Abstract
The diagnosis of invasive aspergillosis (IA) based on the detection of Aspergillus galactomannan (GM) is complicated by the presence of cross-reactive GM epitopes in patient specimens. We have developed a novel and specific Aspergillus antigen-capture enzyme-linked immunosorbent assay (ELISA) by the selection of two well-characterized monoclonal antibodies from 17 candidate antibodies. The epitopes recognized by the monoclonal antibodies were present on the cell walls of the hyphae and the conidia of Aspergillus species, which were circulating or excreted as immunodominant antigens during the acute phase of IA established in the animal models. The detection of experimental Aspergillus-mediated antigenemia was suitably sensitive, and the sensitivity was comparable to that of a commercial GM detection ELISA kit (the Platelia Aspergillus assay). Moreover, the specificity of this assay was 100% when it was used to test 382 serum specimens and 120 urine specimens from healthy individuals. Cross-reactivity with other common opportunistic fungi, such as Penicillium and Candida species, and with purified GM protein derived from Aspergillus was not evident. Therefore, the chemical nature of the epitopes captured in this assay is most likely not associated with the GM structure, indicating that this newly developed Aspergillus antigen-capture ELISA is a promising tool for the diagnosis of IA without the risk of the false-positive results that are problematic with current GM antigen assays.
In recent years, the numbers of cases of invasive aspergillosis (IA) associated with high rates of morbidity and mortality have increased, likely due to the greater prevalence of immunosuppressive therapies being performed (6, 33). IA is most commonly caused by Aspergillus fumigatus and A. flavus and less frequently by A. terreus, A. niger, and A. nidulans (6). The early and accurate diagnosis of IA is critical in improving the prognosis for patients through the delivery of more prompt antifungal therapy and lessening the unnecessary use of toxic antifungal drugs (32). However, the early clinical diagnosis of IA is often difficult, since the signs and symptoms of infection are nonspecific. A positive blood fungal culture is rarely obtained during the early stage of the infection, and antibody detection is often negative, as the majority of immunosuppressed patients have a weak antibody response (13, 38).
Recent efforts to improve the early diagnosis of IA have focused on the detection of circulating antigens. Galactomannan (GM), which is present in the cell walls of most Aspergillus species, is an effective marker for facilitating the early detection of the antigenemia of IA (28). Polyclonal antibodies are capable of detecting the GM of Aspergillus (2, 4, 8). However, assays based on such antibodies are subject to variable intra- and interlaboratory results due to batch-to-batch variations in antisera. In addition, antigen tests based on polyclonal antibodies raised against crude fungal antigens exhibit significant cross-reactivity with several fungal antigens (7). Monoclonal antibody (MAb)-based immunodiagnostic assays are preferred over polyclonal antibody-based assays. Two immunoassays that employ a rat immunoglobulin M (IgM) MAb designated EB-A2 for the detection of circulating Aspergillus GM have recently been developed (24, 25). One of these, designated the Platelia Aspergillus assay (Bio-Rad, Marnes-La-Coquette, France), is a commercially available, double-sandwich enzyme-linked immunosorbent assay (ELISA) that utilizes MAb EB-A2 as both the capture and the detector antibody; the assay enjoys worldwide use for the diagnosis of IA (17). Studies that have evaluated the Platelia Aspergillus assay have documented a high percentage of false-positive results when serum or urine samples from immunocompromised patients without evidence of aspergillosis are tested (29, 30), even though antigen detection is sensitive. Other studies have reported a high incidence of false-positive results (up to 74%) when the assay system is used to test patients treated with Penicillium-derived piperacillin-tazobactam (26, 31). This may be because MAb EB-A2 recognizes the β-(1→5)-linked galactofuranoside side chain residues of Aspergillus GM, but also recognizes cross-reacting epitopes on other fungal polysaccharide cell wall components (i.e., Penicillium species) (13, 25). Thus, the occurrence of false-positive results may be caused by the cross-reactive epitopes in human serum or contamination by other fungal components. Since many antibiotics originate from fungi (i.e., ampicillin-sulbactam, piperacillin-tazobactam, and amoxicillin-clavulanic acid) and since these drugs are commonly used for the management of febrile immunosuppressed patients, the occurrence of false-positive results in patients during the administration of these drugs may limit the utility of the Platelia Aspergillus assay, leading to inappropriate treatment. This concern may also extend to pediatric populations (21), with which false-positive rates are as high as 83% (23). The false-positive results most likely relate to the cross-reacting epitopes of MAb EB-A2 with Bifidobacterium lipoteichoic acid, which is abundant in the neonatal gut and which may be transported through the immature intestinal mucosa into the bloodstream (18). Indeed, cross-reactions of rat anti-GM MAb EB-A2 have been described with other organisms and foods (1, 13, 17, 22, 34). It is conceivable that the passage of food-derived GM through intestinal mucosa damaged as a result of chemotherapy may underlie the cross-reactivity (12).
With the aim of improving the diagnosis of IA, we produced and characterized a set of 17 MAbs against a released Aspergillus cell wall antigen and tested the practicality of their use in the development of a sensitive and specific antigen-capture ELISA. The study involved the use of an experimental rabbit model of aspergillosis for determination of the value of the assay during the acute phase of the disease.
MATERIALS AND METHODS
Strains.
The A. fumigatus (strain QMH5526), A. flavus (strain QMH16962), A. terreus, A. niger, A. nidulans, and Penicillium marneffei (strain PM4) strains used in this study (36) were obtained from the Department of Microbiology, University of Hong Kong. Candida albicans, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis were obtained from the Research Centre for Medical Mycology, Beijing University, China. All of these fungal stains were originally clinical isolates recovered from infectious patients.
Preparation of fungal antigens.
Fungal strains were grown first on Sabouraud agar plates at 37°C for several days to form single colonies. The conidia from the plates were inoculated into 50 ml of RPMI medium in a 500-ml flask on a shaker at 37°C for 3 to 7 days; the cultures were harvested and separated from the mycelia by filtration. For the preparation of mycelial extract antigens (MAs) of A. fumigatus, the mycelia were collected and resuspended in phosphate-buffered saline (PBS). After disruption of the mycelia by sonication, the supernatant was collected by centrifugation. The resultant MAs were stored at −80°C until they were used. For the preparation of excretive antigens (EAs) from the culture supernatant of A. fumigatus, the cells were cultured in a 1-liter flask containing 200 ml RPMI medium, and incubation was continued for 3 to 4 weeks with shaking at 25°C. EAs were recovered by precipitation of the culture filtrates with 4 volumes of ethanol, as described previously (16). The precipitates were washed three times with ethanol, resuspended in water, and frozen at −80°C until they were used. For the preparation of inactivated conidia of A. fumigatus, the conidia were collected from the Sabouraud agar plate cultures and were then washed two times with sterile PBS. The conidia were resuspended in PBS with a final concentration of 3.7% formalin solution. After inactivation overnight at 4°C, the conidia were washed at least 10 times with sterile PBS.
To further purify the mannoproteins from the crude culture filtrate of A. fumigatus, the culture filtrate was passed through a concanavalin A (ConA) Sepharose 4B affinity column (Amersham Biosciences AB, Uppsala, Sweden). The column was first washed with equilibration buffer, as described previously (19), and was then washed extensively with acetate buffer (0.1 M; pH 6.0) until no protein was detected in the column effluent. The bound substances were subsequently eluted with equilibration buffer containing 0.4 M α-methyl-d-mannoside (Sigma-Aldrich, St. Louis, MO). The effluents were concentrated by using centrifugal filter units (Millipore Corporation, Billerica, MA) with a molecular weight cutoff of 10,000 and were stored at −80°C until they were used. The concentration of protein was determined by use of the Coomassie Plus protein assay reagent (Pierce Biotechnology, Rockford, IL), according to the manufacturer's instructions.
Production of MAbs against Aspergillus.
BALB/c mice were immunized by using the MAs, EAs, and the antigens of inactivated conidia from A. fumigatus. Two different protocols were performed. In the first protocol, 4- to 6-week-old female BALB/c mice were immunized subcutaneously with 100 μg of MAs or EAs emulsified with complete Freund's adjuvant (Sigma-Aldrich), followed by four booster doses of 50 μg of the particular antigen in incomplete Freund's adjuvant every 10 days. Three days before the establishment of fusion hybridomas, a booster dose of 100 μg of the particular antigen in saline was injected intravenously. In the second immunization protocol, similarly aged female BALB/c mice received 106 inactivated conidia of A. fumigatus intravenously on days 1, 10, 20, and 30. Three days before the establishment of the fusion hybridomas, a booster dose of 100 μg of EAs in saline was injected intravenously. Splenocytes from the immunized mice were fused with NS-1 myeloma cells (3). The MAbs produced and excreted from the hybridomas were screened by an indirect ELISA with both the MAs and the EAs of A. fumigatus as the coating antigens. Positive hybridoma cells were cloned by limiting dilution. The MAb isotypes were determined by using a commercially available mouse MAb isotyping kit (Zymed Laboratories, Carlsbad, CA). The MAbs were purified from ascitic fluids by ammonium sulfate precipitation and were conjugated with highly purified horseradish peroxidase (HRP; Sigma-Aldrich) by the use of periodate (35).
Indirect immunofluorescent staining.
An indirect immunofluorescent assay (IFA) was carried out as described previously (39), with modifications. In brief, the fungal mycelium growth was washed twice with PBS, resuspended in PBS at a cell density of 106/ml, and placed on wells of Teflon-coated slides. The immobilized cells were fixed for 10 min with prechilled fixed buffer containing acetone (70%, wt/vol) and methanol (30%, vol/vol) and stored at −70°C until they were used. The slides were incubated with the particular MAb at a concentration of 10 μg/ml in a humid chamber for 60 min at 37°C. After the slides were washed with PBS containing 0.1% (vol/vol) Tween 20, fluorescein isothiocyanate-conjugated goat anti-mouse IgG or IgM (Zymed Laboratories) was added and the slides were incubated for 40 min at 37°C. After several washes, a 0.25% (wt/vol) solution of Evans blue in PBS was added for counterstaining; fluorescence was determined by fluorescent laser confocal microscopy with a LEICA TCS SP2 AOBS microscope.
Immunohistochemical staining.
Tissue sections (4 μm) obtained from patients with Aspergillus infections or from the experimental rabbit model of IA were fixed in a 10% formalin solution, embedded in paraffin, and heat fixed at 55°C for 30 min. Slides of the sections, prepared as described previously (9), were incubated with the MAbs (100 μg/ml) in a humid chamber at 4°C overnight. After the slides were washed in PBS, they were incubated with HRP-conjugated goat anti-mouse IgG or IgM (Zymed Laboratories) at 37°C for 30 min. The slides were washed with PBS, counterstained with hematoxylin for 1 min, dehydrated, mounted with Permount (Fisher Scientific, Pittsburgh, PA), and examined by light microscopy.
Western blotting.
The ConA-purified mannoproteins of A. fumigatus were electrophoretically separated in 8% sodium dodecyl sulfate-polyacrylamide gels and transferred to a nitrocellulose membrane. After the membrane was blocking with a blocking reagent, each membrane was incubated with an HRP-conjugated MAb for 1 h at 37°C, washed several times with 0.5% (vol/vol) Tween 20 in PBS, and developed by incubation with a solution of diaminobenzidine (Amresco Inc, Solon, OH).
Competition ELISA.
The binding epitopes of the MAbs were analyzed by use of a previously described competition ELISA (37). Microwell plates (Costar Corning, Corning, NY) were coated with 100 μl of A. fumigatus MAs at a concentration of 30 μg/ml in coating buffer. After the blocking steps were performed, unlabeled MAbs were added to the wells at different concentrations and incubated overnight at 4°C, which allowed saturating amounts of antibody to bind to the immobilized antigen. The plates were washed, and HRP-conjugated MAb was added to the appropriate wells at optimal dilutions determined by titration and incubated for 1 h at 37°C. After the plates were washed, the binding of HRP-labeling MAb was detected by addition of tetramethylbenzidine (Amresco Inc.); the reaction was stopped after 10 min by the addition of 1 N sulfuric acid, and the plates were examined in an ELISA plate reader (Bio-Tek, Winooski, VT). An irrelevant, unlabeled MAb was used as a control. The percentage of inhibition was calculated by the following formula: [1 − (OD450 of the test well/OD450 of the control well)] × 100 (where OD450 is the optical density at 450 nm). The results were described as competition if the inhibition was >75%, inhibition from 25 to 75% represented relative competition, and <25% inhibition was described as noncompetitive (37).
Experimental IA rabbit model.
Female New Zealand White rabbits (weight, 2 to 3 kg) were immunosuppressed by the subcutaneous injection of one dose of cyclophosphamide (25 mg/kg of body weight) and cortisone acetate (15 mg/kg) 2 days before infection and an additional of cortisone acetate (15 mg/kg) 1 day before and on the day of infection, as described previously (14). The rabbits were infected intravenously with 2 × 107 Aspergillus conidia. Blood samples were obtained every day until death. Autopsies were done on all rabbits; both kidneys and a sample of liver tissue were immediately removed, fixed in formalin, and embedded in paraffin wax for histochemical staining with hematoxylin-eosin (9). Immunohistochemistry examination was carried out as described earlier in the text.
Development of MAb-based Aspergillus antigen-capture ELISA.
The procedure for the antigen-capture ELISA was carried out as described previously (37), with modifications. In brief, microwell plates (Corning) were coated with 100 μl/well of capture MAb overnight at 4°C, and then the wells were incubated with a blocking reagent. After removal of the blocking solution, a series of samples diluted to 100 μl/well was added, and the plates were incubated for 1 h at 37°C. After the plates were washed, 100 μl/well of diluted HRP-conjugated MAb was added and the plates were incubated for 30 min at 37°C. After a further wash, 100 μl/well of tetramethylbenzidine (Amresco Inc.) was added, and the reaction was stopped after incubation for 10 min by the addition of 1 N sulfuric acid. The absorbance was determined as described above. When animal or human serum was tested, the samples were diluted 1:2 in PBS containing 4% EDTA and heated at 100°C for 3 min.
Detection of GM.
The GM antigen of Aspergillus was detected by a commercial one-step capture ELISA (the Platelia Aspergillus assay Bio-Rad), according to the manufacturer's instructions.
RESULTS
MAbs against Aspergillus species.
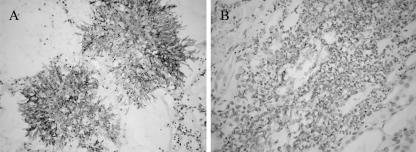
A total of 17 hybridoma cell lines that stably produced MAbs against A. fumigatus were established. They were selected on the basis of their strong positive ELISA reactions with A. fumigatus MAs and EAs, and the reactivities were further confirmed by IFA. All of these hybridoma cell lines were subcloned at least once or sometimes more times. Of the 17 MAbs, 7 were generated from mice immunized with MAs (MAbs MA1, MA2, MA3, MA4, MA5, MA6, and MA7), 8 were generated from mice immunized with EAs (MAbs EA1, EA2, EA3, EA4, EA5, EA6, EA7, and EA8), and the remaining 2 were generated from mice immunized with inactivated conidia (MAbs Con1 and Con2). Isotype determinations revealed that seven MAbs were IgM, eight were IgG1, and two were IgG3. The reactivities of the MAbs with Aspergillus species are summarized in Table 1. All MAbs reacted strongly to A. fumigatus EAs and MAs, displaying OD450s exceeding the value of 1.5 in an ELISA experiment. IFA demonstrated that all MAbs recognized A. fumigatus hyphae, with 13 MAbs additionally recognizing the conidia of this fungal species. In addition to reacting with A. fumigatus, the MAbs also cross-reacted with other Aspergillus species, including A. flavus, A. terreus, A. niger, and A. nidulans. These MAbs were not reactive with P. marneffei. Also, there was no recognition of either the hyphae or the conidia of the five Candida species tested (C. albicans, C. glabrata, C. tropicalis, C. krusei, and C. parapsilosis), with the exception of MAbs Con1 and Con2, which cross-reacted with the conidia but not with the hyphae of the five Candida species (data not shown), suggesting that the cross epitopes recognized by MAbs Con1 and Con2 were present on the conidia of Candida species. To test for the MAb identification of Aspergillus infection in tissue samples, formalin-fixed paraffin-embedded tissue sections obtained from both the animal model of IA and Aspergillus-infected patients were examined by IHC. All MAbs clearly recognized A. fumigatus hyphae in kidney tissue obtained from the rabbit model of IA, 14 MAbs reacted with hyphae in the lung tissue from a patient whose postmortem examination confirmed IA (33), and 13 MAbs reacted with hyphae in the lung tissue from a patient with biopsy-confirmed aspergilloma (Table 1; Fig. 1).
TABLE 1.
Reactivities of MAbs against Aspergillus by IFA, ELISA, and IHC
| MAb | Isotype | Reactivity by IFAa
|
A. fumigatus ELISA result (OD450)a
|
Reactivity by IHCa,b
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
A. fumigatus
|
A. flavus
|
A. niger
|
A. terreus
|
A. nidulans
|
A | B | C | |||||||||
| Conidia | Hyphae | Conidia | Hyphae | Conidia | Hyphae | Conidia | Hyphae | Conidia | Hyphae | EAs | MAs | |||||
| MA7 | IgG3 | ++++ | ++++ | +++ | +++ | − | +++ | − | − | +++ | +++ | 2.038 | 2.304 | +++ | ++ | +++ |
| EA4 | IgM | ++ | +++ | + | ++++ | + | ++ | − | − | + | ++ | 1.576 | 2.058 | ++ | +++ | ++ |
| EA5 | IgM | ++ | +++ | + | ++++ | + | + | − | − | ++ | + | 1.777 | 2.284 | ++ | ++ | +++ |
| EA8 | IgM | ++++ | ++++ | ++++ | ++++ | +++ | ++ | − | − | ++ | + | 1.945 | 2.333 | ++ | + | +++ |
| MA4 | IgM | ++++ | ++++ | ++++ | ++++ | ++ | + | − | − | ++ | ++ | 2.233 | 2.55 | ++ | ++ | +++ |
| EA6 | IgM | ++++ | ++++ | ++ | ++++ | ++ | ++ | − | − | +++ | + | 1.938 | 2.460 | ++ | ++ | ++ |
| EA2 | IgG1 | − | ++ | − | − | − | − | − | + | − | − | 2.261 | 2.861 | +++ | ++ | + |
| EA3 | IgG1 | − | ++ | − | + | − | − | − | + | − | − | 2.350 | 2.950 | + | + | + |
| MA1 | IgG1 | − | ++ | − | − | − | − | − | − | − | + | 1.620 | 2.358 | + | − | + |
| EA1 | IgG1 | + | ++ | − | − | − | + | − | − | − | − | 1.538 | 2.407 | + | +++ | + |
| Con1 | IgM | ++ | ++ | ++ | +++ | + | + | ++ | +++ | +++ | +++ | 1.731 | 2.548 | + | − | − |
| Con2 | IgG3 | +++ | ++ | ++ | ++ | ++ | + | +++ | ++ | ++ | ++ | 1.676 | 2.558 | + | − | ++ |
| EA7 | IgM | +++ | +++ | ++ | ++++ | ++ | ++ | ++ | ++++ | − | +++ | 2.343 | 2.302 | + | + | − |
| MA2 | IgG1 | ++ | ++++ | ++ | +++ | ++ | ++++ | +++ | ++++ | + | ++ | 2.432 | 1.957 | ++ | ++ | − |
| MA3 | IgG1 | + | +++ | + | +++ | ++ | +++ | +++ | +++ | − | − | 1.893 | 2.668 | ++ | +++ | + |
| MA5 | IgG1 | − | ++ | − | + | − | + | − | + | − | − | 1.841 | 2.513 | + | + | + |
| MA6 | IgG1 | ++ | ++++ | ++ | ++ | + | +++ | +++ | ++++ | + | ++++ | 2.344 | 2.344 | ++ | + | − |
The reactivity of each MAb was determined by IFA to the mycelia and conidia of Aspergillus species, by indirect ELISA to the MAs and EAs of A. fumigatus, and by immunohistochemical assay (IHC) to formalin-fixed paraffin section of tissues. −, no reactivity; +, weak reactivity; ++, intermediate reactivity; +++, strong reactivity; ++++, very strong reactivity.
A, kidney tissue from an experimental rabbit model of IA; B, lung tissue from a patient with IA; C, lung tissue from a patient with aspergilloma.
FIG. 1.
Immunohistochemical analyses of MAb EA6 in formalin-fixed paraffin tissue sections. (A) Immunohistochemical staining of lung tissue from a patient with invasive aspergillosis. Magnification, ×200. (B) Immunohistochemical staining of kidney tissue from the experimental rabbit model of IA. Magnification, ×400. No staining occurred with the control MAb.
MAb binding to cell wall antigens of A. fumigatus.
Confocal microscopy of immunofluorescently stained cells demonstrated that all the MAbs were strongly reactive to A. fumigatus hyphae; intense fluorescence was evident in the hyphal cell wall. The conidial cell wall also exhibited fluorescence (Fig. 2).
FIG. 2.
Indirect immunofluorescent analyses of MAb MA6 binding to the hyphae and the conidia of A. fumigatus. Confocal microscopy immunofluorescent images (A and C) and contrast bright images (B and D) demonstrated the binding of the MAb to the inner cell wall and intracellular membranes of A. fumigatus hyphae (A) and conidia (C). Similar labeling was seen with other MAbs, whereas no binding occurred with the control MAbs.
Identification of MAb epitope groups.
The MAb-binding epitopes were determined by competition ELISA with the A. fumigatus MAs as the immobilized antigen. Different concentrations of MAbs were incubated in the microwell plates overnight before a constant concentration of the HRP-labeled MAbs was added. The percentages of inhibition of 17 HRP-labeled MAbs with homologous and heterologous unlabeled MAbs at a maximal concentration of 100 μg/ml are summarized in Table 2. MAbs MA7, EA4, EA5, and EA8 displayed competitive inhibition (almost >75%). Interestingly, the binding of MAbs MA7, EA4, EA5, and EA8 to the coating MAs was abrogated by MAb MA4 or EA6, while the binding of MAb EA6 to the coating MAs was completely inhibited by MAbs MA7, EA4, EA5, and EA8 (≤1% inhibition) and was partially inhibited by MA4 MAb (38 to 70% inhibition). However, EA6 MAb did not interfere with either the homologous or the heterologous binding of any of MAbs. The observations indicated that the aforementioned six MAbs recognized identical or sterically overlapping epitopes, with MAb EA6 being capable of recognizing multiple different epitopes. The same pattern of reactivity with Aspergillus species was apparent by IFA; none of the six MAbs were reactive with A. terreus (Table 1). Similarly, MAbs EA2, EA3, MA1, and EA1 from one group and MAbs Con1 and Con2 from another group may have recognized the same epitope or antigenic determinant in the same region, since they interfered with each others ' binding or inhibited MAb binding by the heterologous MAbs and displayed the same pattern of reactivity with Aspergillus species by IFA (Table 1). This result indicated that MAbs EA2, EA3, MA1, and EA1 recognized prevalent epitopes in A. fumigatus hyphae and that MAbs Con1 and Con2 recognized both hyphal and conidial epitopes in Aspergillus species and conidial epitopes in Candida species. MAbs EA7, MA2, MA3, MA5, and MA6 did not interfere with each others' binding, consistent with their recognition of different epitopes. Accordingly, these 17 MAbs were divided into eight groups, with each group reacting with the same epitope or sterically overlapping A. fumigatus epitopes. None of these MAbs inhibited the binding of MAb EB-A2 (the MAb supplied with the Platelia Aspergillus kit) to the coating MAs (Table 2).
TABLE 2.
Analysis of distinct epitope binding of MAbs by competition ELISA
| MAb | Epitope groupa | % Inhibition of the following HRP-labeled MAbb:
|
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MA7 | EA4 | EA5 | EA8 | MA4 | EA6 | EA2 | EA3 | MA1 | EA1 | Con1 | Con2 | EA7 | MA2 | MA3 | MA5 | MA6 | EB-A2c | ||
| MA7 | I | 86 | 81 | 75 | 81 | 45 | 1 | 23 | 0 | 0 | 0 | 30 | 8 | 0 | 0 | 0 | 0 | 0 | 0 |
| EA4 | I | 82 | 90 | 78 | 85 | 57 | 0 | 13 | 4 | 1 | 3 | 67 | 2 | 50 | 0 | 1 | 0 | 2 | 0 |
| EA5 | I | 83 | 85 | 82 | 77 | 45 | 0 | 5 | 4 | 0 | 0 | 65 | 4 | 27 | 0 | 0 | 0 | 7 | 0 |
| EA8 | I | 76 | 84 | 68 | 78 | 38 | 0 | 7 | 11 | 0 | 0 | 54 | 0 | 21 | 0 | 0 | 0 | 0 | 0 |
| MA4 | I | 78 | 91 | 69 | 88 | 70 | 0 | 9 | 1 | 0 | 0 | 28 | 3 | 18 | 0 | 0 | 0 | 2 | 0 |
| EA6 | I | 84 | 92 | 85 | 87 | 50 | 0 | 6 | 2 | 0 | 0 | 60 | 0 | 28 | 0 | 0 | 0 | 0 | 0 |
| EA2 | II | 0 | 0 | 0 | 0 | 0 | 0 | 96 | 75 | 88 | 88 | 0 | 0 | 0 | 0 | 0 | 12 | 0 | 0 |
| EA3 | II | 0 | 0 | 0 | 0 | 0 | 0 | 95 | 75 | 77 | 82 | 17 | 1 | 0 | 0 | 0 | 13 | 0 | 0 |
| MA1 | II | 0 | 0 | 0 | 0 | 0 | 0 | 67 | 0 | 8 | 1 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 |
| EA1 | II | 0 | 0 | 0 | 0 | 0 | 0 | 88 | 10 | 75 | 36 | 0 | 0 | 0 | 0 | 0 | 9 | 0 | 0 |
| Con1 | III | 65 | 12 | 0 | 0 | 4 | 0 | 2 | 0 | 0 | 0 | 93 | 47 | 15 | 0 | 0 | 0 | 0 | 0 |
| Con2 | III | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 93 | 18 | 16 | 0 | 0 | 0 | 0 | 0 |
| EA7 | IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MA2 | V | 0 | 0 | 7 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 29 | 0 | 2 | 0 | 0 | 0 |
| MA3 | VI | 0 | 0 | 0 | 0 | 0 | 0 | 57 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 |
| MA5 | VII | 0 | 0 | 17 | 0 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| MA6 | VIII | 0 | 0 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
The competition ELISA was performed to analyze the binding epitopes of the different MAbs. By use of the criteria described in Materials and Methods, 17 MAbs were divided into eight groups, with each group reacting with the same epitope or sterically overlapping epitopes on Aspergillus antigens. The experiment was repeated with similar results.
Competitive inhibition values ≥75% are in boldface.
EB-A2, a rat anti-GM MAb supplied with the commercial Platelia Aspergillus kit.
Identification of MAb reactivity with A. fumigatus mannoprotein.
Western blot analysis with ConA-purified mannoprotein from the crude culture filtrate of A. fumigatus revealed that eight MAbs (MAbs EA4, EA5, EA8, MA4, EA6, EA7, MA2, and MA6) reacted with two major components of mannoprotein with molecular masses of approximately 50 and 75 kDa. In addition, MAbs MA2 and MA6 reacted with several bands between 25 and 75 kDa of ConA-purified mannoprotein; the pattern of reactivity was similar to that apparent with ConA-purified mannoprotein obtained by using MAb EB-A2 supplied with the Platelia Aspergillus kit (Fig. 3). Another nine MAbs did not show reacting bands with ConA-purified mannoprotein (data not shown).
FIG. 3.
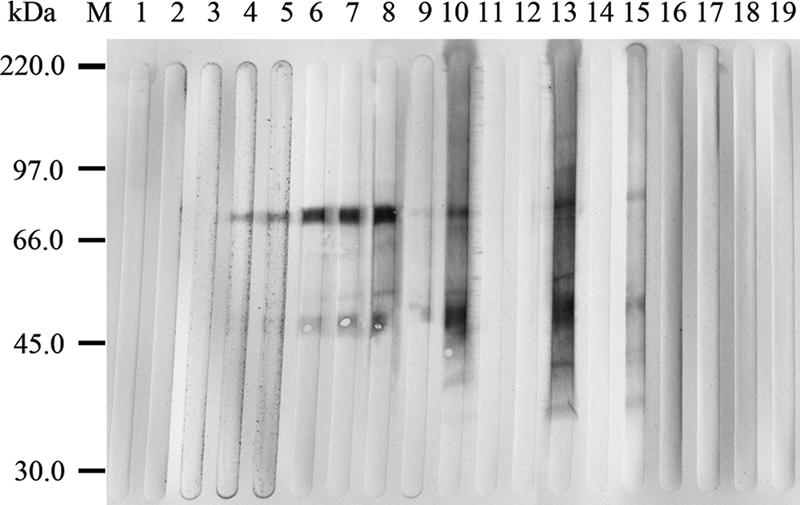
Western blot analyses of the binding of MAbs to ConA-purified mannoprotein derived from A. fumigatus. Lanes: 1, Con1; 2, Con2; 3, MA7; 4, EA4; 5, EA5; 6, EA8; 7, MA4; 8, EA6; 9, EA7; 10, MA2; 11, MA3; 12, MA5; 13, MA6; 14, control MAb; 15, EB-A2; 16, EA1; 17, EA2; 18, EA3; 19, MA1; M, markers. The Western blot demonstrated the binding of MAbs EA4, EA5, EA8, MA4, EA6, EA7, MA2, and MA6 to proteins at 50 and 75 kDa. MAbs MA2 and MA6 also bound to several bands between 25 and 75 kDa, and the pattern of reactivity was similar to that seen in the rat anti-GM MAb EB-A2 supplied with the Platelia Aspergillus assay kit.
Development of the antigen-capture ELISA.
To establish a sensitive antigen-capture ELISA for the detection of Aspergillus antigen, the optimum capture-detector MAb pair that recognized distinct epitopes was selected by a two-step sandwich ELISA (37) on the basis of the epitope groups among these MAbs (Table 1). The use of MAb MA6 as the solid-phase immobilized capture antibody and MA2 MAb as the labeled detecting antibody gave the highest combination of sensitivity and specificity for the detection of dilutions of sera from rabbits displaying A. fumigatus antigenemia and a panel of sera from healthy humans.
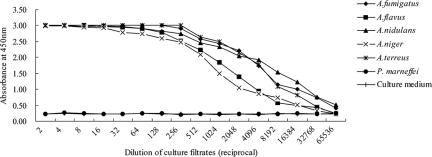
To further analyze the cross-reactivity within the Aspergillus species, serial dilutions of the culture filtrates from the five Aspergillus species were analyzed in the antigen-capture ELISA. Culture filtrates from the Candida species tested and P. marneffei were also examined. The culture filtrates from the Aspergillus species gave a very strong positive signal. The sensitivity of the positive assay signal could be detected even when filtrates from cultures grown to 2 × 106 cells/ml were diluted 1:10,000. In contrast, the P. marneffei culture filtrate was not detectable even at a dilution of 1:2 (Fig. 4). Also, none of the culture filtrates from C. albicans, C. glabrata, C. tropicalis, C. krusei, or C. parapsilosis could be detected (data not shown). To further determine whether the assay would recognize the heat-resistant or hydrolysate epitopes from the ConA-purified mannoprotein, the mannoprotein was treated at 100°C for 30 min or with 0.01 N HCl at 100°C overnight. The assay could detect either intact or heated mannoprotein, as well as mildly acidic (0.01 N HCl) treated mannoprotein, with comparable sensitivities without reducing the binding activity (data not shown). Interestingly, this Aspergillus antigen assay could not capture the purified GM protein supplied with the Platelia Aspergillus kit.
FIG. 4.
Evaluation of the sensitivity and the specificity of the Aspergillus antigen-capture ELISA for the detection of culture filtrates of fungal antigens. Culture filtrates from five Aspergillus species gave a very strong positive signal following the dilution (approximately 1:10,000) of cell cultures containing 2 × 106 cells/ml. The culture filtrate from Penicillium marneffei was not detectable beyond a dilution of 1:2.
Furthermore, the reproducibility of the antigen-capture ELISA was evaluated with three samples from A. fumigatus-infected immunosuppressed rabbit serum diluted 1:500, 1:1,000, and 1:5,000; these were run numerous times within and between assays. The coefficients of variation for 10 replicates tested in the same assay were 1.85% (1:500), 4.12% (1:1,000), and 2.27% (1:5,000); and the test-to-test coefficients of variation determined by measuring the same samples daily over a 20-day period were 7.96% (1:500), 10.46% (1:1,000), and 8.03% (1:5,000).
Detection of antigenemia in experimental IA.
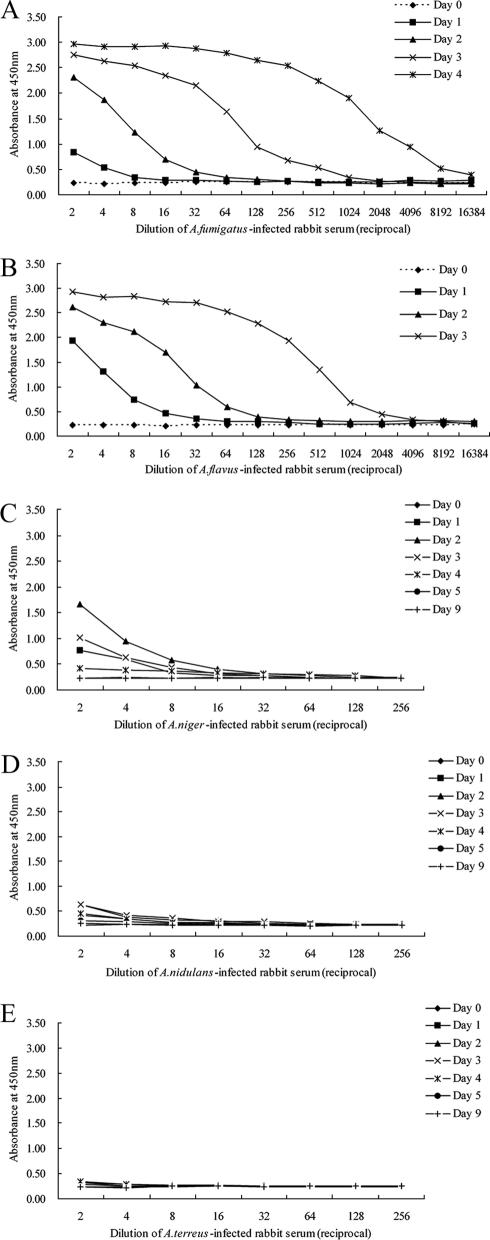
Rabbit IA models were used to further evaluate the sensitivity of the antigen-capture ELISA. Five immunosuppressed rabbits were infected intravenously with either A. fumigatus, A. flavus, A. terreus, A. niger, or A. nidulans. Prior to infection (day 0), the assay did not detect Aspergillus antigen in heated serum from any of the five immunosuppressed rabbits (Fig. 5). An increased amount of antigen was detectable in heated serum from two immunosuppressed rabbits infected with A. fumigatus beginning on days 1 to 4 and rabbits infected with A. flavus beginning on days 1 to 3. The slight increase in detectable Aspergillus antigen present in the serum was also observed in an immunosuppressed rabbit infected with A. niger within 2 days of the initiation of infection, but the antigen titer was low and dropped quickly over the intervening 1 to 3 days. A lower antigen titer was evident in the A. nidulans-infected rabbit 2 to 4 days after the initiation of infection. The antigen was slightly detectable in serum from the A. terreus-infected rabbit 1 to 4 days after infection. The increase in antigen detectable in the serum correlated with disease progression. Immunosuppressed rabbits that had been infected with either A. flavus or A. fumigatus died on days 3 and 4, respectively, after infection. Autopsies revealed areas of necrosis and hemorrhage on the surfaces of the kidneys, spleens, and livers of both animals. Disseminated aspergillosis was confirmed histopathologically by hematoxylin-eosin staining and IHC of the organs. In contrast, three other immunosuppressed rabbits infected with either A. terreus, A. niger, or A. nidulans initially exhibited signs of discomfort but recovered within 1 week after infection and remained alive until they were euthanized.
FIG. 5.
Detection of antigenemia in the experimental rabbit model of IA by a two-step Aspergillus antigen-capture ELISA. Blood samples were drawn prior to the induction of infection (day 0) and daily thereafter until the death of each rabbit. The panels display the appearances of the infections caused by A. fumigatus (A), A. flavus (B), A. niger (C), A. nidulans (D), and A. terreus (E).
Comparison of sensitivities and specificities of the two-step antigen-capture ELISA and one-step antigen-capture ELISA (Platelia Aspergillus assay).
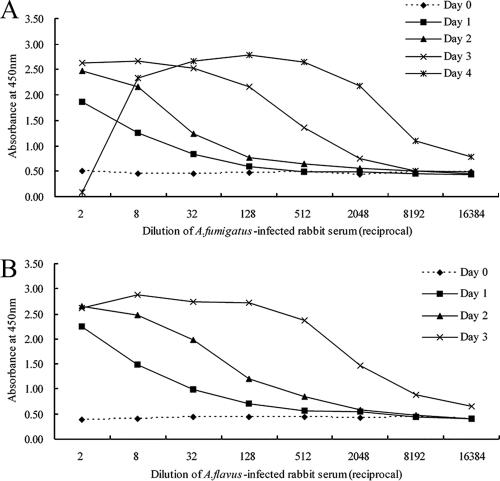
The kinetics of antigenemia detection by the commercially available one-step antigen-capture ELISA and our two-step capture ELISA method were similar when they were used to test sera from A. fumigatus- and A. flavus-infected rabbits (Fig. 6). Although the detection of antigen by the one-step ELISA method was more sensitive than that by the two-step procedure, the background produced with preinfected rabbit serum in the one-step procedure was higher (OD450, 0.40 to 0.50) than that produced in the two-step method developed in the present study (OD450, 0.21 to 0.25). If the end-point titer of detectable antigenemia was defined as the highest serum dilution that resulted in an OD450 value twofold higher than that of preinfected rabbit serum, the highest serum dilutions in which antigenemia in rabbits infected with A. fumigatus was detectable on days 1 to 4 were 1:4, 1:16, 1:512, and 1:8,192, respectively, in our two-step ELISA, and 1:8, 1:32, 1:512, and 1:8,192, respectively, in the one-step ELISA. The dilutions in which antigenemia was detectable in rabbits infected with A. flavus on days 1 to 3 were 1:16, 1:64, and 1:2,048, respectively, in the two-step ELISA and 1:32, 1:512, and 1:8,192, respectively, in the one-step ELISA. Although the one-step assay format was convenient to perform, the “hook effect” was observed at high antigen concentrations when serum obtained at day 4 from an A. fumigatus-infected rabbit was used.
FIG. 6.
Detection of antigenemia in the experimental rabbit model of IA by a one-step commercial GM detection ELISA (the Platelia Aspergillus assay). Blood samples were drawn prior to the induced infection (day 0) and daily thereafter until the death of each rabbit. The times of appearance of the infection in rabbits infected with A. fumigatus (A) and A. flavus (B) are shown.
To study the background of the two-step antigen-capture ELISA for further clinical evaluation, serum and urine specimens from 382 and 120 healthy blood donors, respectively, were analyzed. The mean OD450 values with the standard deviations were 0.200 ± 0.032 for serum samples and 0.141 ± 0.014 for urine samples. The cutoff was set as the average value of the control sera by adding 5 standard deviations, which produced cutoff OD450 values of 0.36 for serum and 0.211 for urine. The result was considered positive if a sample yielded an OD450 value above the cutoff value. By these criteria, no false-positive results were obtained by the antigen-capture ELISA. With very few clinical samples with proven infection available, our assay could detect two serial serum samples and one urine sample (OD450 values, >1.6 for three samples) from two cases of proven IA. These clinical samples demonstrated positive results by using the commercial Platelia Aspergillus GM assay with a cutoff index of 0.5.
DISCUSSION
In this study, 17 murine MAbs against cell wall antigens of A. fumigatus were produced by different immunization protocols with A. fumigatus crude extract antigens. The reaction profiles of each antibody were well characterized by ELISA, immunofluorescence, immunoblotting, and immunohistochemical analyses. The MAbs were initially divided into eight groups on the basis of their reactivities with antigens extracted from hyphae. Each group had the same pattern of reactivity with Aspergillus species without cross-reactivity to Candida species or P. marneffei, with the exception of two MAbs from the same group. Some groups of MAbs reacted with more than one species of Aspergillus, including A. flavus, A. terreus, A. niger, and A. nidulans, which commonly cause aspergillosis in humans (6). Confocal microscopy images of immunofluorescent samples demonstrated that the epitopes recognized by these MAbs were present on the cell walls of the hyphae and the conidia of A. fumigatus. In the immunohistochemical staining assay, most of the MAbs recognized the Aspergillus hyphae in clinical specimens. Collectively, our observations suggest that the MAbs are useful as immunoprobes for the histological diagnosis of Aspergillus infection or for the grouping of Aspergillus species and may additionally be valuable in the establishment of an Aspergillus antigen detection assay.
Recognizing these potential values, a concerted effort was made to develop a sensitive and specific antigen-capture immunoassay for the detection of circulating Aspergillus antigen. A necessary part of this study was the validation of the MAbs. The presence of different Aspergillus antigen epitopes recognized by these 17 MAbs made possible the development of a double-sandwich ELISA and, therefore, the testing of various combinations of MAbs. The result of these tests was the selection of the MA6-MA2 MAb pair as the capture and detector antibodies, respectively, for the development of a two-step antigen-capture ELISA. This combination produced the highest sensitivity of detection of the experimentally induced antigenemia among the various combinations of MAbs. In rabbits infected with A. fumigatus and A. flavus, the most common agents of aspergillosis, our two-step assay detected an infection-related antigen increase as early as 1 day following establishment of the infection and continued to detect increasing amounts of antigen as the infection progressed. The amount of detectable antigen over time was also consistent with disease progression in rabbits infected with A. niger, A. nidulans, and A. terreus. The assay could detect transient antigenemia in A. niger-, A. nidulans-, and A. terreus-infected rabbits weakly, even though the assay yielded a high sensitivity for the detection of the culture filtrates from the three species. Comparison of the commercial one-step ELISA (the Platelia Aspergillus assay) with our two-step ELISA by using an experimental animal model of IA infection revealed similar sensitivities of detection in A. fumigatus-infected rabbits over time; the sensitivity of the one-step ELISA was higher than that of the two-step ELISA for A. flavus-infected rabbits.
MAbs MA2 and MA6 had the same patterns of reactivity with ConA-purified mannoprotein that were seen with the EB-A2 MAb, with molecular masses of 25 to 75 kDa. Yet, the epitopes recognized by the two MAbs differed from the epitope recognized by MAb EB-A2, which binds to an epitope located on the β(1→5) galactofuranose-containing side chains of the Aspergillus GM molecule (25). An ELISA inhibition experiment demonstrated that MAbs MA2 and MA6 do not inhibit the binding of MAb EB-A2 to the coating MAs of A. fumigatus, even when the MAbs were used at a high concentration of 100 μg/ml. Further analyses with mild acid-treated, ConA-purified mannoprotein, which contains mannose and galactose, has indicated that our assay can capture the heat-resistant or hydrolysate epitopes in ConA-purified mannoprotein without reducing the binding activity. Previous studies showed that MAb EB-A2 loses its GM binding activity after mild hydrolysis, since the immunoreactivity of GM disappears after removal of the galactoside side chains of A. fumigatus exopolysaccharides (25) but not when other linkages of the mannan core are altered (15). In addition, our assay did not react with the purified GM protein derived from Aspergillus. Taken together, these results indicate that the epitopes recognized by our MAbs are most likely not associated with the GM structure. Although the immunoreactive epitopes in our MAbs cannot yet be precisely identified, the assay detected the circulating Aspergillus antigens present in experimental animal models of IA, indicating that the Aspergillus antigens captured by our assay are the immunodominant glycoproteins released from Aspergillus-infected tissues.
The Platelia Aspergillus commercial GM detection ELISA system utilizes a rat anti-GM MAb (MAb EB-A2), which is widely used in the diagnosis of IA. However, a major problem with the MAb EB-A2-based ELISA is the occurrence of false-positive results, since the anti-GM MAb presents a wide range of cross-reactivity with other opportunistic fungi; bacteria such as Bifidobacterium species; and drugs, including amoxicillin-clavulanic acid and piperacillin-tazobactam (5, 10, 11, 20, 27). The consequence may be increased difficulty with the selection of a specific antifungal therapy. The Aspergillus antigen detection assay described in this paper displays acceptable sensitivity and specificity and an absence of cross-reactivity with other common opportunistic fungi, such as Penicillium and Candida.
There have been few reports on the utility of MAbs in the development of an Aspergillus antigen assay. The development of a new two-step antigen-capture ELISA with well-characterized MAbs described here enables the detection of Aspergillus epitopes that are associated with aspergillosis. The assay should prove valuable in the diagnosis of systemic Aspergillus infections, especially among patients receiving treatment with antibacterial drugs and premature infants. Future studies in our laboratory will more precisely examine the MAb-binding epitopes and evaluate the efficacy of this Aspergillus antigen assay with clinical samples.
Acknowledgments
This work was supported by grant 06024435 of the Research Program of the Natural Science Foundation from the Guangdong Science and Technology Council, Guangdong Province, People's Republic of China.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Ansorg, R., R. van den Boom, and P. M. Rath. 1997. Detection of Aspergillus galactomannan antigen in foods and antibiotics. Mycoses 40:353-357. [DOI] [PubMed] [Google Scholar]
- 2.Becker, M. J., S. de Marie, D. Willemse, H. A. Verbrugh, and I. A. Bakker-Woudenberg. 2000. Quantitative galactomannan detection is superior to PCR in diagnosing and monitoring invasive pulmonary aspergillosis in an experimental rat model. J. Clin. Microbiol. 38:1434-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Che, X. Y., L. W. Qiu, Y. X. Pan, K. Wen, W. Hao, L. Y. Zhang, Y. D. Wang, Z. Y. Liao, X. Hua, V. C. Cheng, and K. Y. Yuen. 2004. Sensitive and specific monoclonal antibody-based capture enzyme immunoassay for detection of nucleocapsid antigen in sera from patients with severe acute respiratory syndrome. J. Clin. Microbiol. 42:2629-2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chumpitazi, B. F., C. Pinel, B. Lebeau, P. Ambroise-Thomas, and R. Grillot. 2000. Aspergillus fumigatus antigen detection in sera from patients at risk for invasive aspergillosis. J. Clin. Microbiol. 38:438-443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalle, F., P. E. Charles, K. Blanc, D. Caillot, P. Chavanet, F. Dromer, and A. Bonnin. 2005. Cryptococcus neoformans galactoxylomannan contains an epitope(s) that is cross-reactive with Aspergillus galactomannan. J. Clin. Microbiol. 43:2929-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denning, D. W. 1998. Invasive aspergillosis. Clin. Infect. Dis. 26:781-803. [DOI] [PubMed] [Google Scholar]
- 7.de Repentigny, L. 1992. Serodiagnosis of candidiasis, aspergillosis, and cryptococcosis. Clin. Infect. Dis. 14(Suppl. 1):S11-S22. [DOI] [PubMed] [Google Scholar]
- 8.de Repentigny, L., M. Boushira, L. Ste-Marie, and G. Bosisio. 1987. Detection of galactomannan antigenemia by enzyme immunoassay in experimental invasive aspergillosis. J. Clin. Microbiol. 25:863-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding, Y., L. He, Q. Zhang, Z. Huang, X. Che, J. Hou, H. Wang, H. Shen, L. Qiu, Z. Li, J. Geng, J. Cai, H. Han, X. Li, W. Kang, D. Weng, P. Liang, and S. Jiang. 2004. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: implications for pathogenesis and virus transmission pathways. J. Pathol. 203:622-630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Food and Drug Administration. 2005. Practice caution in using Bio-Rad Platelia Aspergillus EIA. Food and Drug Administration, Washington, DC. http://www.fda.gov/cdrh/oivd/laboratory.html#tip8. Accessed 17 August 2005.
- 11.Giacchino, M., N. Chiapello, S. Bezzio, F. Fagioli, P. Saracco, A. Alfarano, V. Martini, G. Cimino, P. Martino, and C. Girmenia. 2006. Aspergillus galactomannan enzyme-linked immunosorbent assay cross-reactivity caused by invasive Geotrichum capitatum. J. Clin. Microbiol. 44:3432-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Herbrecht, R., V. Letscher-Bru, C. Oprea, B. Lioure, J. Waller, F. Campos, O. Villard, K. L. Liu, S. Natarajan-Ame, P. Lutz, P. Dufour, J. P. Bergerat, and E. Candolfi. 2002. Aspergillus galactomannan detection in the diagnosis of invasive aspergillosis in cancer patients. J. Clin. Oncol. 20:1898-1906. [DOI] [PubMed] [Google Scholar]
- 13.Hope, W. W., T. J. Walsh, and D. W. Denning. 2005. Laboratory diagnosis of invasive aspergillosis. Lancet Infect. Dis. 5:609-622. [DOI] [PubMed] [Google Scholar]
- 14.Hurst, S. F., G. H. Reyes, D. W. McLaughlin, E. Reiss, and C. J. Morrison. 2000. Comparison of commercial latex agglutination and sandwich enzyme immunoassays with a competitive binding inhibition enzyme immunoassay for detection of antigenemia and antigenuria in a rabbit model of invasive aspergillosis. Clin. Diagn. Lab. Immunol. 7:477-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latgé, J. P., J. P. Debeaupuis, M. Moutaouakil, M. Diaquin, J. Sarfati, M. C. Prevost, J. M. Wieruszeski, Y. Leroy, and B. Fournet. 1991. Galactomannan and the circulating antigens of Aspergillus fumigatus, p. 143-155. In J. P. Latge and D. Boucias (ed.), Fungal cell wall and immune response. Springer-Verlag, Berlin, Germany.
- 16.Latgé, J. P., H. Kobayashi, J. P. Debeaupuis, M. Diaquin, J. Sarfati, J. M. Wieruszeski, E. Parra, J. P. Bouchara, and B. Fournet. 1994. Chemical and immunological characterization of the extracellular galactomannan of Aspergillus fumigatus. Infect. Immun. 62:5424-5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mennink-Kersten, M. A., J. P. Donnelly, and P. E. Verweij. 2004. Detection of circulating galactomannan for the diagnosis and management of invasive aspergillosis. Lancet Infect. Dis. 4:349-357. [DOI] [PubMed] [Google Scholar]
- 18.Mennink-Kersten, M. A., R. R. Klont, A. Warris, H. J. Op den Camp, and P. E. Verweij. 2004. Bifidobacterium lipoteichoic acid and false ELISA reactivity in Aspergillus antigen detection. Lancet 363:325-327. [DOI] [PubMed] [Google Scholar]
- 19.Reiss, E., and P. F. Lehmann. 1979. Galactomannan antigenemia in invasive aspergillosis. Infect. Immun. 25:357-365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rimek, D., T. Zimmermann, M. Hartmann, C. Prariyachatigul, and R. Kappe. 1999. Disseminated Penicillium marneffei infection in an HIV-positive female from Thailand in Germany. Mycoses 42(Suppl. 2):25-28. [PubMed] [Google Scholar]
- 21.Rohrlich, P., J. Sarfati, P. Mariani, M. Duval, A. Carol, C. Saint-Martin, E. Bingen, J. P. Latge, and E. Vilmer. 1996. Prospective sandwich enzyme-linked immunosorbent assay for serum galactomannan: early predictive value and clinical use in invasive aspergillosis. Pediatr. Infect. Dis. J. 15:232-237. [DOI] [PubMed] [Google Scholar]
- 22.Shao, P. L., L. M. Huang, and P. R. Hsueh. 2006. Invasive fungal infection—laboratory diagnosis and antifungal treatment. J. Microbiol. Immunol. Infect. 39:178-188. [PubMed] [Google Scholar]
- 23.Siemann, M., M. Koch-Dorfler, and M. Gaude. 1998. False-positive results in premature infants with the Platelia Aspergillus sandwich enzyme-linked immunosorbent assay. Mycoses 41:373-377. [DOI] [PubMed] [Google Scholar]
- 24.Stynen, D., A. Goris, J. Sarfati, and J. P. Latge. 1995. A new sensitive sandwich enzyme-linked immunosorbent assay to detect galactofuran in patients with invasive aspergillosis. J. Clin. Microbiol. 33:497-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stynen, D., J. Sarfati, A. Goris, M. C. Prevost, M. Lesourd, H. Kamphuis, V. Darras, and J. P. Latge. 1992. Rat monoclonal antibodies against Aspergillus galactomannan. Infect. Immun. 60:2237-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sulahian, A., S. Touratier, and P. Ribaud. 2003. False positive test for Aspergillus antigenemia related to concomitant administration of piperacillin and tazobactam. N. Engl. J. Med. 349:2366-2367. [DOI] [PubMed] [Google Scholar]
- 27.Swanink, C. M., J. F. Meis, A. J. Rijs, J. P. Donnelly, and P. E. Verweij. 1997. Specificity of a sandwich enzyme-linked immunosorbent assay for detecting Aspergillus galactomannan. J. Clin. Microbiol. 35:257-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Verdaguer, V., T. J. Walsh, W. Hope, and K. J. Cortez. 2007. Galactomannan antigen detection in the diagnosis of invasive aspergillosis. Expert. Rev. Mol. Diagn. 7:21-32. [DOI] [PubMed] [Google Scholar]
- 29.Verweij, P. E., J. P. Donnelly, B. E. De Pauw, and J. F. G. M. Meis. 1996. Prospects for the early diagnosis of invasive aspergillosis in the immunocompromised patient. Rev. Med. Microbiol. 7:105-113. [Google Scholar]
- 30.Verweij, P. E., D. Stynen, A. J. Rijs, B. E. de Pauw, J. A. Hoogkamp-Korstanje, and J. F. Meis. 1995. Sandwich enzyme-linked immunosorbent assay compared with Pastorex latex agglutination test for diagnosing invasive aspergillosis in immunocompromised patients. J. Clin. Microbiol. 33:1912-1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viscoli, C., M. Machetti, P. Cappellano, B. Bucci, P. Bruzzi, M. T. Van Lint, and A. Bacigalupo. 2004. False-positive galactomannan Platelia Aspergillus test results for patients receiving piperacillin-tazobactam. Clin. Infect. Dis. 38:913-916. [DOI] [PubMed] [Google Scholar]
- 32.von Eiff, M., N. Roos, R. Schulten, M. Hesse, M. Zuhlsdorf, and J. van de Loo. 1995. Pulmonary aspergillosis: early diagnosis improves survival. Respiration 62:341-347. [DOI] [PubMed] [Google Scholar]
- 33.Wang, H., Y. Ding, X. Li, L. Yang, W. Zhang, and W. Kang. 2003. Fatal aspergillosis in a patient with SARS who was treated with corticosteroids. N. Engl. J. Med. 349:507-508. [DOI] [PubMed] [Google Scholar]
- 34.Wheat, L. J. 2003. Rapid diagnosis of invasive aspergillosis by antigen detection. Transplant. Infect. Dis. 5:158-166. [DOI] [PubMed] [Google Scholar]
- 35.Wilson, M. B., and P. K. Nakane. 1978. Recent developments in the periodate method of conjugating horseradish peroxidase (HPRO) to antibodies, p. 215-224. In W. Knapp, K. Holubar, and G. Wicks (ed.), Immunofluorescence and related staining techniques. Elsevier, Amsterdam, The Netherlands.
- 36.Woo, P. C., C. M. Chan, A. S. Leung, S. K. Lau, X. Y. Che, S. S. Wong, L. Cao, and K. Y. Yuen. 2002. Detection of cell wall galactomannoprotein Afmp1p in culture supernatants of Aspergillus fumigatus and in sera of aspergillosis patients. J. Clin. Microbiol. 40:4382-4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu, H., B. Di, Y. X. Pan, L. W. Qiu, Y. D. Wang, W. Hao, L. J. He, K. Y. Yuen, and X. Y. Che. 2006. Serotype 1-specific monoclonal antibody-based antigen capture immunoassay for detection of circulating nonstructural protein NS1: implications for early diagnosis and serotyping of dengue virus infections. J. Clin. Microbiol. 44:2872-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yeo, S. F., and B. Wong. 2002. Current status of nonculture methods for diagnosis of invasive fungal infections. Clin. Microbiol. Rev. 15:465-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuen, K. Y., C. M. Chan, K. M. Chan, P. C. Woo, X. Y. Che, A. S. Leung, and L. Cao. 2001. Characterization of AFMP1: a novel target for serodiagnosis of aspergillosis. J. Clin. Microbiol. 39:3830-3837. [DOI] [PMC free article] [PubMed] [Google Scholar]