clear round vesicles forms a synapse with a pronounced postsynaptic specialization. In B, a large bouton (b) tightly packed with small vesicles and also containing dense core vesicles (one is labeled by an open arrowhead) forms a synapse with a thin postsynaptic specialization. This axon terminal is likely to be a GABAergic afferent from extrinsic sources such as the nucleus accumbens and contains neuropeptides like enkephalin or dynorphin. In C, the upper bouton (b) is morphologically similar to the glutamatergic one presented in A and forms a synapse with a prominent postsynaptic density (double arrowhead). In contrast, the lower axon terminal (b) contains only a few vesicles, often with a flattened shape, lacks dense core vesicles and most importantly forms a symmetric synapse typical of GABAergic axons (double arrow). This type of bouton may represent GABAergic axon terminals deriving from local intrinsic GABAergic cells. D–G) High-resolution preembedding immunogold staining demonstrates that gold particles (arrows) representing the precise subcellular localization of DGL-α are always attached to the intracellular surface of the plasma membrane of dendrites (d) adjacent to asymmetric (in D) and symmetric (in E–G) synapses formed by all three, morphologically distinct axon terminal types (b): small axon terminal containing small round vesicles and with a pronounced postsynaptic density (double arrowhead; “probable extrinsic glutamatergic type” in D); axon terminal containing flattened vesicles forming clear symmetric synapses (double arrows; “probable intrinsic GABAergic type” in E); axon terminal containing tightly packed synaptic vesicles along with dense core vesicles (open arrows) and giving rise to synaptic specialization with a very thin postsynaptic density (double arrows; “probable extrinsic GABAergic”; in F–G). Scale bars: A–G, 0.5μm.
To analyze further this intriguing staining pattern, we carried out silver-enhanced immunogold staining in the next set of experiments, which enables a higher resolution localization of DGL-α in relation to the synaptic specializations of glutamatergic and GABAergic boutons (Fig. 3D–G). Immunogold particles representing the precise subcellular localization of DGL-α were predominantly attached to the intracellular side of the plasma membrane in accordance with the predicted subcellular position of the epitopes. Importantly, most of these immunogold particles occurred in proximity to the postsynaptic specializations of both asymmetric and symmetric synapses, but rarely appeared within the active zone (Fig. 3D–G).
Based on morphological criteria, three main types of axon terminals could be distinguished in the present study. These formed synapses equipped with postsynaptically positioned DGL-α immunolabeling around the synaptic specializations. First, small or medium-sized axon terminals containing several small round vesicles formed synapses on thin dendritic shafts and expressed a characteristically thick postsynaptic density (Fig. 3A, C, D and F). These axon terminals may belong to glutamatergic afferent fibers of the VTA (Sesack and Pickel, 1992; Carr and Sesack, 2000; Omelchenko and Sesack, 2007). Second, large axon terminals densely packed with numerous small, round or often ovoid vesicles and frequently containing large dense core vesicles as well, indicating the presence of neuropeptides, formed synapses mainly on thick dendritic branches and expressed an erratic postsynaptic density that varied in thickness from very thin to thick (Fig. 3B and G). These profiles may represent predominantly GABAergic, but occasionally GABA-negative axon terminals deriving from extrinsic sources like the nucleus accumbens, and may belong to the well-characterized enkephalin- and dynorphin-containing axon terminals (Pickel et al., 1993; Sesack and Pickel, 1992 and 1995). Finally, boutons forming symmetric synapses on all types of somatic and dendritic profiles and expressing a hardly visible postsynaptic density contained numerous small flattened vesicles, but were always devoid of large dense core vesicles (Fig. 3C and E). These boutons may derive from local intrinsic GABAergic interneurons, as well as from various extrinsic sources (Bayer and Pickel, 1991).
Postsynaptic DGL-α occurs on both dopaminergic and non-dopaminergic dendrites
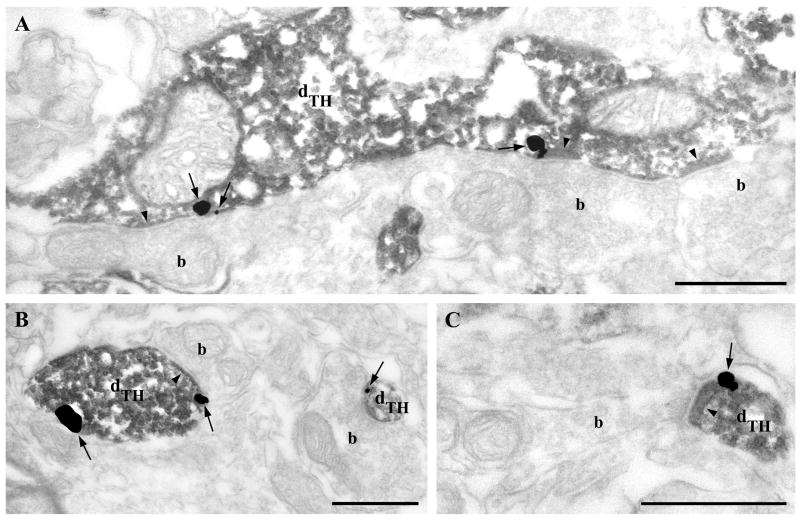
To elucidate the cellular identity of VTA neurons expressing DGL-α on their plasma membrane, DGL-α immunogold labeling was combined with immunoperoxidase staining for TH, an exclusive marker of dopaminergic neurons in the VTA. Electron microscopic analysis revealed that DGL-α is present on the plasma membrane of dopaminergic neurons often close to the edge of putative glutamatergic and GABAergic synapses (Fig. 4A–C), although in this experiment the synaptic specializations could not be always unequivocally determined due to the masking effect of the DAB precipitate. It should be noted that several DGL-α-labeled dendrites were found to be TH-immunonegative indicating that DGL-α may occur on non-dopaminergic cells as well (data not shown).
Fig. 4.
Dopaminergic neurons express DGL-α in the mouse ventral tegmental area. A) A dendrite of a dopaminergic neuron identified by tyrosine hydroxylase (TH)-immunostaining (dTH) is immunolabeled for DGL-α indicated by the presence of silver intensified immunogold particles (arrows) on the plasma membrane. Remarkably, immunogold particles are predominantly present at the perisynaptic annulus of synaptic specializations (arrowheads) formed by immunonegative axon terminals (b). B–C) DGL-α (arrows) is also present in small diameter TH-positive dendrites (dTH) receiving synaptic contacts (arrowheads) from unlabeled boutons (b). Scale bars: A–C, 0.5μm.
Presynaptic CB1 cannabinoid receptors are localized on both glutamatergic and GABAergic axon terminals in the VTA
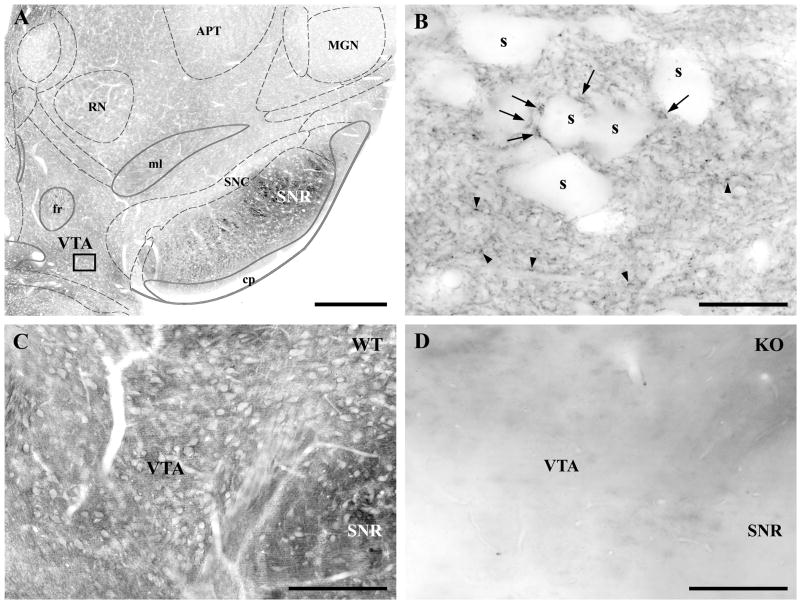
The widespread perisynaptic distribution of DGL-α on the dendritic arbor of dopaminergic and non-dopaminergic neurons taken together with extensive electrophysiological evidence suggest that cannabinoid receptors might be present on both glutamatergic and GABAergic axon terminals in the VTA (Lupica et al., 2004). Surprisingly though, previous anatomical studies reported a sparse distribution or a complete lack of CB1 cannabinoid receptors in the VTA (Herkenham et al., 1991b; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998). To resolve this disparity, we used a recently developed polyclonal antibody (Fukudome et al., 2004), which has significantly higher sensitivity over previously used antibodies for CB1 receptors (Kawamura et al., 2006; Katona et al., 2006). Immunostaining using this novel CB1 antibody revealed a dense neuropil labeling in the VTA of wild type animals (Fig. 5A–C), which was absent in the midbrain section from CB1 knockout mice (Fig. 5D). At higher magnification, punctate labeling was observed in wild type animals with two different sizes of granules at the light microscopic level (Fig. 5B). Larger puncta were scattered predominantly around CB1-immunonegative cell bodies and main dendritic trunks, whereas a meshwork of smaller CB1-immunoreactive profiles were evenly distributed throughout the neuropil (Fig. 5B).
Fig. 5.
Distribution of the CB1 cannabinoid receptors in the ventral tegmental area. A) Moderately dense CB1-immunolabeling is found in the ventral tegmental area (VTA) containing the A10 dopaminergic cell in the midbrain. Note that the other dopaminergic midbrain nucleus, the substantia nigra pars compacta (SNC) has much weaker labeling, whereas the adjacent substantia nigra pars reticulata (SNR) has a very high density of CB1-immunostaining. B) High magnification view of the framed area in A reveals a punctate staining pattern for CB1 with two different sizes of granules: large ones (marked by arrows) are mainly present around the cell bodies of CB1-immunonegative neurons (s), whereas the smaller ones (depicted by arrowheads) are widely distributed throughout the neuropil. C–D) The specificity of the CB1 antibody was tested using littermate wild type (WT) and knockout (KO) mice. Note that the neuropil staining for CB1 is present only in sections from the WT animals (C), and is completely absent in KO mice (D), which confirms the selectivity of the antibody. Abbreviations: APT, anterior pretectal nucleus; cp, cerebral peduncle, basal part; fr, fasciculus retroflexus; MGN, medial geniculate nucleus; ml, lemniscus medialis; RN, red nucleus. Scale bars: A, 500 μm; B, 20 μm; C–D, 200 μm.
To determine the identity of the immunostained profiles in the punctate pattern, we performed an electron microscopic analysis (Fig. 6–7). Both immunoperoxidase and immunogold techniques were utilized and revealed a similar distribution of immunoreactivity. CB1-immunostaining was restricted to axon terminals and the immunogold technique revealed that presynaptic CB1 receptors were located on the plasma membrane of boutons, mainly in extrasynaptic position. Moreover, immunogold particles representing the precise subcellular localization of CB1 were always attached to the intracellular side of the plasma membrane as predicted by the known intracellular localization of the C-terminus epitope further confirming the validity of these findings.
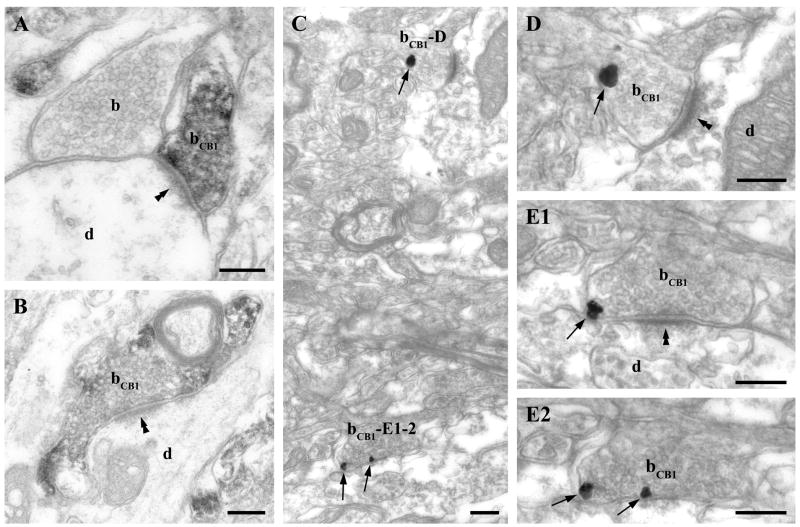
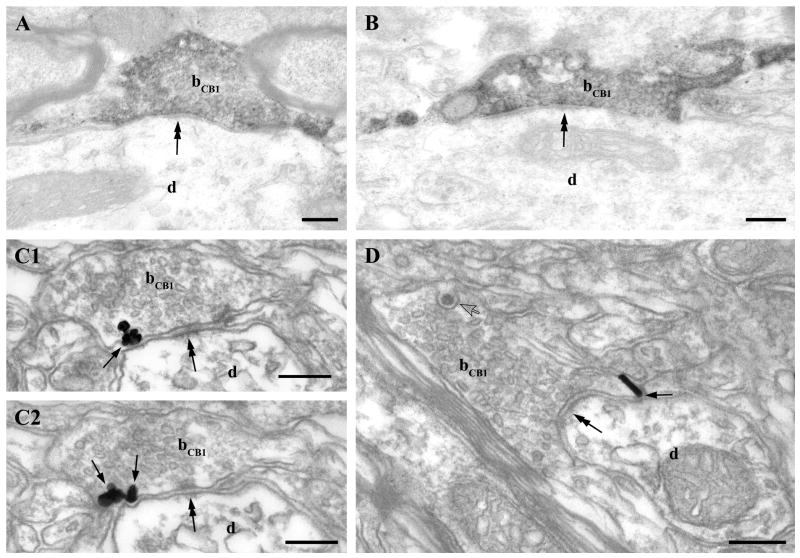
Fig. 6.
CB1 cannabinoid receptors are located presynaptically on glutamatergic axon terminals in the ventral tegmental area. A–B) The punctate immunostaining for CB1 observed at the light microscopic level in Fig. 5. partly corresponds to boutons (bCB1) giving rise to asymmetric synapses (double arrowheads) terminating on large (d in A) and small diameter (d in B) CB1-immunonegative dendrites. C-E2) shows weak but consistent labeling for CB1 with immunogold particles (depicted by arrows) in axon terminals (bCB1). D and E1–E2) Two CB1-positive axon terminals (bCB1) labeled with immunogold particles (arrows) in panel C are shown at higher magnification. These boutons form asymmetrical synapses with a robust postsynaptic density (double arrowheads) on immunonegative dendrites (d). E1-2 present two consecutive sections of the lower axon terminal in C (bCB1-E1-2). Note that all immunogold particles (arrows) representing the localization site of the CB1 receptors are attached to the intracellular surface of the plasma membrane in the axon terminals. Unlabeled axon terminal is indicated by ”b”. Scale bars: A-E2, 0.2μm.
Fig. 7.
CB1 cannabinoid receptors are located presynaptically on GABAergic axon terminals in the ventral tegmental area. A–B) Immunoperoxidase (DAB) staining for CB1 shows that CB1 is also present on axon terminals (bCB1) forming symmetric synapses (double arrows) on immunonegative thick proximal dendrites (d) – corresponding probably to the large-sized granules observed in the light microscopic image in Fig. 5. C1–2) Two consecutive sections demonstrate that CB1 receptors labeled with immunogold particles (arrows) are present presynaptically on putative intrinsic GABAergic axon terminals (bCB1) giving rise to symmetric synapses (double arrows). D shows another CB1-positive bouton (bCB1) giving rise to GABAergic, symmetric synaptic contact onto an unlabeled dendrite (d). However this axon terminal differs from the one depicted in panel C1–C2, because it contains a large dense core vesicle (open arrow) and it is densely packed with small, often flattened synaptic vesicles. Scale bars: A–D, 0.2μm.
The same three types of axon terminals identified as presynaptic to DGL-α positive profiles were found to be CB1-positive. First, smaller axon terminals forming synapses with thick postsynaptic density were found throughout the VTA (Fig. 6A, C–D). These boutons may belong to glutamatergic afferents of the VTA (Sesack and Pickel, 1992; Carr and Sesack, 2000; Omelchenko and Sesack, 2007). Second, large terminals containing numerous dense core vesicles formed both asymmetric (Fig. 7D) and symmetric synapses on thick dendritic shafts. The presence of dense core vesicles indicates that these axon terminals represent enkephalin- or dynorphin-positive extrinsic afferents of the VTA (Pickel et al., 1993; Sesack and Pickel, 1992 and 1995). Finally, CB1-bearing axon terminals with a moderate number of small flattened and round vesicles, but without large dense core vesicles were also encountered throughout the VTA. These boutons always formed symmetric synapses (Fig. 7C1–C2) and may belong to local intrinsic GABAergic neurons (Bayer and Pickel, 1991).
Postsynaptic DGL-α and presynaptic CB1 cannabinoid receptors are colocalized at both glutamatergic and GABAergic synapses
Retrograde endocannabinoid signaling mediates CB1 receptor-dependent short-term depression of both glutamatergic and GABAergic neurotransmission in the VTA (Riegel and Lupica, 2004; Melis et al., 2004b). The probable synaptic endocannabinoid responsible for this phenomenon is 2-AG (Melis et al., 2004a and 2006). These findings indicate that the underlying molecular machinery for retrograde 2-AG signaling might occur together at distinct types of synapses in the VTA. Indeed, combined double immunostaining confirmed that DGL-α is present perisynaptically on the postsynaptic side of both asymmetric and symmetric synapses, which are formed by CB1-immunoreactive axon terminals (Fig. 8). This observation confirms that distinct types of synapses are equipped with the basic molecular elements required for retrograde synaptic 2-AG signaling in the VTA.
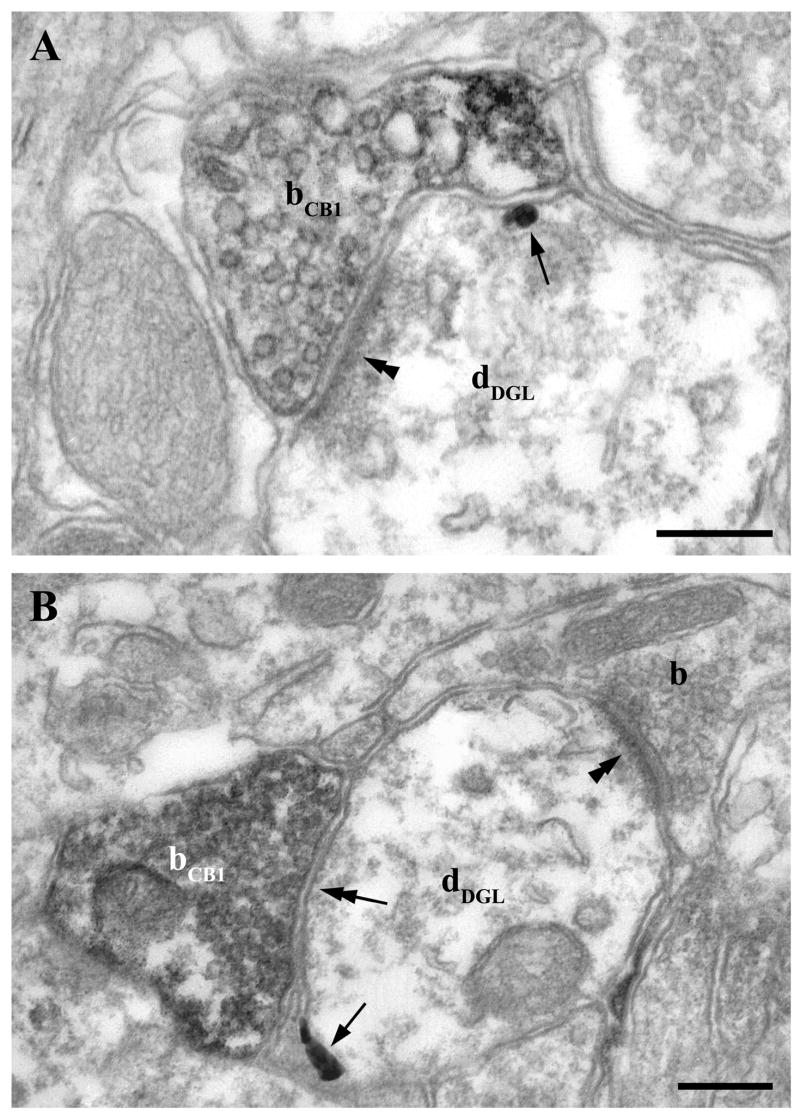
Fig. 8.
Colocalization of postsynaptic DGL-α enzymes and presynaptic CB1 receptors at both glutamatergic and GABAergic synapses. A–B) High-power electron micrographs confirm that DGL-α, the synthesizing enzyme for the endocannabinoid 2-AG, and CB1 cannabinoid receptor, the molecular target of 2-AG are colocalized at the same synapses. Combination of preembedding immunogold labeling for DGL-α and immunoperoxidase staining for CB1 reveals the postsynaptic position of this enzyme (arrows; dDGL) and presynaptic localization of the receptor (DAB; bCB1). This molecular architecture of the endocannabinoid system is present at both glutamatergic (asymmetric synaptic specialization; double arrowheads; in A and GABAergic (symmetric synaptic specialization; double arrows; in B) synapses. Scale bars: A–B, 0.2 μm.
Discussion
It is well established that the endocannabinoid system controls the primary rewarding effects of several drugs of abuse and thus plays a central role in drug addiction (Maldonado et al., 2006). Most of these drugs increase the burst firing activity of dopaminergic neurons in the ventral tegmental area (VTA), therefore cannabinoid modulation of the various types of synaptic afferents of dopaminergic neurons may be a potential neurobiological substrate to explain this striking phenomenon (Lupica and Riegel, 2005). In contrast, previous comparative anatomical studies did not find a widespread presence of cannabinoid receptors or other molecular elements of the endocannabinoid system in the VTA, although the adjacent substantia nigra pars reticulata has one of the highest density of cannabinoid receptors in the brain, which excludes technical reasons for the lack of labeling (Herkenham et al., 1991b; Mailleux and Vanderhaeghen, 1992; Tsou et al., 1998; Egertova and Elphick, 2000; Julian et al., 2003).
Here we report anatomical findings that resolve this discrepancy, and provide direct evidence for the presence of diacylglycerol lipase-alpha (DGL-α), the primary biosynthetic enzyme of 2-arachidonoylglycerol, as well as its target, the CB1 cannabinoid receptor, in several types of synapses in the VTA. The major findings of the present study are the following: (1) DGL-α mRNA is expressed by most neurons in the VTA; (2) DGL-α protein is concentrated around the postsynaptic specializations of both glutamatergic and GABAergic synapses on the dendrites of both dopaminergic and non-dopaminergic cells; (3) axon terminals forming these synapses are equipped with presynaptic CB1 cannabinoid receptors.
One important implication of the above findings is the central role of 2-AG at multiple synapses of the ventral tegmental area. A plethora of evidence suggests that 2-AG is the major endogenous ligand of the neuronal cannabinoid receptor CB1, and this endocannabinoid is present in the brain at much higher concentrations than its counterpart anandamide (for review see Sugiura et al., 2006). For example, Bisogno and colleagues found, using biochemical measurements, 100 times higher levels of 2-AG than anandamide in the mesencephalon (Bisogno et al., 1999). Beyond its regional distribution, the recent discovery of its biosynthetic enzyme sn-1-DGL-α paved the way for high-resolution anatomical studies, which can greatly facilitate the understanding of 2-AG’s role at the cellular and subcellular level as well (Bisogno et al., 2003). Our present findings along with earlier reports suggest that it is a widely expressed lipase in the brain (Bisogno et al., 2003; Katona et al., 2006; Yoshida et al., 2006, Uchigashima et al., 2007). In the VTA, we found that nearly all neurons express DGL-α mRNA, albeit at a lower level than principal neurons in the hippocampus (Katona et al., 2006). In accordance with a recent study, which have calculated that only 55% of the neurons are dopaminergic in the VTA (Margolis et al., 2006), our double immunostaining experiments confirmed directly the presence of DGL-α in both tyrosine hydroxylase (TH)-positive dopaminergic and TH-negative non-dopaminergic neurons. Thus, this ubiquitous distribution of DGL-α indicates that 2-AG may be released from several distinct cells in the VTA to modulate at least three distinct types of synapses.
Importantly, the subcellular distribution of 2-AG’s site of synthesis seems to follow also common principles in the brain. Previous electron microscopic analysis demonstrated that DGL-α is present on the plasma membrane, and its level is highest on the head of dendritic spines in a perisynaptic annulus around the postsynaptic density of glutamatergic synapses in case of hippocampal pyramidal cells and striatal medium spiny neurons (Katona et al., 2006; Uchigashima et al., 2007), whereas it is concentrated around the spine neck in case of Purkinje cells of the cerebellum (Yoshida et al., 2006). It is noteworthy that although VTA dopaminergic and non-dopaminergic neurons are generally aspiny and bear only a few spine-like protrusions called spinules (Grace and Onn, 1989; Steffensen et al., 1998), still, DGL-α immunostaining showed a punctate distribution in the VTA similarly to other brain areas, where principal neurons are densely covered with dendritic spines. The granular staining pattern corresponded to the accumulation of DGL-α adjacent to synapses located on dendritic shafts instead of spine heads. This striking positioning of DGL-α indicates that 2-AG release and signaling may be restricted to specific subcellular zones called the perisynaptic domains (Baude et al., 1993), which monitor the strength of presynaptic activity (e.g. by measuring transmitter spillover) and ensure synapse-specific feed-back signaling. The phenomenon has been well described in spine heads, but has only recently been demonstrated on aspiny dendrites in case of cerebellar stellate cells (Soler-Llavina and Sabatini, 2006).
The compartmentalization of endocannabinoid signaling is especially intriguing in light of the current findings that DGL-α is localized perisynaptically at various types of synapses received by dopaminergic and non-dopaminergic neurons. Moreover, our results have also revealed that 2-AG’s main target, the CB1 cannabinoid receptor occurs presynaptically on the opposite side of these various types of synapses in the VTA. The direct demonstration of the presence of CB1 receptors on glutamatergic afferents as well as on GABAergic afferents - deriving probably both from extrinsic sources like the nucleus accumbens and from intrinsic GABAergic neurons - corroborates several previous electrophysiological findings. CB1 receptors, which are generally presynaptic receptors throughout the central nervous system (Freund et al., 2003), have been shown to regulate synaptic neurotransmission derived from local GABAergic interneurons (Cheer et al., 2000; Szabó et al., 2002), from extrinsic GABAergic afferents (Riegel and Lupica, 2004), and from extrinsic glutamatergic afferents (Melis et al., 2004a). In contrast to the widespread presynaptic localization, CB1 receptor immunolabeling did not reach detection threshold on the somatic and dendritic profiles in the VTA. Nevertheless, this does not rule out the possibility that CB1 receptors may be expressed by dopaminergic neurons and are targeted to the terminal segments of their axons in the nucleus accumbens or in the prefrontal cortex, as has been suggested previously (Wenger et al., 2003; but see contradicting results from Herkenham et al., 1991a; Szabó et al., 1999; Kofalvi et al., 2005).
Taken together, the findings of the present study suggest that synaptic cannabinoid signaling has multiple substrates in the drug-reward circuitry of the VTA. At first glance, this heterogeneous distribution pattern renders the interpretation of the results of pharmacological treatments that lack any cellular or subcellular (synaptic) resolution more difficult. It may also hinder the generation of a simple, unifying framework to explain the contribution of cannabinoids to rewarding effects and drug addiction. However, a potential resolution and interpretation of these findings may be gained from recent electrophysiological observations (see for review Lupica and Riegel, 2005). Recently, two laboratories provided independent evidence that instead of an exclusively depolarization-dependent and spatially unrestricted release mechanism, endocannabinoids are released from dopaminergic neurons under special circumstances within certain types of synapses (Melis et al., 2004a, 2004b; Riegel and Lupica, 2004). It implies, very importantly, that instead of dopaminergic neurons non-specifically releasing endocannabinoids that simultaneously affect both glutamatergic and GABAergic synaptic inputs (which would not have a significant net alteration of dopaminergic firing activity), the synapse-specific activation of endocannabinoid release by coincidence of cellular depolarization and relevant incoming synaptic input seems to be the case in the VTA drug-reward circuitry. This may provide a more reasonable framework to explain cannabinoid effects on the activity of dopaminergic neurons, and its contribution to drug addiction (Lupica et al., 2004; Gardner, 2005). Based on this model, Lupica and Riegel (2005) have proposed that exclusively those dopaminergic neurons, which encode environmental stimuli with strong reward salience (Schultz, 1998), may enhance their own burst firing activity by escaping from tonic GABAergic inhibition using endocannabinoids. It is appealing to note the similarity of this proposal and the hypothesis by Freund et al. (2003), which suggests that endocannabinoid release may have a role in the formation of neuronal assemblies in the hippocampus encoding specific spatial position of the animal during exploration. As CB1-positive GABAergic interneurons fire coincidentally with the emergence of these neuronal assemblies in the hippocampus (Klausberger et al., 2005), a key experiment would be to test whether the in vivo firing pattern of local GABAergic neurons in the VTA ensures the precisely timed tonic inhibition on dopaminergic neurons to be counteracted by endocannabinoid release upon environmental stimuli or drug-associated inputs. This similarity in the molecular organization, the neural circuitry and the potential physiological significance further suggests that endocannabinoid signaling may have a conserved fundamental physiological role both at the synaptic and network level throughout the brain.
In conclusion, our findings reveal that multiple substrates for synaptic cannabinoid signaling exist in the VTA. Importantly, they all share a unifying principle in the molecular and anatomical organization of the endocannabinoid system, which involves a synthetic enzyme, DGL-α and a receptor, CB1 on the postsynaptic and presynaptic sides of the synapses, respectively.
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (T.F.F.), Országos Tudományos Kutatási Alapprogramok (OTKA) Grants T046820 (T.F.F.) and F046407 (I.K.), Egészségügyi Tudományos Tanács (ETT 561/2006) (I.K.), Nemzeti Kutatási és Fejlesztési Pályázatok (NKFP) Grant 1A/002/2004 (T.F.F.), National Institutes of Health Grants DA00286 and DA11322 (K.M.), NS30549 (T.F.F) and European Union Contract LSHM-CT-2004-005166 (T.F.F. and A.Z.). I.K. is a grantee of the János Bolyai scholarship. We are grateful to Katalin Lengyel, Em ke Simon, Katalin Iványi and Gy z Goda for excellent technical assistance and to Rita Nyilas for help with brain samples.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anthony JC, Warner LA, Kessler RC. Comparative epidemiology of dependence on tobacco, alcohol, controlled substances, and inhalants: Basic findings from the National Comorbidity Survey. Exp and Clin Psychopharmacol. 1994;2:244–268. [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Bayer VE, Pickel VM. GABA-labeled terminals form proportionally more synapses with dopaminergic neurons containing low densities of tyrosine hydroxylase-immunoreactivity in rat ventral tegmental area. Brain Res. 1991;559:44–55. doi: 10.1016/0006-8993(91)90285-4. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Berrendero F, Ambrosino G, Cebeira M, Ramos JA, Fernandez-Ruiz JJ, Di Marzo V. Brain regional distribution of endocannabinoids: implications for their biosynthesis and biological function. Biochem Biophys Res Commun. 1999;256:377–380. doi: 10.1006/bbrc.1999.0254. [DOI] [PubMed] [Google Scholar]
- Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DB, Sesack SR. Projections from the rat prefrontal cortex to the ventral tegmental area: target specificity in the synaptic associations with mesoaccumbens and mesocortical neurons. J Neurosci. 2000;20:3864–3873. doi: 10.1523/JNEUROSCI.20-10-03864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Marsden CA, Kendall DA, Mason R. Lack of response suppression follows repeated ventral tegmental cannabinoid administration: an in vitro electrophysiological study. Neuroscience. 2000;99:661–667. doi: 10.1016/s0306-4522(00)00241-4. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Kendall DA, Mason R, Marsden CA. Differential cannabinoid-induced electrophysiological effects in rat ventral tegmentum. Neuropharmacology. 2003;44:633–641. doi: 10.1016/s0028-3908(03)00029-7. [DOI] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Heien ML, Phillips PE, Wightman RM. Cannabinoids enhance subsecond dopamine release in the nucleus accumbens of awake rats. J Neurosci. 2004;24:4393–4400. doi: 10.1523/JNEUROSCI.0529-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheer JF, Wassum KM, Sombers LA, Heien ML, Ariansen JL, Aragona BJ, Phillips PE, Wightman RM. Phasic dopamine release evoked by abused substances requires cannabinoid receptor activation. J Neurosci. 2007;27:791–795. doi: 10.1523/JNEUROSCI.4152-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Paredes W, Lowinson JH, Gardner EL. Delta 9-tetrahydrocannabinol enhances presynaptic dopamine efflux in medial prefrontal cortex. Eur J Pharmacol. 1990;190:259–262. doi: 10.1016/0014-2999(90)94136-l. [DOI] [PubMed] [Google Scholar]
- Egertová M, Elphick MR. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J Comp Neurol. 2000;422:159–171. doi: 10.1002/(sici)1096-9861(20000626)422:2<159::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- French ED. delta9-Tetrahydrocannabinol excites rat VTA dopamine neurons through activation of cannabinoid CB1 but not opioid receptors. Neurosci Lett. 1997;226:159–162. doi: 10.1016/s0304-3940(97)00278-4. [DOI] [PubMed] [Google Scholar]
- French ED, Dillon K, Wu X. Cannabinoids excite dopamine neurons in the ventral tegmentum and substantia nigra. Neuroreport. 1997;8:649–652. doi: 10.1097/00001756-199702100-00014. [DOI] [PubMed] [Google Scholar]
- Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signaling. Physiol Rev. 2003;83:1017–1066. doi: 10.1152/physrev.00004.2003. [DOI] [PubMed] [Google Scholar]
- Fukudome Y, Ohno-Shosaku T, Matsui M, Omori Y, Fukaya M, Tsubokawa H, Taketo MM, Watanabe M, Manabe T, Kano M. Two distinct classes of muscarinic action on hippocampal inhibitory synapses: M2-mediated direct suppression and M1/M3-mediated indirect suppression through endocannabinoid signalling. Eur J Neurosci. 2004;19:2682–2692. doi: 10.1111/j.0953-816X.2004.03384.x. [DOI] [PubMed] [Google Scholar]
- Gardner EL. Endocannabinoid signaling system and brain reward: emphasis on dopamine. Pharmacol Biochem Behav. 2005;81:263–284. doi: 10.1016/j.pbb.2005.01.032. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Melis M, Muntoni AL, Diana M. Cannabinoids activate mesolimbic dopamine neurons by an action on cannabinoid CB1 receptors. Eur J Pharmacol. 1998;341:39–44. doi: 10.1016/s0014-2999(97)01442-8. [DOI] [PubMed] [Google Scholar]
- Grace AA, Onn SP. Morphology and electrophysiological properties of immunocytochemically identified rat dopamine neurons recorded in vitro. J Neurosci. 1989;9:3463–3481. doi: 10.1523/JNEUROSCI.09-10-03463.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, de Costa BR, Richfield EK. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991a;547:267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, de Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in rat brain: a quantitative in vitro autoradiographic study. J Neurosci. 1991b;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian MD, Martin AB, Cuellar B, Rodriguez De Fonseca F, Navarro M, Moratalla R, Garcia-Segura LM. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119:309–318. doi: 10.1016/s0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- Katona I, Urbán GM, Wallace M, Ledent C, Jung KM, Piomelli D, Mackie K, Freund TF. Molecular composition of the endocannabinoid system at glutamatergic synapses. J Neurosci. 2006;26:5628–5637. doi: 10.1523/JNEUROSCI.0309-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura Y, Fukaya M, Maejima T, Yoshida T, Miura E, Watanabe M, Ohno-Shosaku T, Kano M. The CB1 cannabinoid receptor is the major cannabinoid receptor at excitatory presynaptic sites in the hippocampus and cerebellum. J Neurosci. 2006;26:2991–3001. doi: 10.1523/JNEUROSCI.4872-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausberger T, Marton LF, O’Neill J, Huck JH, Dalezios Y, Fuentealba P, Suen WY, Papp E, Kaneko T, Watanabe M, Csicsvari J, Somogyi P. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofalvi A, Rodrigues RJ, Ledent C, Mackie K, Vizi ES, Cunha RA, Sperlagh B. Involvement of cannabinoid receptors in the regulation of neurotransmitter release in the rodent striatum: a combined immunochemical and pharmacological analysis. J Neurosci. 2005;25:2874–2884. doi: 10.1523/JNEUROSCI.4232-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC, Hoffman AF. Marijuana and cannabinoid regulation of brain reward circuits. Br J Pharmacol. 2004;143:227–234. doi: 10.1038/sj.bjp.0705931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupica CR, Riegel AC. Endocannabinoid release from midbrain dopamine neurons: a potential substrate for cannabinoid receptor antagonist treatment of addiction. Neuropharmacology. 2005;48:1105–1116. doi: 10.1016/j.neuropharm.2005.03.016. [DOI] [PubMed] [Google Scholar]
- Mailleux P, Vanderhaeghen JJ. Distribution of neuronal cannabinoid receptor in the adult rat brain: a comparative receptor binding radioautography and in situ hybridization histochemistry. Neuroscience. 1992;48:655–668. doi: 10.1016/0306-4522(92)90409-u. [DOI] [PubMed] [Google Scholar]
- Maldonado R, Valverde O, Berrendero F. Involvement of the endocannabinoid system in drug addiction. Trends Neurosci. 2006;29:225–232. doi: 10.1016/j.tins.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Lock H, Hjelmstad GO, Fields HL. The ventral tegmental area revisited: is there an electrophysiological marker for dopaminergic neurons? J Physiol. 2006;577:907–924. doi: 10.1113/jphysiol.2006.117069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Perra S, Muntoni AL, Pillolla G, Lutz B, Marsicano G, Di Marzo V, Gessa GL, Pistis M. Prefrontal cortex stimulation induces 2-arachidonoyl-glycerol-mediated suppression of excitation in dopamine neurons. J Neurosci. 2004a;24:10707–10715. doi: 10.1523/JNEUROSCI.3502-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pistis M, Perra S, Muntoni AL, Pillolla G, Gessa GL. Endocannabinoids Mediate Presynaptic Inhibition of Glutamatergic Transmission in Rat Ventral Tegmental Area Dopamine Neurons through Activation of CB1 Receptors. J Neurosci. 2004b;24:53–62. doi: 10.1523/JNEUROSCI.4503-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Bisogno T, Minassi A, Petrosino S, Perra S, Muntoni AL, Lutz B, Gessa GL, Marsicano G, Di Marzo V, Pistis M. Protective activation of the endocannabinoid system during ischemia in dopamine neurons. Neurobiol Dis. 2006;24:15–27. doi: 10.1016/j.nbd.2006.04.010. [DOI] [PubMed] [Google Scholar]
- Omelchenko N, Sesack SR. Glutamate synaptic inputs to ventral tegmental area neurons in the rat derive primarily from subcortical sources. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.02.016. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Sesack SR. Cellular substrates for interactions between dynorphin terminals and dopamine dendrites in rat ventral tegmental area and substantia nigra. Brain Res. 1993;602:275–289. doi: 10.1016/0006-8993(93)90693-h. [DOI] [PubMed] [Google Scholar]
- Riegel AC, Lupica CR. Independent presynaptic and postsynaptic mechanisms regulate endocannabinoid signaling at multiple synapses in the ventral tegmental area. J Neurosci. 2004;24:11070–11078. doi: 10.1523/JNEUROSCI.3695-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Prefrontal cortical efferents in the rat synapse on unlabeled neuronal targets of catecholamine terminals in the nucleus accumbens septi and on dopamine neurons in the ventral tegmental area. J Comp Neurol. 1992;320:145–160. doi: 10.1002/cne.903200202. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Pickel VM. Ultrastructural relationships between terminals immunoreactive for enkephalin, GABA, or both transmitters in the rat ventral tegmental area. Brain Res. 1995;672:261–275. doi: 10.1016/0006-8993(94)01391-t. [DOI] [PubMed] [Google Scholar]
- Soler-Llavina GJ, Sabatini BL. Synapse-specific plasticity and compartmentalized signaling in cerebellar stellate cells. Nat Neurosci. 2006;9:798–806. doi: 10.1038/nn1698. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Svingos AL, Pickel VM, Henriksen SJ. Electrophysiological characterization of GABAergic neurons in the ventral tegmental area. J Neurosci. 1998;18:8003–8015. doi: 10.1523/JNEUROSCI.18-19-08003.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Office of Applied Studies, NHSDA Series H-22, DHHS Publication No. SMA 03-3836. Rockville, MD: 2003. Results from the 2002 National Survey on Drug Use and Health: National Findings. [Google Scholar]
- Sugiura T, Kishimoto S, Oka S, Gokoh M. Biochemistry, pharmacology and physiology of 2-arachidonoylglycerol, an endogenous cannabinoid receptor ligand. Prog Lipid Res. 2006;45:405–446. doi: 10.1016/j.plipres.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Szabó B, Muller T, Koch H. Effects of cannabinoids on dopamine release in the corpus striatum and the nucleus accumbens in vitro. J Neurochem. 1999;73:1084–1089. doi: 10.1046/j.1471-4159.1999.0731084.x. [DOI] [PubMed] [Google Scholar]
- Szabó B, Siemes S, Wallmichrath I. Inhibition of GABAergic neurotransmission in the ventral tegmental area by cannabinoids. Eur J Neurosci. 2002;15:2057–2061. doi: 10.1046/j.1460-9568.2002.02041.x. [DOI] [PubMed] [Google Scholar]
- Tanda G, Pontieri FE, Di Chiara G. Cannabinoid and heroin activation of mesolimbic dopamine transmission by a common mu1 opioid receptor mechanism. Science. 1997;276:2048–2050. doi: 10.1126/science.276.5321.2048. [DOI] [PubMed] [Google Scholar]
- Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- Uchigashima M, Narushima M, Fukaya M, Katona I, Kano M, Watanabe M. Subcellular Arrangement of Molecules for 2-Arachidonoyl-Glycerol-Mediated Retrograde Signaling and Its Physiological Contribution to Synaptic Modulation in the Striatum. J Neurosci. 2007;27:3663–3676. doi: 10.1523/JNEUROSCI.0448-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenger T, Moldrich G, Furst S. Neuromorphological background of cannabis addiction. Brain Res Bull. 2003;61:125–128. doi: 10.1016/s0361-9230(03)00081-9. [DOI] [PubMed] [Google Scholar]
- Wu X, French ED. Effects of chronic delta9-tetrahydrocannabinol on rat midbrain dopamine neurons: an electrophysiological assessment. Neuropharmacology. 2000;39:391–398. doi: 10.1016/s0028-3908(99)00140-9. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Fukaya M, Uchigashima M, Miura E, Kamiya H, Kano M, Watanabe M. Localization of diacylglycerol lipase-alpha around postsynaptic spine suggests close proximity between production site of an endocannabinoid, 2-arachidonoyl-glycerol, and presynaptic cannabinoid CB1 receptor. J Neurosci. 2006;26:4740–4751. doi: 10.1523/JNEUROSCI.0054-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zangen A, Solinas M, Ikemoto S, Goldberg SR, Wise RA. Two brain sites for cannabinoid reward. J Neurosci. 2006;26:4901–4907. doi: 10.1523/JNEUROSCI.3554-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]