Abstract
Glycoconjugate vaccines have dramatically reduced the incidence of encapsulated bacterial diseases in toddlers under 2 years of age, but vaccine-induced antibody levels in this age group wane rapidly. We immunized adults and 12-month-old toddlers with heptavalent pneumococcal conjugate vaccine to determine differences in B-cell and antibody responses. The adults and 12-month-old toddlers received a pneumococcal conjugate vaccine. The toddlers received a second dose at 14 months of age. The frequencies of diphtheria toxoid and serotype 4, 14, and 23F polysaccharide-specific plasma cells and memory B cells were determined by enzyme-linked immunospot assay. The toddlers had no preexisting polysaccharide-specific memory B cells or serum immunoglobulin G (IgG) antibody but had good diphtheria toxoid-specific memory responses. The frequencies of plasma cells and memory B cells increased by day 7 (P < 0.0001) in the adults and the toddlers following a single dose of conjugate, but the polysaccharide responses were significantly lower in the toddlers than in the adults (P = 0.009 to <0.001). IgM dominated the toddler antibody responses, and class switching to the IgG was serotype dependent. A second dose of vaccine enhanced the antibody and memory B-cell responses in the toddlers but not the ex vivo plasma cell responses. Two doses of pneumococcal conjugate vaccine are required in toddlers to generate memory B-cell frequencies and antibody class switching for each pneumococcal polysaccharide equivalent to that seen in adults.
Streptococcus pneumoniae is a major respiratory pathogen of toddlers and elderly adults, causing 1 million childhood deaths per year worldwide (19). The peak incidence of invasive pneumococcal disease is between 4 and 18 months, when maternal antibody has waned and before the immune responsiveness to polysaccharide antigens develops (59).
The introduction of a new heptavalent, conjugated pneumococcal capsular polysaccharide vaccine (Pnc7) in the United States in 2000 led to a major reduction in invasive pneumococcal disease cases among immunized toddlers (7, 79) and more widely in the population as a result of “herd immunity,” which arises because of the reduced transmission of the organism through the blockage of nasopharyngeal carriage (32, 41, 45, 78).
Toddlers immunized at 2, 4, and 6 months of age generate immunoglobulin G (IgG) antibody responses to Pnc7 (16), but the serum antibody wanes rapidly, with some serotype-specific antibody levels falling below the protective threshold within a matter of months (47, 64). Similarly, in early infancy antibody wanes rapidly after immunization with other glycoconjugate vaccines, such as the Haemophilus influenzae type b (30) and serogroup C Neisseria meningitidis glycoconjugate vaccines (68), and there is a corresponding loss of vaccine effectiveness (56, 70). This failure of persistence of IgG to capsular polysaccharides after immunization in infancy may be overcome by the subsequent administration of a booster dose of a conjugate vaccine at 12 to 15 months of age, which results in a marked rise in IgG antibody levels, demonstrating that immunological memory had been induced by priming (2, 3, 58).
In the United Kingdom, Pnc7 was introduced into the primary immunization schedule at the end of 2006 as two doses given at 2 months and 4 months of age, with a booster dose given at 13 months of age. Children between 12 months and 2 years of age at the time that Pnc7 was introduced were included in a single-dose catch-up campaign. However, at 12 months of age, a single dose of Pnc7 may not be sufficient to induce protective levels (a protective level has been variously described as >0.2 μg/ml or as 0.35 μg/ml or 1.0 μg/ml [4, 27]) of antibodies to all seven serotypes included in the current vaccine (47), and there is little information about the persistence of antibody after this single-dose priming regimen and the subsequent memory responses.
By contrast, in adults a single dose of Pnc7 is sufficient to induce protective levels of IgG to all seven serotypes included in Pnc7, although the levels also wane somewhat (1, 33, 62, 80) and no further increase in response is demonstrated following reimmunization (75), perhaps because the polysaccharide antigens (conjugated as well as purified) stimulate predominantly marginal zone B (MZB)-cell responses in this age group (9, 37, 74, 75). These cells accumulate with age and require a mature splenic marginal zone to function. They are also capable of rapid isotype switching to IgG positivity during the first week after immunization (21). Thus, fewer of these cells in early infancy and the immature phenotype expressed by these cells may also contribute to the lack of the long-term maintenance of serum IgG levels in toddlers (81).
During the first 7 days of the immune response to a booster dose of glycoconjugate vaccine there is a rapid but transient rise in the frequency of antigen-specific antibody-forming cells (AFCs) in the peripheral blood of adults by day 7 (12). These cells disappear from the circulation by day 9 of the vaccine response. A similar time course has also been reported in response to plain pneumococcal polysaccharide vaccines, tetanus toxoid, and influenza vaccines (17, 25); and it is likely that these AFCs are plasma cells generated from preexisting memory cells. However, various subsets of B cells are presumed to circulate through the peripheral blood following immunization, including mature plasma cells, nonsecreting antigen-specific memory B cells, and long-lived plasma cell precursors migrating to the bone marrow (20, 44); and uncertainty remains about which of these cell subsets is responsible for both the early rise in antibody levels after immunization and the prolonged production of antibody over the subsequent months and years.
While the phenotype of the B-cell response and the maturity of bone marrow niches probably do vary with age, the differences in the magnitudes of the B-cell response at different ages have not been studied extensively. In this investigation, the antigen-specific memory B-cell response and the antibody response following immunization with Pnc7 in adults and toddlers were compared. The main aim of this study was to determine whether the frequency of memory B cells and the timing of their appearance in peripheral blood were the same in the 12-month-old toddlers and the adults. The response in the toddlers after a single or second dose of Pnc7 was compared with that in the adults after a single dose. The reason for these comparisons was to show how effective the catch-up campaign of a single dose at 12 months was at inducing memory to the different serotypes.
Due to the restricted sample sizes for the toddler group, we chose pneumococcal serotypes based on the previously published rates of nasopharyngeal carriage and invasive disease (10, 24, 65). Serotypes 4, 14, and 23F represented common, intermediate, and rare serotypes for each of these factors, respectively. We also looked at the CRM197 (cross-reactive material; mutant diphtheria toxoid) carrier response, represented in our assays by the diphtheria toxoid-specific AFCs in the enzyme-linked immunospot (ELISPOT) assay.
MATERIALS AND METHODS
Subjects and clinical procedures.
Twenty healthy adult volunteers (age range, 23 to 49 years; 8 males and 12 females) with no history of pneumococcal vaccination received a single dose of the heptavalent pneumococcal-CRM197 conjugate vaccine (Pnc7; Wyeth Vaccines, Pearl River, MA) by intramuscular injection in the left deltoid. The 0.5-ml dose of the vaccine contained a concentration of saccharides of 2.0 μg/ml for each of serotypes 4, 9V, 4, 18C, 19F, and 23F and 4 μg/ml for serotype 6B. Each saccharide is conjugated to CRM197 and is adsorbed on aluminum phosphate. A 20-ml venous blood sample was collected (2 ml was placed into a tube for clotted serum and 18 ml was placed into a tube containing heparin for isolation of peripheral blood mononuclear cells [PBMCs]) prior to vaccination and again on days 6, 7, 15, and 28 following immunization.
Forty healthy toddlers aged 12 months received two doses of heptavalent pneumococcal-CRM197 conjugate vaccine at a 2-month interval. A 5-ml venous blood sample was collected prior to vaccination, then day 6 or 7 later, then on day 56 (prior to the second dose), and finally on day 6 or 7 (day 62 or 63 after the first vaccination) following the second immunization. The toddler blood was allocated for serum (1 ml clotted serum) and for isolation of PBMCs (4 ml in a tube containing heparin). Due to ethical constraints, it was not possible to obtain a further blood sample on day 56 after the second dose of Pnc7. All of the toddlers had previously received all of their routine immunizations during infancy.
Informed consent was obtained from the volunteers, and the protocol was approved by the Oxfordshire Research Ethics Committee (OxREC number C02.005).
Antibodies used. (i) ELISPOT assay.
The capture antibody was polyvalent goat anti-human Ig (H17000; Caltag, Buckingham, United Kingdom) (14). The detection antibody was a goat anti-human IgG, γ-chain-specific-alkaline phosphatase conjugate (401442; Calbiochem-Novabiochem, Nottingham, United Kingdom).
(ii) Pneumococcal ELISA detection antibodies.
Goat anti-human IgG (AHI0305), IgA (AHI0105), and IgM (AHI0605) all conjugated to alkaline-phosphatase, were obtained from Biosource.
Preparation and in vitro stimulation of PBMCs for memory B-cell detection by ELISPOT assay.
PBMCs were isolated from peripheral blood by density gradient centrifugation; and 2 × 105 cells/well were cultured in RPMI with penicillin- streptomycin with 10% newborn bovine serum, with final well concentrations of the Staphylococcus aureus Cowan strain at a 1:5,000 dilution of the Pansorbin cell suspension (Calbiochem-Novabiochem), GpG DNA at 2.5 μg/ml (Autogen-Bioclear UK Ltd., Calne, United Kingdom), and pokeweed mitogen at 83 ng/ml (Sigma, Poole, United Kingdom) for 5 days at 37°C in 5% CO2 and 95% humidity. These cells were then harvested, washed, and resuspended at 2 × 106 cells/ml for seeding into the ELISPOT assay plates as described below.
Enumeration of antigen-specific ex vivo plasma cells and in vitro-stimulated memory B cells.
Multiscreen hydrophobic polyvinylidene difluoride membrane plates (Millipore, Watford, United Kingdom) were coated with anti-IgG, tetanus toxoid, diphtheria toxoid (Statens Seruminstitut, Copenhagen, Denmark), or pneumococcal polysaccharides (serotypes 4, 14, and 23F; LGC Promochem, Teddington, United Kingdom) conjugated to methylated human serum albumin (National Institute for Standards and Controls, Potters Bar, United Kingdom) (13). Anti-IgG, diphtheria toxoid, and polysaccharides 4 and 14 were coated at 10 μg/ml in phosphate-buffered saline; and tetanus toxoid and serotype 23F were coated at 5 and 20 μg/ml, respectively. Diphtheria toxoid was used to represent the CRM197 carrier protein response. Since the availability of PBMCs was limited, only three of the seven vaccine serotypes were studied. Washed B cells were seeded at 200 to 2 × 105 cells per well on ELISPOT assay plates that had previously been blocked with 10% newborn calf serum (Sigma), and the plates were incubated overnight at 37°C in 5% CO2 and 95% humidity. The cells were washed with phosphate-buffered saline-Tween, and bound IgG antibody was detected by using an alkaline phosphatase conjugate and alkaline phosphatase conjugate substrate kit (nitroblue tetrazolium plus 5-bromo-4-chloro-3-indolylphosphate in dimethyl formamide; Bio-Rad Laboratories, Hemel Hempstead, United Kingdom), before the cells were counted by using an AID ELISPOT reader ELR02 and software, version 3.2.3 (Cadama Medical Ltd., Stourbridge, United Kingdom). The spots were characterized by the use of preestablished settings and the AID software. The total frequency of IgG-secreting AFCs (IgG AFCs) was used as the positive control for the memory B-cell ELISPOT assay. Samples with <1,000 total IgG AFCs/106 cultured PBMCs were removed from the analysis. Tetanus toxoid (a nonvaccine antigen) was used as a negative control in the ex vivo plasma cell ELISPOT assay and as a positive control in the memory B-cell ELISPOT assay.
A positive response to immunization was defined as a more than twofold increase in AFC frequency following immunization.
Pneumococcal ELISA for the detection of anti-polysaccharide Igs.
The World Health Organization standard protocol was used for the detection of human IgG, IgA, and IgM antibodies against S. pneumoniae capsular polysaccharides (77; http://www.vaccine.uab.edu). In brief, serum samples were preabsorbed with 10 μg/ml of cell wall polysaccharide (Statens Seruminstitut) and 10 μg/ml of non-vaccine-related polysaccharide 22F (LGC Promochem, Teddington, United Kingdom). Standard serum 89SF (FDA) was used for the concentration curve, and control sera were obtained from National Institute for Standards and Controls. Antibodies specific for capsular polysaccharides 4, 14, and 23F were detected by using alkaline phosphatase conjugates, and the antibody concentrations (in μg/ml) were calculated from a four-point, sigmoidal plot by using Revelation software (ThermoLabs Inc., Basingstoke, United Kingdom).
Antibodies against tetanus and diphtheria toxoids will be quantified as part of an ongoing study.
Statistics.
Comparisons of the results between individuals and time points were carried out by using the Wilcoxon signed-rank test and the Mann-Whitney test with SPSS software (version 12), Graphpad Prism software (version 4.0), and the Microsoft Excel software TTEST function.
RESULTS
The spontaneous IgG AFC response following a primary (day 0) and secondary (day 56) dose of Pnc7 in toddlers.
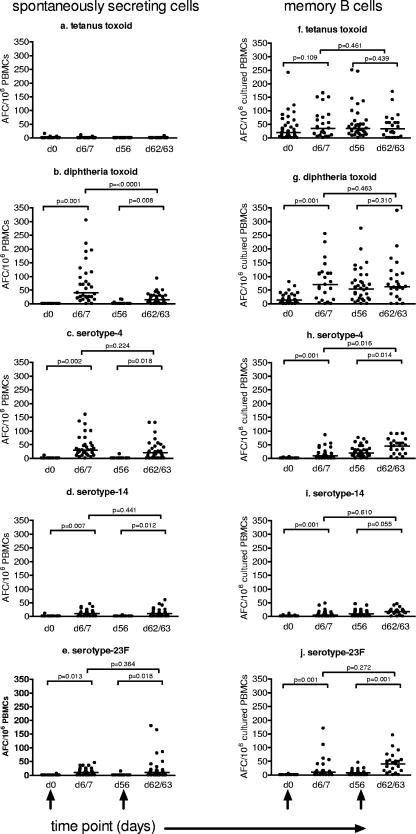
For all of the antigens tested except diphtheria toxoid, there was at least one individual who had some ex vivo AFCs on day 0, prior to immunization (Fig. 1a to e). There was no effect of Pnc7 immunization on the frequency of tetanus toxoid-specific AFCs (Fig. 1a). However, at day 6 or 7 following immunization, there was a significant rise in the frequency of AFCs in response to diphtheria toxoid and serotypes 4, 14, and 23F (P = 0.001, P = 0.002, P = 0.007, and P = 0.013, respectively) (Fig. 1b to e, respectively). The overall AFC response 1 week after immunization was high, with between 62 and 100% of the toddlers demonstrating a more than twofold increase in AFCs specific for the vaccine antigens and with 0% increases for the nonvaccine antigen tetanus toxoid. The strongest responses following the primary dose of Pnc7 were to diphtheria toxoid and serotype 4 (Fig. 1b and c, respectively), with 100% and 92% of the toddlers achieving more than twofold increases in the AFC frequency, respectively. In response to serotypes 14 and 23F, only 62% and 67% of the toddlers achieved more than twofold increases in the AFC frequency (Fig. 1d to e).
FIG. 1.
Frequency of spontaneously secreting IgG AFCs (a to e) and memory B-cell-derived IgG AFCs (f to j) isolated from the peripheral blood of 12-month-old toddlers prior to and 1 week following a primary and secondary dose of Pnc7, as determined by ELISPOT assay. Immunizations were given on day 0 (d0) and day 56 (black arrows), with blood draws performed on days 0, 6 or 7, 56, and 62 or 63. IgG AFCs specific for the control antigen (tetanus toxoid) and the vaccine antigens (diphtheria toxoid and polysaccharide serotypes 4, 14, and 23F) were enumerated. The data represent the numbers of IgG AFCs/106 cultured PBMCs, and the bars represent the medians.
Following the second dose of Pnc7, no significant difference in the magnitude of the AFC response was seen compared with that seen at 1 week after the first or the second dose of Pnc7 (Fig. 1b to e). The percentage of toddlers achieving a more than twofold increase in the AFC frequency in response to serotypes 14 and 23F rose to 80% and 70%, respectively, following the second dose of vaccine, although this increase was not significant. The ex vivo AFC response to diphtheria toxoid was lower after the second dose than after the first dose of Pnc7 (P = 0.002), with the proportion of toddlers achieving more than twofold increases in the AFC frequency dropping from 100% to 80%. Similarly, the frequencies of responders to serotype 4 dropped from 92% to 70%.
Memory B-cell response following immunization of toddlers with a primary and secondary dose of Pnc7.
In vitro stimulation of B cells isolated from the peripheral blood allowed the detection of IgG AFC derived from memory B cells. Prior to immunization, 86% of the toddlers already had diphtheria toxoid-specific memory cells, while only 2.5 to 5.6% had any polysaccharide-specific memory. There was no significant rise in the frequency of tetanus toxoid-specific IgG AFCs after immunization (Fig. 1f).
Seven days after primary Pnc7 immunization of the toddlers, there was a significant increase (P = 0.001) in the frequency of in vitro-inducible, antigen-specific AFCs derived from peripheral blood memory B cells (Fig. 1 g to j). The median numbers of spots on day 6 or 7 were 70, 10, 5, and 10 per 106 cultured PBMCs for diphtheria toxoid and serotypes 4, 14, and 23F, respectively. Between 67% and 81% of the toddlers made a more than twofold increase in AFC frequency during the first week. By day 56 the frequency remained significantly elevated or even increased further above the baseline levels for serotype 4 (Fig. 1h) (P = 0.016).
The second Pnc7 dose generated a further, significant increase in inducible, polysaccharide-specific AFCs by 1 week after immunization (Fig. 1g to j). However, the increase in AFC frequency was lower than that after the first dose (except in response to serotype 23F), so that at 1 week after the second dose (day 62 or 63), only 41% to 59% of the toddlers responded with a more than twofold rise in the AFC frequency. In response to serotype 23F, the proportion of responders increased from 67% to 71%. In response to the carrier protein (represented by diphtheria toxoid), only 12% of the toddlers showed a further twofold rise in AFC numbers after the second dose (Fig. 1g). The overall frequencies of memory B cells at this time were the highest in response to diphtheria toxoid, followed by serotypes 4, 23F, and 14, while the percentage of toddlers achieving a more than twofold increase was greater in response to serotype 23F (71%) and was the least for diphtheria toxoid (12%).
Memory B-cell response to a single dose of Pnc7 in adults versus that in toddlers.
Total IgG-secreting cells were enumerated as a control for the in vitro differentiation of AFCs from peripheral blood memory B cells. The median frequency of total IgG-secreting cells was significantly lower in the toddlers than in the adults (P = <0.001). Within each age group, however, the frequency remained constant throughout the time course (data not shown).
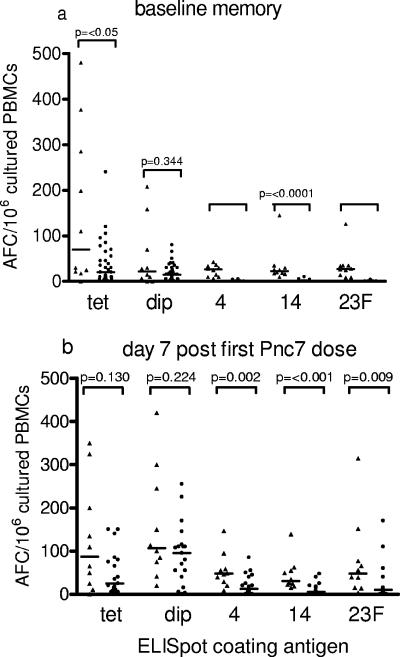
The frequency of polysaccharide-specific IgG AFCs derived from memory B cells was significantly lower in the toddlers than in the adults at the baseline (Fig. 2a). The median counts of spots specific for serotypes 4, 14, and 23F in the adults and toddlers were 26 and 0, 23 and 0, and 27 and 0 AFCs/106 cultured PBMCs, respectively. However, the diphtheria toxoid-specific IgG AFC frequency did not differ significantly between the adults and the toddlers (21 and 14 spots/106 cultured PBMCs, respectively) (Fig. 2a). The wide range of the adult tetanus toxoid-specific memory B-cell frequency meant that the median was significantly higher in the adults than in the toddlers (Fig. 2a).
FIG. 2.
Frequency of memory B-cell-derived IgG AFCs isolated from the peripheral blood of adults (triangles) and 12-month-old toddlers (circles). In vitro-stimulated PBMCs were isolated at day 0 for the baseline memory B-cell-derived IgG AFC frequency (a) and 1 week after a single dose of Pnc7 (b). IgG AFCs specific for the control antigen (tetanus toxoid [tet]) and the vaccine antigens (diphtheria toxoid [dip] and polysaccharide serotypes 4, 14, and 23F) were enumerated. The data represent the numbers of IgG AFCs/106 cultured PBMCs, and the bars represent the medians.
Seven days after a single dose of Pnc7 vaccine the antigen-specific memory cell frequency remained significantly lower in the toddlers than in the adults for serotypes 4, 14. and 23F (P = 0.002, <0.001, and 0.009, respectively) (Fig. 2b). In response to serotypes 14 and 23F, more toddlers than adults made a more than twofold response (59% and 30%, respectively, for the toddlers and 71% and 40%, respectively, for the adults), while the percentage of responders to serotype 4 was similar between the age groups (41% for the toddlers and 50% for the adults).
In response to diphtheria toxoid (the carrier response), there was no difference in the frequency of memory B cells between the toddlers and the adults (P = 0.224) (Fig. 2b), with 90% of the adults and 81% of the toddlers achieving a more than twofold rise in memory cell frequency. There was also no difference in the frequency of tetanus toxoid (nonvaccine antigen) memory B cells between the age groups (P = 0.130) (Fig. 2b), and only 38% of the adults and 10% of the toddlers showed a response (a more than twofold rise).
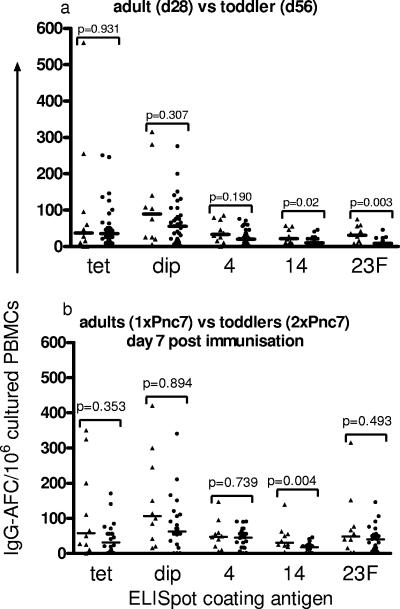
The maintenance of the memory response following the first dose was determined in the toddlers on day 56 after the first dose and was compared to the adult memory response at day 28 after a single Pnc7 dose (Fig. 3a). By day 56 none of the toddlers had maintained the more than twofold rise in AFC frequency seen at day 7 after immunization, while between 20 and 80% of the adults still had a more than twofold AFC frequency above that at the baseline at day 28. There was no significant difference between the adult and the toddler IgG AFC frequency in response to serotype 4 or diphtheria toxoid, and the frequency remained significantly elevated above that at the baseline in both age groups (Fig. 3a).
FIG. 3.
Comparison of in vitro-stimulated memory B-cell derived IgG AFC frequency in adults (triangles) and 12-month-old toddlers (circles). The responses to the single dose of Pnc7 in the adults and the toddlers at day 28 were compared to those at day 56 (a). The frequency of memory IgG AFCs achieved in the adults 1 week after one Pnc7 dose was compared with that achieved by the toddlers 1 week after a second Pnc7 dose (b). IgG AFCs specific for the control antigen (tetanus toxoid [tet]) and the vaccine antigens (diphtheria toxoid [dip] and polysaccharide serotypes 4, 14, and 23F) were enumerated. The data represent the numbers of IgG AFCs/106 cultured PBMCs, and the bars represent the medians.
The frequencies of serotype 14 and serotype 23F IgG AFCs remained significantly lower in the toddlers than in the adults prior to the second dose of Pnc7 in the toddler group (Fig. 3a).
Memory B-cell response following a second dose of Pnc7 in toddlers.
One week after a second dose of conjugate (day 63), the toddlers (who were then 14 months of age) attained frequencies of memory B cells equivalent to those seen in adult peripheral blood after a single dose for all antigens except serotype 14, the response to which was still significantly lower in the toddlers (P = 0.004) (Fig. 3b). The percentage of the toddlers responding with a more than twofold rise in the IgG AFC frequency compared to that for the adults was similar for serotype 4 (41% and 50%, respectively), but the frequencies in the toddlers remained slightly higher than those in the adults in response to serotypes 14 (59% and 30%, respectively) and 23F (71% and 40%, respectively). The response to diphtheria toxoid was lower in the toddlers after the second dose than in the adults after a single dose (12% and 90%, respectively).
Spontaneous secretion of IgG by AFCs from adults and toddlers.
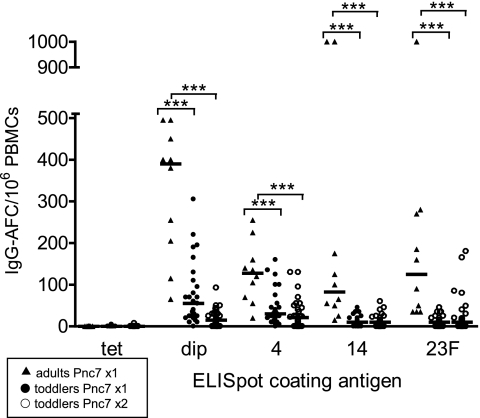
The frequency of spontaneously secreting, antigen-specific AFCs was significantly greater in the adults than in the 12-month-old toddlers on day 7 after the primary dose of Pnc7 (P = <0.001 for all comparisons) (Fig. 4). This significant difference between the age groups remained even after the toddlers received a second dose of Pnc7 (P = <0.001 for diphtheria toxoid and serotypes 4, 14, and 23F) (Fig. 4). The percentage of the toddlers achieving a more than twofold increase in spontaneously secreting AFCs rose after the second dose but was still less than that of the adults for diphtheria toxoid (80% and 100%, respectively) and serotypes 4 (70% and 100%, respectively), 14 (80% and 100%, respectively), and 23F (70% and 100%, respectively).
FIG. 4.
Frequency of spontaneously secreting IgG AFCs in adults after a single dose of Pnc7 (triangles) and 12-month-old toddlers after a single dose (closed circles) and a second dose (open circles) of Pnc7. PBMCs were isolated prior to and 1 week after each dose of vaccine and were allowed to spontaneously secrete IgG onto ELISPOT assay plate wells coated with the control antigen (tetanus toxoid [tet]) and the vaccine antigens (diphtheria toxoid [dip] and polysaccharide serotypes 4, 14, and 23F). The spots were enumerated and expressed as the numbers of IgG AFCs/106 PBMCs, and the bars represent the medians (***, P ⩽ 0.0001).
Serum antipolysaccharide antibody response following a primary or secondary dose of Pnc7 in toddlers.
A single dose of Pnc7 in the 12-month-old toddlers led to a significant increase in the levels of IgG, IgA, and IgM antipolysaccharide antibodies to each of the three serotypes tested at 1 week after immunization (Table 1). The percentage of the toddlers responding with a more than twofold increase in antibody concentration by day 7 for serotypes 4, 14, and 23F were 38%, 19%, and 29%, respectively, for IgG; 29%, 33%, and 10%, respectively, for IgA; and 91%, 81%, and 57%, respectively, for IgM.
TABLE 1.
Quantification of antipneumococcal polysaccharide serum antibody (IgG, IgA, and IgM) from 12-month-old toddlers prior to and following each of two doses of Pnc7 vaccine
| Serotype | Ig | GMC (μg/ml) of anti-pneumococcal polysaccharide serum antibody from 12-mo-old toddlers at the indicated times aftera:
|
|||
|---|---|---|---|---|---|
| First Pnc7 dose
|
Second Pnc7 dose
|
||||
| Day 0 | Day 6 or 7b | Day 56b | Day 62 or 63b | ||
| 4 | IgG | 0.02 (0.01-0.04) | 0.77 (0.52-1.16) | 2.16 (1.65-2.83) | 6.23 (4.42-8.78) |
| IgA | 0.02 (0.01-0.02) | 0.49 (0.34-0.71) | 0.20 (0.14-0.71) | 0.70 (0.51-0.97) | |
| IgM | 0.14 (0.11-0.17) | 1.12 (0.85-1.47) | 0.54 (0.42-0.68) | 0.98 (0.75-1.29) | |
| 14 | IgG | 0.05 (0.02-0.10) | 0.66 (0.38-1.14) | 2.78 (1.72-4.50) | 12.11 (9.44-15.55) |
| IgA | 0.02 (0.01-0.03) | 0.28 (0.20-0.40) | 0.15 (0.11-0.21) | 0.81 (0.62-1.07) | |
| IgM | 1.22 (0.98-1.52) | 5.90 (4.65-7.50) | 4.10 (3.22-5.21) | 8.34 (6.57-10.60) | |
| 23F | IgG | 0.02 (0.02-0.04) | 0.19 (0.10-0.36) | 0.33 (0.19-0.55) | 2.26 (1.35-3.78) |
| IgA | 0.01 (0.01-0.01) | 0.10 (0.07-0.16) | 0.04 (0.03-0.05) | 0.28 (0.18-0.45) | |
| IgM | 0.48 (0.38-0.59) | 1.16 (0.88-1.52) | 1.11 (0.87-1.42) | 2.07 (1.59-2.70) | |
The levels of antibodies against pneumococcal serotypes 4, 14, and 23F were quantified on days 0, 6 or 7, 56, and 62 or 63 after vaccination by ELISA. The 95% confidence intervals are given in parentheses.
These values represent a significant (P ≤ 0.0001) rise in the GMC above that from the baseline following the first dose of conjugate at day 0 and the second dose at day 56.
By day 56 (2 months postimmunization), IgG levels remained elevated above the day 7 levels for serotypes 4 and 14 (geometric mean concentrations [GMCs], 2.16 μg/ml and 2.78 μg/ml, respectively). The increases in the levels of antibodies to serotypes 4, 14, and 23F were greater in the toddlers after the second dose: 70%, 83%, and 83%, respectively, for IgG and 83%, 91%, and 57%, respectively, for IgA. The IgM response was lower than that after the first dose, with 30%, 44%, and 39% of the toddlers responding to the three serotypes, respectively. Thus, a single dose of Pnc7 did not generate an IgG response to 23F that was as good during the same time period. The IgA and IgM levels over the same 2-month period declined from the day 6 or 7 levels. The IgG, IgA, and IgM antibody concentrations remained above those at the baseline throughout the time course.
A second dose of Pnc7 induced a significant increase in the levels of IgG, IgA, and IgM to all three serotypes, with serotype 14 generating the greatest IgG response and serotype 23F generating the least. The serotype 23F response (and, to a lesser extent, the serotype 14 response) appeared to consist of IgG and IgM equally.
The first dose induced more polysaccharide-specific IgM than IgG to all three serotypes. By day 56 this was still the case for serotypes 14 and 23F, while the serotype 4 response appeared to have switched to a more IgG-mediated response.
Serum antipolysaccharide antibody in adults and toddlers prior to immunization.
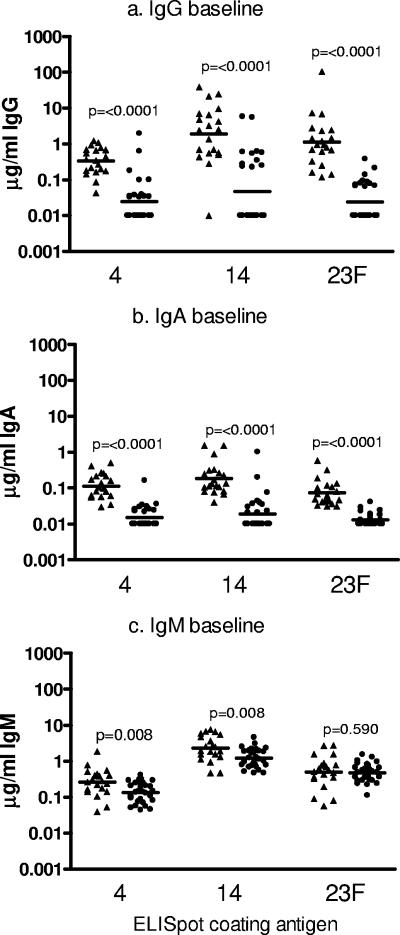
The adults had significantly more serotype 4-, 14-, and 23F-specific IgG than the toddlers at the baseline (Fig. 5a). This was also the case for serum IgA (Fig. 5b). The levels of serum IgM specific for serotypes 4 and 14 differed significantly between the age groups, with the toddlers having higher concentrations (Fig. 5c). Similar levels of IgM against serotype 23F were present in both age groups (P = 0.590) (Fig. 5c).
FIG. 5.
Quantification of preimmunization, antipneumococcal polysaccharide serum IgG (a), IgA (b), and IgM (c) antibody levels in adults (n = 20; triangles) and 12-month-old toddlers (n = 23; circles). Antibody levels against pneumococcal serotypes 4, 14, and 23F were quantified by ELISA. The data are plotted as the log of the individual antibody concentrations (in μg/ml), and the bars represent the GMCs.
Serum antipolysaccharide antibody in adults and toddlers following a single dose of Pnc7.
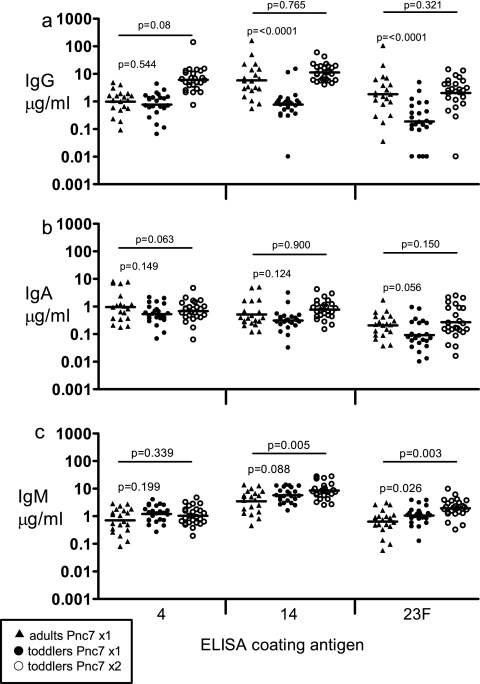
Seven days following the first Pnc7 dose, the GMCs of serotype 4-specific IgG were similar in both age groups (P = 0.544) (Fig. 6a). Significantly higher concentrations of IgG specific for serotypes 14 and 23F were achieved by the adults than by the toddlers (P ⩽ 0.0001 in both cases) (Fig. 6a). There was no significant difference between the two age groups in the IgA antibody responses to any of the serotypes generated by the first dose of Pnc7 (P = 0.149, 0.124, and 0.056 for serotypes 4, 14, and 23F, respectively) (Fig. 6b). This was also true for the IgM induced by serotypes 4 and 14 (P = 0.199 and 0.08, respectively) (Fig. 6c). The level of IgM specific for serotype 23F was significantly higher in the toddlers than in the adults (P = 0.026) (Fig. 6c).
FIG. 6.
Quantification of postimmunization, antipneumococcal polysaccharide serum IgG (a), IgA (b), and IgM (c) antibody levels. The adults received a single dose of Pnc7 (n = 20; triangles), and the 12-month-old toddlers received a single dose (n = 23; closed circles), followed by a second dose (n = 23; open circles) 2 months later. The levels of antibodies against pneumococcal serotypes 4, 14, and 23F were quantified by ELISA. The data are plotted as the log of the individual antibody concentrations (in μg/ml), and the bars represent the GMCs.
Serum antipolysaccharide antibody in toddlers following a second dose of Pnc7 compared to that in adults following the single dose.
One week after a second dose of Pnc7 was administered to the toddlers, there were no significant differences in the GMC of the serum IgG compared with that in the adults 1 week after they received their single dose (Fig. 6a). This was also true for the levels of serum IgA to each of the capsular polysaccharides (Fig. 6b). After the second dose, the toddlers' IgM response to serotypes 14 and 23F was significantly higher than that after the first dose (Fig. 6c), but there was no difference for serotype 4.
DISCUSSION
This is the first comparative study of B-cell responses to the pneumococcal conjugate vaccine in adults and toddlers and has shown that although both adults and 12-month-old toddlers mount significant immune responses to a single dose of a pneumococcal conjugate vaccine, the magnitudes of the initial IgG memory responses to the polysaccharide antigens were significantly lower in the toddlers and were serotype dependent. One week after the second Pnc7 dose in the toddlers, the memory B-cell-derived IgG AFC frequencies were equivalent to the adult memory B-cell frequencies seen 1 week after a single dose. The exception was the memory response to serotype 14, which remained lower in the toddlers than in the adults.
The antibody response showed evidence of class switching from IgM to IgG in the toddlers by day 56 after the first dose, suggesting the development of a memory response. This was serotype dependent, and IgM still dominated the response to serotypes 14 and 23F. After the second dose of Pnc7 in the toddlers, serotype 4 induced a mainly IgG-mediated response, although the magnitude of the response was lower than that to serotype 14. Serotype 23F induced equal amounts of IgG and IgM following the second dose and was the poorest immunogen in the toddlers.
Induction of antigen-specific B-cell memory is important for sustained antibody responses and for the rapid rises in antibody seen following reexposure to the antigen. A priming schedule at 2 and 4 months of age has now been introduced in the United Kingdom, with a booster dose given at 12 to 15 months of age. This schedule induces demonstrable memory responses with rapid elevations in antibody concentration, even when prebooster antibody levels are negligible. Following the introduction of the Pnc7 immunization into the United Kingdom toddler immunization schedule, 12-month-old toddlers received a single dose of conjugate in a catch-up campaign. Therefore, it was important to know whether memory would be generated in response to all components in the vaccine after a single dose.
Toddlers had no preexisting polysaccharide-specific IgG memory.
Prior to immunization, 100% of the adults in this study had IgG-producing memory B cells specific for the capsular polysaccharides of the three pneumococcal serotypes that were studied, while only 2.8 to 5.6% of the toddlers had any capsule-specific memory. Similarly, the adults had preexisting serum IgG levels above the protective levels (4, 27) for all three serotypes tested, while IgG was undetectable in the toddlers. Interestingly, in our study the adults demonstrated higher levels of naturally acquired IgG to the more commonly carried serotypes 14 and 23F than to the rarely detected serotype 4 (Fig. 5a) (11, 24, 65).
Nasopharyngeal carriage of pneumoccoci is known to induce IgG memory in older children and adults. The continued exposure to pneumococci over many years enhances the adult immune response to Pnc7, and it may be necessary to require maintenance of detectable levels of IgG memory (55) This has been demonstrated in mouse models of protection (55, 71).
For the toddlers in this study, the pattern of IgM at the baseline mirrored the pattern of carriage, with higher levels of anti-serotype 14 and 23F IgM. Thus, the response to the primary dose of conjugate in this age group looks more like a naïve response than that seen in adults, even though the vaccine is conjugated. The absence of naturally acquired, polysaccharide-specific IgG B-cell memory in the toddlers prior to immunization might be related to a lack of priming via the nasopharyngeal carriage of pneumococci, except that a number of epidemiological studies have clearly shown that 80% of toddlers have carried the vaccine or vaccine-related serotypes of pneumococci by 1 year of age (10, 36, 40, 61, 65). These observations suggest that the amount of pneumococcal carriage experienced during the first year of life may not be sufficient to maintain the levels of IgG or the elevated frequencies of IgG memory B cells against all serotypes in this age group. However, infants have been shown to be capable of antibody generation and maturation in response to pneumococcal surface proteins following nasopharyngeal carriage and disease (57, 73). It has also been reported that adults are capable of making polysaccharide-specific memory responses, as characterized by increased Ig gene diversity, following nasopharyngeal exposure (5). The higher responses in adults might reflect priming from prior exposure through nasopharyngeal carriage. Therefore, the immaturity of B-cell immunity that is specifically associated with T-cell-independent responses, such as low frequencies of MZB cells, low levels of expression of CD21 by MZB cells, and immature splenic marginal structure, in 12-month-old toddlers results in a lack of preexisting serum IgG and memory B-cell responses, which then delays the memory response to primary immunization with glycoconjugates compared to the time of the response in adults. This is discussed in more detail later, in relation to the cells involved in the immune response to polysaccharide antigens. The link between the effectiveness of conjugate immunization and carriage was recently suggested in the United Kingdom, where a lack of exposure to Haemophilus influenzae type b carriage led to an increased incidence of disease among children given a priming course of immunizations but no booster (30).
A single dose of Pnc7 induced polysaccharide-specific B-cell memory responses in a serotype-dependent manner.
Following a single dose of Pnc7, there was a significant rise in the frequency of polysaccharide- and diphtheria toxoid-specific memory B cells in both the toddlers and the adults (Fig. 1f to j). However, 1 week after immunization the frequency of polysaccharide-specific B cells was still significantly lower in the toddlers than in the adults (Fig. 2b). The lack of priming from nasopharyngeal carriage already discussed may be the reason for the lower IgG response in the toddlers at this time point. This is supported by the antibody data, which showed that IgM dominated the antipolysaccharide serum antibody response to the primary dose of Pnc7 vaccine in the toddlers (Table 1). The antipolysaccharide serum IgG responses after the first dose of Pnc7 observed in the toddlers in this study were also similar to those observed in 2- to 5-year-old children primed with the pneumococcal polysaccharide vaccine (60).
There was evidence of a maturing response to serotype 4 by day 56 after a single Pnc7 dose in toddlers. First, the memory IgG AFC frequency in response to serotype 4 in the toddlers on day 56 after immunization was similar to that in the adults at day 28 after a single dose (Fig. 3a). By contrast, the frequencies of memory B cells specific for serotypes 14 and 23F were still lower in the toddlers than in the adults. Second, the antibody data show a change from an IgM-mediated response to an IgG-mediated response to serotype 4 that was equivalent in the adults and the toddlers at day 7 (Fig. 6a).
In a recent Oxford University study of pneumococcal conjugate vaccine immunization of toddlers, the highest IgG antibody response was generated against serotype 4 and the lowest response was generated against serotype 23F. This is in contrast to the carriage data obtained for the same children, where serotype 23F was isolated frequently and serotype 4 was never isolated (61). The responses of toddler B cells to the polysaccharides in the vaccine may be affected by the immunogenicity of the antigen. In immunized adults the polysaccharide of serotype 14 is more immunogenic than that of serotype 23F, and that of serotype 4 is poorly immunogenic (42). The data reported here show the opposite for toddlers, with the immunogenicity of serotype 4 followed by that of serotype 14 and then that of serotype 23F.
The serum IgA concentrations generated following immunization differed by serotype, and the relationship was the same in the adults and the toddlers. Serotype 4 induced the best IgA response following the primary immunization. This may be one reason for the low rates of detection of this serotype in carriage studies, and a lack of carriage in adults and toddlers may explain the similarity in response to this serotype following primary immunization (11, 24, 61, 65).
A second dose of Pnc7 was required for toddlers to generate IgG memory responses to all three serotypes tested.
While a single dose of Pnc7 had initiated IgG memory formation in the toddlers, it was enhanced by the second dose. Seven days after the second dose of conjugate, the serum IgG response to all three serotypes was the same as that in the adults at the same time point after one dose (Fig. 6a). The serotype 14 memory B-cell response remained lower in the toddlers at this time point.
When the memory IgG AFC response of the toddlers was compared to that of the adults on day 28, there was no significant difference in the frequencies for any of the antigens tested (data not shown). This suggests that the toddlers were able to respond to the conjugated forms of the capsular polysaccharides equally as well as the adults following preexposure via the first dose.
Pnc7 immunization induces the circulation of spontaneously secreting plasma cells in toddlers, but reimmunization does not improve the response.
Previous studies have shown that the reimmunization of adults with the polysaccharide vaccines does not boost the frequency of spontaneously secreting plasma cells (29).
We have previously observed the same phenomenon with two doses of conjugate pneumococcal vaccines in adults (unpublished observations). Similarly, we found that despite the generation of increased memory B-cell frequencies, the reimmunization of the toddlers failed to increase the frequency of spontaneously secreting plasma cells generated 1 week after immunization (Fig. 4). This suggests that the plasma cell response is generated by a mechanism separate from that for the memory response and is not affected by immunization.
A single dose of Pnc7 induced carrier protein-specific B-cell memory in adults and toddlers.
Follicular B cells rather than MZB cells may have a greater role in immunity in 12-month-old toddlers (75), and immunity may require the recruitment of T-cell help by a carrier protein. We found that more than 80% of the 1-year-old toddlers had preexisting memory to the carrier protein (diphtheria toxoid) (Fig. 1g), presumably induced by the three-dose priming schedule with diphtheria and tetanus toxoids and pertussis vaccines during infancy. The frequencies at the baseline were similar in the adults and the toddlers at the baseline. Following the first dose of Pnc7, all of the toddlers responded to diphtheria toxoid, and the percentage achieving a more than twofold rise increase in memory B-cell frequencies was equivalent to that in seen in the adults (Fig. 2b). Thus, the differences in the magnitudes of the antipolysaccharide response between the adults and the toddlers did not result from poor carrier immunity or a lack of preexisting immunity to the CRM197 carrier protein in toddlers.
Carrier protein-specific memory B-cell frequency in toddlers was not improved by reimmunization.
The diphtheria toxoid memory IgG AFC response in the toddlers did not increase following reimmunization, and this is supported by evidence from a previous study in which an inverse relationship between the AFC frequency and the number of doses of diphtheria vaccine in adults was found (46). Preexisting antibody to carrier proteins has also been shown to either enhance or impair the anticapsular responses following glycoconjugate immunization (6, 15, 18, 22, 49, 51). Obukhanych et al. (48) demonstrated that serum IgG levels regulate the responsiveness of specific B cells to their antigens. Therefore, toddlers with preexisting and possibly high-avidity antibody to diphtheria toxoid may have more limited responses to Pnc7 reimmunization (26, 43). However, observations of carrier suppression have mainly been seen when conjugate vaccines are used with the tetanus toxoid as the carrier protein (15, 18, 51). Quantification of the diphtheria toxoid/CRM197 antibodies was not undertaken as part of this study, but this is the subject of future work. From such data it will be possible to determine the positive or negative effect of high anticarrier antibody levels on the polysaccharide response.
Other studies have shown that polysaccharides of different serotypes induce different antibody isotypes and have variable immunogenicities, even in the presence of good anti-carrier protein T- and B-cell responses (28, 39). Different cytokine responses induced by individual polysaccharide-protein conjugates in the vaccine (38) alter the observed antibody isotype response.
The maturation status of the toddler immune system delays the development of the antipolysaccharide B-cell memory responses induced by Pnc7.
MZB cells, implicated in adult responses to polysaccharide antigens, increase in frequency and maturity with age and secrete IgM and IgG or IgA. Factors such as bystander cytokine secretion rather than direct T-cell contact are required to induce a switch of MZB cells from IgM to IgG or IgA (31, 50, 72, 74-76). In a previous study we found that Pnc7 induced B cells with a phenotype consistent with that of MZB cells in adults (12). MZB cells are present only at low frequencies in children <2 years old (63), and the splenic structure lacks some of the functional features present in adults, such as CD27+ B cells (81) and CD21 expression (8, 52, 59), both of which are highly expressed on mature MZB cells. The low frequency of CD27+ memory B cells combined with the low level of expression of CD21 (complement receptor CR2) reduces the strength of signaling via the B-cell receptor-coreceptor complex (23, 59, 69). Thus, MZB cell activation in toddlers is less efficient than that in adults. Together, this may be an explanation for the delayed antipolysaccharide response seen in the toddlers in this study.
The long-term survival of plasma cells in the bone marrow is thought to be another mechanism for the maintenance of long-term antibody production (34). It has been suggested that differentiated plasma cells leave the germinal center following Pnc7 immunization and migrate through the peripheral blood to the bone marrow, where the long-term survival of antigen-specific plasma cells is maintained via contact with factors expressed by bone marrow stromal cells (35, 66, 67). The situation in toddlers is less clear, but Pihlgren et al. (53, 54) demonstrated that there is a lack of suitable bone marrow niches for the maintenance of plasma cells in infant mice, and it is postulated that this may contribute to the more rapid waning of antibody in infancy. Although we did not look at the long-term antibody responses in this study, the combination of immature MZB cells and splenic structure combined with a lack of suitable bone marrow niches for plasma cell survival may be why priming of the immune response via nasopharyngeal carriage is less efficient in young children and therefore may affect the response to the initial dose of Pnc7.
Thus, the data shown here have revealed that human toddlers require two priming doses of Pnc7 conjugate vaccine to induce memory B-cell and serum antibody responses similar to those seen in adults after a single dose of the vaccine. Priming of the immune system via continued exposure to nasopharyngeal carriage of pneumococci, the nature of the polysaccharide capsule, and the age and maturation status of the immune system are important factors in determining the generation of IgG memory.
Acknowledgments
This work was funded by a grant awarded by the Edward Jenner Institute for Vaccine Research, Compton, Berkshire, United Kingdom.
We are grateful to the staff at the Oxford Vaccine Group for their support in collecting the samples and recruiting volunteers for the study and also to the volunteers and children who participated.
A. J. Pollard and P. C. L. Beverley are Jenner Institute investigators. A. J. Pollard acts as the chief investigator for clinical trials conducted on behalf of Oxford University and sponsored by vaccine manufacturers (Novartis Vaccines, GlaxoSmithKline, Sanofi-Pasteur, Sanofi-Pasteur MSD, and Wyeth Vaccines) and has received assistance from vaccine manufacturers to attend scientific meetings. Industry-sourced honoraria for lecturing or writing are paid directly to an independent charity or an educational/administrative fund held by the Department of Paediatrics, University of Oxford.
Footnotes
Published ahead of print on 11 November 2007.
REFERENCES
- 1.Abraham-Van Parijs, B. 2004. Review of pneumococcal conjugate vaccine in adults: implications on clinical development. Vaccine 22:1362-1371. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, E. L., D. J. Kennedy, K. M. Geldmacher, J. Donnelly, and P. M. Mendelman. 1996. Immunogenicity of heptavalent pneumococcal conjugate vaccine in infants. J. Pediatr. 128:649-653. [DOI] [PubMed] [Google Scholar]
- 3.Barnett, E. D., S. I. Pelton, H. J. Cabral, R. D. Eavey, C. Allen, M. J. Cunningham, E. R. McNamara, and J. O. Klein. 1999. Immune response to pneumococcal conjugate and polysaccharide vaccines in otitis-prone and otitis-free children. Clin. Infect. Dis. 29:191-192. [DOI] [PubMed] [Google Scholar]
- 4.Barzilay, E. J., K. L. O'Brien, Y. S. Kwok, R. M. Hoekstra, E. R. Zell, R. Reid, M. Santosham, C. G. Whitney, and D. R. Feikin. 2006. Could a single dose of pneumococcal conjugate vaccine in children be effective? Modeling the optimal age of vaccination. Vaccine 24:904-913. [DOI] [PubMed] [Google Scholar]
- 5.Baxendale, H. E., Z. Davis, H. N. White, M. B. Spellerberg, F. K. Stevenson, and D. Goldblatt. 2000. Immunogenetic analysis of the immune response to pneumococcal polysaccharide. Eur. J. Immunol. 30:1214-1223. [DOI] [PubMed] [Google Scholar]
- 6.Bergquist, C., T. Lagergard, and J. Holmgren. 1997. Anticarrier immunity suppresses the antibody response to polysaccharide antigens after intranasal immunization with the polysaccharide-protein conjugate. Infect. Immun. 65:1579-1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Black, S., H. Shinefield, R. Baxter, R. Austrian, L. Bracken, J. Hansen, E. Lewis, and B. Fireman. 2004. Postlicensure surveillance for pneumococcal invasive disease after use of heptavalent pneumococcal conjugate vaccine in Northern California Kaiser Permanente. Pediatr. Infect. Dis. J. 23:485-489. [DOI] [PubMed] [Google Scholar]
- 8.Bondada, S., H.-J. Wu, D. A. Robertson, and R. L. Chelvarajan. 2000. Accessory cell defect in unresponsiveness of neonates and aged to polysaccharide vaccines. Vaccine 19:557-565. [DOI] [PubMed] [Google Scholar]
- 9.Breukels, M. A., A. Zandvoort, G. T. Rijkers, M. E. Lodewijk, P. A. Klok, G. Harms, and W. Timens. 2005. Complement dependency of splenic localization of pneumococcal polysaccharide and conjugate vaccines. Scand. J. Immunol. 61:322-328. [DOI] [PubMed] [Google Scholar]
- 10.Brueggemann, A. B., D. T. Griffiths, E. Meats, T. Peto, D. W. Crook, and B. G. Spratt. 2003. Clonal relationships between invasive and carriage Streptococcus pneumoniae and serotype- and clone-specific differences in invasive disease potential. J. Infect. Dis. 187:1424-1432. [DOI] [PubMed] [Google Scholar]
- 11.Brueggemann, A. B., T. E. Peto, D. W. Crook, J. C. Butler, K. G. Kristinsson, and B. G. Spratt. 2004. Temporal and geographic stability of the serogroup-specific invasive disease potential of Streptococcus pneumoniae in children. J. Infect. Dis. 190:1203-1211. [DOI] [PubMed] [Google Scholar]
- 12.Clutterbuck, E. A., P. Salt, S. Oh, A. Marchant, P. Beverley, and A. J. Pollard. 2006. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology 119:328-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Concepcion, N., and C. E. Frasch. 1998. Evaluation of previously assigned antibody concentrations in pneumococcal polysaccharide reference serum 89SF by the method of cross-standardization. Clin. Diagn. Lab. Immunol. 5:199-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crotty, S., R. D. Aubert, J. Glidewell, and R. Ahmed. 2004. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J. Immunol. Methods 286:111-122. [DOI] [PubMed] [Google Scholar]
- 15.Dagan, R., J. Eskola, C. Leclerc, and O. Leroy. 1998. Reduced response to multiple vaccines sharing common protein epitopes that are administered simultaneously to infants. Infect. Immun. 66:2093-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ekstrom, N., H. Ahman, J. Verho, J. Jokinen, M. Vakevainen, T. Kilpi, and H. Kayhty. 2005. Kinetics and avidity of antibodies evoked by heptavalent pneumococcal conjugate vaccines PncCRM and PncOMPC in the Finnish Otitis Media Vaccine Trial. Infect. Immun. 73:369-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Falkoff, R. J., L. P. Zhu, and A. S. Fauci. 1983. The relationship between immunization and circulating antigen-specific plaque-forming cells. Cell. Immunol. 78:392-399. [DOI] [PubMed] [Google Scholar]
- 18.Fattom, A., Y. H. Cho, C. Chu, S. Fuller, L. Fries, and R. Naso. 1999. Epitopic overload at the site of injection may result in suppression of the immune response to combined capsular polysaccharide conjugate vaccines. Vaccine 17:126-133. [DOI] [PubMed] [Google Scholar]
- 19.Fedson, D. S., and J. A. Scott. 1999. The burden of pneumococcal disease among adults in developed and developing countries: what is and is not known. Vaccine 17(Suppl. 1):S11-S18. [DOI] [PubMed] [Google Scholar]
- 20.Gatto, D., S. W. Martin, J. Bessa, E. Pellicioli, P. Saudan, H. J. Hinton, and M. F. Bachmann. 2007. Regulation of memory antibody levels: the role of persisting antigen versus plasma cell life span. J. Immunol. 178:67-76. [DOI] [PubMed] [Google Scholar]
- 21.Gatto, D., C. Ruedl, B. Odermatt, and M. F. Bachmann. 2004. Rapid response of marginal zone B cells to viral particles. J. Immunol. 173:4308-4316. [DOI] [PubMed] [Google Scholar]
- 22.Granoff, D. M., M. H. Rathore, S. J. Holmes, P. D. Granoff, and A. H. Lucas. 1993. Effect of immunity to the carrier protein on antibody responses to Haemophilus influenzae type b conjugate vaccines. Vaccine 11(Suppl. 1):S46-S51. [DOI] [PubMed] [Google Scholar]
- 23.Griffioen, A. W., S. W. Franklin, B. J. Zegers, and G. T. Rijkers. 1993. Expression and functional characteristics of the complement receptor type 2 on adult and neonatal B lymphocytes. Clin. Immunol. Immunopathol. 69:1-8. [DOI] [PubMed] [Google Scholar]
- 24.Hausdorff, W. P., D. R. Feikin, and K. P. Klugman. 2005. Epidemiological differences among pneumococcal serotypes. Lancet Infect. Dis. 5:83-93. [DOI] [PubMed] [Google Scholar]
- 25.Heilmann, C., J. Henrichsen, and F. K. Pedersen. 1987. Vaccination-induced circulation of human B cells secreting type-specific antibodies against pneumococcal polysaccharides. Scand. J. Immunol. 25:61-67. [DOI] [PubMed] [Google Scholar]
- 26.Heyman, B. 2000. Regulation of antibody responses via antibodies, complement, and Fc receptors. Annu. Rev. Immunol. 18:709-737. [DOI] [PubMed] [Google Scholar]
- 27.Jodar, L., J. Butler, G. Carlone, R. Dagan, D. Goldblatt, H. Kayhty, K. Klugman, B. Plikaytis, G. Siber, R. Kohberger, I. Chang, and T. Cherian. 2003. Serological criteria for evaluation and licensure of new pneumococcal conjugate vaccine formulations for use in infants. Vaccine 21:3265-3272. [DOI] [PubMed] [Google Scholar]
- 28.Kamboj, K. K., H. L. Kirchner, R. Kimmel, N. S. Greenspan, and J. R. Schreiber. 2003. Significant variation in serotype-specific immunogenicity of the seven-valent Streptococcus pneumoniae capsular polysaccharide-CRM197 conjugate vaccine occurs despite vigorous T cell help induced by the carrier protein. J. Infect. Dis. 187:1629-1638. [DOI] [PubMed] [Google Scholar]
- 29.Kehrl, J. H., and A. S. Fauci. 1983. Activation of human B lymphocytes after immunization with pneumococcal polysaccharides. J. Clin. Investig. 71:1032-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kelly, D. F., E. R. Moxon, and A. J. Pollard. 2004. Haemophilus influenzae type b conjugate vaccines. Immunology 113:163-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kruetzmann, S., M. M. Rosado, H. Weber, U. Germing, O. Tournilhac, H. H. Peter, R. Berner, A. Peters, T. Boehm, A. Plebani, I. Quinti, and R. Carsetti. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J. Exp. Med. 197:939-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lexau, C. A., R. Lynfield, R. Danila, T. Pilishvili, R. Facklam, M. M. Farley, L. H. Harrison, W. Schaffner, A. Reingold, N. M. Bennett, J. Hadler, P. R. Cieslak, and C. G. Whitney for the Active Bacterial Core Surveillance Team. 2005. Changing epidemiology of invasive pneumococcal disease among older adults in the era of pediatric pneumococcal conjugate vaccine. JAMA 294:2043-2051. [DOI] [PubMed] [Google Scholar]
- 33.Lottenbach, K. R., C. M. Mink, S. J. Barenkamp, E. L. Anderson, S. M. Homan, and D. C. Powers. 1999. Age-associated differences in immunoglobulin G1 (IgG1) and IgG2 subclass antibodies to pneumococcal polysaccharides following vaccination. Infect. Immun. 67:4935-4938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manz, R. A., A. E. Hauser, F. Hiepe, and A. Radbruch. 2005. Maintenance of serum antibody levels. Annu. Rev. Immunol. 23:367-386. [DOI] [PubMed] [Google Scholar]
- 35.Manz, R. A., A. Thiel, and A. Radbruch. 1997. Lifetime of plasma cells in the bone marrow. Nature 388:133-134. [DOI] [PubMed] [Google Scholar]
- 36.Marchisio, P., S. Esposito, G. C. Schito, A. Marchese, R. Cavagna, and N. Principi. 2002. Nasopharyngeal carriage of Streptococcus pneumoniae in healthy children: implications for the use of heptavalent pneumococcal conjugate vaccine. Emerg. Infect. Dis. 8:479-484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin, F., A. M. Oliver, and J. F. Kearney. 2001. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity 14:617-629. [DOI] [PubMed] [Google Scholar]
- 38.Mawas, F., I. M. Feavers, and M. J. Corbel. 2000. Serotype of Streptococcus pneumoniae capsular polysaccharide can modify the Th1/Th2 cytokine profile and IgG subclass response to pneumococal-CRM(197) conjugate vaccines in a murine model. Vaccine 19:1159-1166. [DOI] [PubMed] [Google Scholar]
- 39.McCool, T. L., C. V. Harding, N. S. Greenspan, and J. R. Schreiber. 1999. B- and T-cell immune responses to pneumococcal conjugate vaccines: divergence between carrier- and polysaccharide-specific immunogenicity. Infect. Immun. 67:4862-4869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meats, E., A. B. Brueggemann, M. C. Enright, K. Sleeman, D. T. Griffiths, D. W. Crook, and B. G. Spratt. 2003. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae. J. Clin. Microbiol. 41:386-392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Metlay, J. P., N. O. Fishman, M. Joffe, and P. H. Edelstein. 2006. Impact of pediatric vaccination with pneumococcal conjugate vaccine on the risk of bacteremic pneumococcal pneumonia in adults. Vaccine 24:468. [DOI] [PubMed] [Google Scholar]
- 42.Mikolajczyk, M. G., N. F. Concepcion, T. Wang, D. Frazier, B. Golding, C. E. Frasch, and D. E. Scott. 2004. Characterization of antibodies to capsular polysaccharide antigens of Haemophilus influenzae type b and Streptococcus pneumoniae in human immune globulin intravenous preparations. Clin. Diagn. Lab. Immunol. 11:1158-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moller, G. 1985. Antibody-mediated suppression of the immune response is determinant specific. Eur. J. Immunol. 15:409-412. [DOI] [PubMed] [Google Scholar]
- 44.Moser, K., K. Tokoyoda, A. Radbruch, I. MacLennan, and R. A. Manz. 2006. Stromal niches, plasma cell differentiation and survival. Curr. Opin. Immunol. 18:265-270. [DOI] [PubMed] [Google Scholar]
- 45.Musher, D. M. 2006. Pneumococcal vaccine—direct and indirect (“herd”) effects. N. Engl. J. Med. 354:1522-1524. [DOI] [PubMed] [Google Scholar]
- 46.Nanan, R., D. Heinrich, M. Frosch, and H. W. Kreth. 2001. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine 20:498-504. [DOI] [PubMed] [Google Scholar]
- 47.O'Brien, K. L., A. J. Swift, J. A. Winkelstein, M. Santosham, B. Stover, R. Luddy, J. E. Gootenberg, J. T. Nold, A. Eskenazi, S. J. Snader, H. M. Lederman, et al. 2000. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM(197) among infants with sickle cell disease. Pediatrics 106:965-972. [DOI] [PubMed] [Google Scholar]
- 48.Obukhanych, T. V., and M. C. Nussenzweig. 2006. T-independent type II immune responses generate memory B cells. J. Exp. Med. 203:305-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olander, R.-M., T. Wuorimaa, H. Kayhty, O. Leroy, R. Dagan, and J. Eskola. 2001. Booster response to the tetanus and diphtheria toxoid carriers of 11-valent pneumococcal conjugate vaccine in adults and toddlers. Vaccine 20:336. [DOI] [PubMed] [Google Scholar]
- 50.Oliver, A. M., F. Martin, and J. F. Kearney. 1999. IgMhighCD21high lymphocytes enriched in the splenic marginal zone generate effector cells more rapidly than the bulk of follicular B cells. J. Immunol. 162:7198-7207. [PubMed] [Google Scholar]
- 51.Peeters, C. C., A. M. Tenbergen-Meekes, J. T. Poolman, M. Beurret, B. J. Zegers, and G. T. Rijkers. 1991. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect. Immun. 59:3504-3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peset Llopis, M. J., G. Harms, M. J. Hardonk, and W. Timens. 1996. Human immune response to pneumococcal polysaccharides: complement-mediated localization preferentially on CD21-positive splenic marginal zone B cells and follicular dendritic cells. J. Allergy Clin. Immunol. 97:1015-1024. [DOI] [PubMed] [Google Scholar]
- 53.Pihlgren, M., M. Friedli, C. Tougne, A. F. Rochat, P. H. Lambert, and C. A. Siegrist. 2006. Reduced ability of neonatal and early-life bone marrow stromal cells to support plasmablast survival. J. Immunol. 176:165-172. [DOI] [PubMed] [Google Scholar]
- 54.Pihlgren, M., N. Schallert, C. Tougne, P. Bozzotti, J. Kovarik, A. Fulurija, M. Kosco-Vilbois, P. H. Lambert, and C. A. Siegrist. 2001. Delayed and deficient establishment of the long-term bone marrow plasma cell pool during early life. Eur. J. Immunol. 31:939-946. [DOI] [PubMed] [Google Scholar]
- 55.Rabquer, B., A. K. Shriner, S. L. Smithson, and M. A. Westerink. 2007. B cell mediated priming following pneumococcal colonization. Vaccine 25:2036-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramsay, M. E., J. McVernon, N. J. Andrews, P. T. Heath, and M. P. Slack. 2003. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 188:481-485. [DOI] [PubMed] [Google Scholar]
- 57.Rapola, S., V. Jantti, R. Haikala, R. Syrjanen, G. M. Carlone, J. S. Sampson, D. E. Briles, J. C. Paton, A. K. Takala, T. M. Kilpi, and H. Kayhty. 2000. Natural development of antibodies to pneumococcal surface protein A, pneumococcal surface adhesin A, and pneumolysin in relation to pneumococcal carriage and acute otitis media. J. Infect. Dis. 182:1146-1152. [DOI] [PubMed] [Google Scholar]
- 58.Rennels, M. B., K. M. Edwards, H. L. Keyserling, K. S. Reisinger, D. A. Hogerman, D. V. Madore, I. Chang, P. R. Paradiso, F. J. Malinoski, and A. Kimura. 1998. Safety and immunogenicity of heptavalent pneumococcal vaccine conjugated to CRM197 in United States infants. Pediatrics 101:604-611. [DOI] [PubMed] [Google Scholar]
- 59.Rijkers, G. T., E. A. M. Sanders, M. A. Breukels, and B. J. M. Zegers. 1998. Infant B cell responses to polysaccharide determinants. Vaccine 16:1396. [DOI] [PubMed] [Google Scholar]
- 60.Rose, M. A., R. Schubert, N. Strnad, and S. Zielen. 2005. Priming of immunological memory by pneumococcal conjugate vaccine in children unresponsive to 23-valent polysaccharide pneumococcal vaccine. Clin. Diagn. Lab Immunol. 12:1216-1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Salt, P., C. Banner, S. Oh, L. M. Yu, S. Lewis, D. Pan, D. Griffiths, B. Ferry, and A. Pollard. 2007. Social mixing with other children during infancy enhances antibody response to a pneumococcal conjugate vaccine in early childhood. Clin. Vaccine Immunol. 14:593-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shelly, M. A., H. Jacoby, G. J. Riley, B. T. Graves, M. Pichichero, and J. J. Treanor. 1997. Comparison of pneumococcal polysaccharide and CRM197-conjugated pneumococcal oligosaccharide vaccines in young and elderly adults. Infect. Immun. 65:242-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi, Y., K. Agematsu, H. D. Ochs, and K. Sugane. 2003. Functional analysis of human memory B-cell subpopulations: IgD+CD27+ B cells are crucial in secondary immune response by producing high affinity IgM. Clin. Immunol. 108:128-137. [DOI] [PubMed] [Google Scholar]
- 64.Shinefield, H. R., S. Black, P. Ray, I. Chang, N. Lewis, B. Fireman, J. Hackell, P. R. Paradiso, G. Siber, R. Kohberger, D. V. Madore, F. J. Malinowski, A. Kimura, C. Le, I. Landaw, J. Aguilar, and J. Hansen. 1999. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 18:757-763. [DOI] [PubMed] [Google Scholar]
- 65.Sleeman, K. L., D. Griffiths, F. Shackley, L. Diggle, S. Gupta, M. C. Maiden, E. R. Moxon, D. W. Crook, and T. E. Peto. 2006. Capsular serotype-specific attack rates and duration of carriage of Streptococcus pneumoniae in a population of children. J. Infect. Dis. 194:682-688. [DOI] [PubMed] [Google Scholar]
- 66.Slifka, M. K., and R. Ahmed. 1998. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr. Opin. Immunol. 10:252-258. [DOI] [PubMed] [Google Scholar]
- 67.Slifka, M. K., M. Matloubian, and R. Ahmed. 1995. Bone marrow is a major site of long-term antibody production after acute viral infection. J. Virol. 69:1895-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Snape, M. D., and A. J. Pollard. 2005. Meningococcal polysaccharide-protein conjugate vaccines. Lancet Infect. Dis. 5:21-30. [DOI] [PubMed] [Google Scholar]
- 69.Timens, W., A. Boes, T. Rozeboom-Uiterwijk, and S. Poppema. 1989. Immaturity of the human splenic marginal zone in infancy. Possible contribution to the deficient infant immune response. J. Immunol. 143:3200-3206. [PubMed] [Google Scholar]
- 70.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 71.van Rossum, A. M., E. S. Lysenko, and J. N. Weiser. 2005. Host and bacterial factors contributing to the clearance of colonization by Streptococcus pneumoniae in a murine model. Infect. Immun. 73:7718-7726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vinuesa, C. G., D. M. Sze, M. C. Cook, K. M. Toellner, G. G. Klaus, J. Ball, and I. C. MacLennan. 2003. Recirculating and germinal center B cells differentiate into cells responsive to polysaccharide antigens. Eur. J. Immunol. 33:297-305. [DOI] [PubMed] [Google Scholar]
- 73.Virolainen, A., W. Russell, M. J. Crain, S. Rapola, H. Kayhty, and D. E. Briles. 2000. Human antibodies to pneumococcal surface protein A in health and disease. Pediatr. Infect. Dis. J. 19:134-138. [DOI] [PubMed] [Google Scholar]
- 74.Weller, S., M. C. Braun, B. K. Tan, A. Rosenwald, C. Cordier, M. E. Conley, A. Plebani, D. S. Kumararatne, D. Bonnet, O. Tournilhac, G. Tchernia, B. Steiniger, L. M. Staudt, J. L. Casanova, C. A. Reynaud, and J. C. Weill. 2004. Human blood IgM “memory” B cells are circulating splenic marginal zone B cells harboring a prediversified immunoglobulin repertoire. Blood 104:3647-3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Weller, S., C. A. Reynaud, and J. C. Weill. 2005. Vaccination against encapsulated bacteria in humans: paradoxes. Trends Immunol. 26:85-89. [DOI] [PubMed] [Google Scholar]
- 76.Werner-Favre, C., F. Bovia, P. Schneider, N. Holler, M. Barnet, V. Kindler, J. Tschopp, and R. H. Zubler. 2001. IgG subclass switch capacity is low in switched and in IgM-only, but high in IgD+IgM+, post-germinal center (CD27+) human B cells. Eur. J. Immunol. 31:243-249. [DOI] [PubMed] [Google Scholar]
- 77.Wernette, C. M., C. E. Frasch, D. Madore, G. Carlone, D. Goldblatt, B. Plikaytis, W. Benjamin, S. A. Quataert, S. Hildreth, D. J. Sikkema, H. Kayhty, I. Jonsdottir, and M. H. Nahm. 2003. Enzyme-linked immunosorbent assay for quantitation of human antibodies to pneumococcal polysaccharides. Clin. Diagn. Lab Immunol. 10:514-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, N. M. Bennett, R. Lynfield, A. Reingold, P. R. Cieslak, T. Pilishvili, D. Jackson, R. R. Facklam, J. H. Jorgensen, and A. Schuchat. 2003. Decline in invasive pneumococcal disease after the introduction of protein-polysaccharide conjugate vaccine. N. Engl. J. Med. 348:1737-1746. [DOI] [PubMed] [Google Scholar]
- 79.Whitney, C. G., T. Pilishvili, M. M. Farley, W. Schaffner, A. S. Craig, R. Lynfield, A. C. Nyquist, K. A. Gershman, M. Vazquez, N. M. Bennett, A. Reingold, A. Thomas, M. P. Glode, E. R. Zell, J. H. Jorgensen, B. Beall, and A. Schuchat. 2006. Effectiveness of seven-valent pneumococcal conjugate vaccine against invasive pneumococcal disease: a matched case-control study. Lancet 368:1495-1502. [DOI] [PubMed] [Google Scholar]
- 80.Wuorimaa, T., H. Kayhty, O. Leroy, and J. Eskola. 2001. Tolerability and immunogenicity of an 11-valent pneumococcal conjugate vaccine in adults. Vaccine 19:1863-1869. [DOI] [PubMed] [Google Scholar]
- 81.Zandvoort, A., M. E. Lodewijk, N. K. de Boer, P. M. Dammers, F. G. Kroese, and W. Timens. 2001. CD27 expression in the human splenic marginal zone: the infant marginal zone is populated by naive B cells. Tissue Antigens 58:234-242. [DOI] [PubMed] [Google Scholar]