Abstract
The Brucella species type IV secretion system, encoded by the virB1-12 locus, is required for intracellular replication and persistent infection in vivo. The requirement of VirB proteins for infection suggests that they are expressed in vivo and may therefore represent serological markers of infection. To test this idea, we purified recombinant VirB1, VirB5, VirB11, and VirB12 and tested for their recognition by antibodies in sera from experimentally infected mice and goats by using an indirect enzyme-linked immunosorbent assay. Antibody responses to VirB12 but not to VirB1, VirB5, or VirB11 were detected in 20/20 mice experimentally inoculated with Brucella abortus and 12/12 goats experimentally infected with Brucella melitensis. The potential use of VirB12 as a serological tool for the diagnosis of brucellosis was evaluated in the natural bovine host. Serum samples from 145 cattle of known serology (29% negative and 71% positive) were analyzed for the production of antibody responses to VirB12. One hundred two cattle samples (70.3%) were positive for antibodies to VirB12, while 43 samples were negative (29.7%). A positive serological response to VirB12 correlated with positive serology to whole B. abortus antigen in 99% of samples tested. These results show that VirB12 is expressed during infection of both experimental and natural hosts of Brucella species, and they suggest that VirB12 may be a useful serodiagnostic marker for brucellosis.
Brucella strains are the causative agents of brucellosis, which can affect both animals and humans. The disease is characterized by bacterial persistence in the reticuloendothelial system, including the liver, spleen, and lymph nodes, and infection may result in abortion in infected ruminants (34). Zoonotic transmission of the bacteria to humans is caused by accidental contamination from unpasteurized animal products or infected animals.
The type IV secretion system (T4SS) is encoded by the virB locus, located on chromosome II, and includes virB1 to virB12 (2, 3). The T4SS of Brucella spp. has been shown to be an essential virulence factor, and T4SS mutants are highly attenuated in tissue culture models of intracellular survival, in the mouse model of persistent infection (3, 5-8, 10, 14, 23, 30, 32), and in the goat, a natural host for Brucella melitensis (12). A functional T4SS is not required for initial colonization in mice but is required for evasion of immune responses activated during the first week of infection (25, 27).
Several diagnostic tools are used for the detection of brucellosis. While bacteriological isolation is the most specific diagnostic test, the frequency of isolation is usually low, and results are not available immediately. For this reason, serological tests are widely used for diagnosis of human and animal brucellosis (1). Classical serological techniques rely on the detection of smooth lipopolysaccharide (LPS), but false-positive reactions may occur due to cross-reactivity with LPS from other bacteria (21, 22, 33). The shortcomings of the classical serological tests have sparked increasing interest in finding alternative antigens to detect brucellosis.
Secreted or cell envelope-associated bacterial proteins are often antigenic in the context of infection. Proteins associated with the cell envelope of Brucella species have previously been shown to elicit antibody responses in both experimentally and naturally infected animals (4, 17-19, 24, 26). Further, VirB proteins from other bacteria, including Ehrlichia canis and Anaplasma marginale, have been shown to be immunogenic (9, 20). Since the Brucella abortus T4SS has been shown to be expressed during infection and encodes an apparatus that assembles at the surface of the cell, we tested whether the components of the T4SS apparatus are immunogenic and whether responses to these proteins can be used as serological indicators of infection with Brucella species. We have previously shown that mice infected with B. abortus 2308 produced an antibody response to the protein encoded by the virB12 gene (31). In this study, we used purified recombinant VirB1, VirB5, VirB11, and VirB12 to assess the antibody responses to these proteins in sera from mice experimentally infected with B. abortus, from goats experimentally infected with B. melitensis, and from cattle of known serological status for Brucella.
MATERIALS AND METHODS
Bacterial strains, media, and culture conditions.
Escherichia coli cultures were grown on Luria-Bertani (Difco, Becton Dickinson, Sparks, MD) plates or in Luria-Bertani broth at 37°C with or without antibiotics. Brucella abortus 2308, Brucella melitensis 16M, and isogenic mutant strains were cultured on tryptic soy agar (Difco, Becton Dickinson, Sparks, MD) or in tryptic soy broth at 37°C on a rotary shaker. Their characteristics are listed in Table 1. Bacterial inocula for infection of mice were cultured on tryptic soy agar plus 5% blood. All work with live Brucella species was performed at biosafety level 3.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Source or reference |
|---|---|---|
| Strains | ||
| E. coli BL21(DE3) | F′ ompT gal [dcm] [lon] hsdSB(rB− mB−) DE3 | 29 |
| Brucella abortus | ||
| 2308 | Bovine isolate | B. Deyoe |
| ADH4 | ΔvirB1-12::Km | 8 |
| Brucella melitensis | ||
| 16M | Wild type | 13 |
| BMΔasp24 | Δasp24 | 13 |
| BMΔvirB2 | ΔvirB2 (nonpolar) | 13 |
| BMΔcydBA | ΔcydBA | 12 |
| Plasmids | ||
| pGEX-4T-2 | Apr, N-terminal GST tag, lacIq | Pharmacia |
| pAV5.1 | VirB5 cloned into pGEX-4T-1 | 8 |
| pMO4 | VirB1 cloned into pGEX-4T-2 | 8 |
| pIVEX2.4bNdeI | Apr, T7 promoter, N-terminal six-His tag | Roche |
| pDS1 | VirB12 cloned into pIVEX2.4bNdeI | 31 |
| pT7-7Strep::VirB11 | VirB11 cloned into pT7-7Strep | 11 |
Infection of mice.
Female C57BL/6J and BALB/c ByJ mice were obtained from the Jackson Laboratory (Bar Harbor, ME) and used at the ages of 8 to 10 weeks. Mice were held in microisolator cages with sterile bedding and water and irradiated feed in a biosafety level 3 facility. For infection experiments, groups of 10 to 20 mice were inoculated intraperitoneally with 0.1 ml of phosphate-buffered saline (PBS) containing 5 × 105 CFU of B. abortus 2308 or an isogenic ΔvirB1-12 mutant (complete deletion of the virB locus). Ten age-matched mice were injected with PBS. For assaying specific antibody responses, blood samples were collected from the saphenous vein before infection (infected mice) and once a week for 10 weeks after infection for both infected and PBS-treated mice. All animal experiments were approved by the Texas A&M University Laboratory Animal Care and Use Committee and were conducted in accordance with institutional guidelines.
Goat serum samples.
Twelve serum samples from experimentally infected goats were used in this study. Animals were infected, and sera were collected, as described previously by us (12). Briefly, female Angora goats averaging 110 days of gestation were infected at 1 × 107 CFU via bilateral conjunctival inoculation, and sera were collected 8 weeks after infection. Goats (three per group) were infected with B. melitensis 16M or the BMΔasp24, BMΔvirB2 (nonpolar), or BMΔcydBA isogenic mutant. Serum samples from 12 naïve goats were also used.
Bovine serum samples.
One hundred forty-five serum samples of known serology (as determined by the brucellosis card test and an enzyme-linked immunosorbent assay [ELISA] with whole Brucella antigen) were used in this study. One hundred three seropositive samples were from the investigators' specimen collection. The 42 known negative samples were obtained from male calves housed at a dairy calf-rearing operation in Texas, since male dairy calves are not vaccinated against brucellosis.
Purification of GST-VirB1 and GST-VirB5.
E. coli BL21 carrying pMO4 (glutathione S-transferase [GST]-VirB1) or E. coli BL21 carrying pAV5.1 (GST-VirB5) was grown in 20 ml of LB containing 100 μg/liter of carbenicillin (LB plus Carb) at 37°C. Expression of GST-VirB1 or GST-VirB5 was induced with 1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 37°C. Bacteria were resuspended in 1 ml of PBS buffer containing 2% Tween 20 and protease inhibitor cocktail (Sigma). Cells were lysed by sonication and centrifuged, and the supernatant was mixed with 2 ml of glutathione Sepharose 4B (Amersham Bioscience, Piscataway, NJ) beads and incubated for 1 h at 4°C. The slurry was transferred to a column and washed three times with cold PBS. The protein was eluted by adding 1 ml of elution buffer (0.031 g reduced glutathione in 10 ml of 10 mM Tris-HCl [pH 8.0]). GST-VirB1 and GST-VirB5 were concentrated using Amicon Ultra-15 (Millipore) concentration tubes.
Removal of the GST tag from VirB1 and VirB5.
To avoid measuring serological responses to the GST moiety of GST fusion proteins, the GST tag was removed from GST-VirB1 and GST-VirB5 by incubation with thrombin at room temperature for 2 h. The reaction mixture was mixed with 1 ml of glutathione Sepharose 4B (Amersham Bioscience, Piscataway, NJ) beads and incubated for 1 h at 4°C. The slurry was transferred to a column and washed once with cold PBS (the wash contained the protein of interest). VirB1 and VirB5 were concentrated using Amicon Ultra-15 (Millipore) concentration tubes and were dialyzed twice against PBS (pH 7.4).
Purification of recombinant VirB11.
Strep-VirB11 was purified according to the manufacturer's (IBA, Germany) protocol. Briefly, E. coli BL21 carrying pT7-7Strep::virB11 was grown for 16 h in 2 liters of LB plus Carb at 37°C. On the following day, expression of Strep-VirB11 was induced with 1 mM IPTG for 16 h at room temperature. Bacteria were resuspended in 40 ml of washing buffer with 0.5 mM phenylmethylsulfonyl fluoride. Bacteria were lysed with a French press and pelleted twice at 11,000 rpm, and the supernatant was transferred to a 5-ml column of streptactin beads (IBA) that was equilibrated with 2 column volumes (CV) of buffer W (100 mM Tris-HCl [pH 8], 150 mM NaCl, 1 mM EDTA). The beads were washed twice with 5 CV of buffer W, and the protein was eluted with 6× 0.5 CV buffer E (buffer W plus 2.5 mM desthiobiotin). Strep-VirB11 was concentrated using Amicon Ultra-15 (Millipore) concentration tubes and was dialyzed twice against PBS (pH 7.4).
Purification of recombinant VirB12.
E. coli BL21 carrying pDS1 (His6-VirB12) was grown in 20 ml of LB plus Carb at 37°C. Expression of His6-VirB12 was induced with 1 mM IPTG at 37°C. VirB12 was purified under denaturing conditions using reagents from Qiagen (Valencia, CA). Briefly, after induction, cells were lysed with lysis buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea [pH 8.0]), passed through a column, washed with wash buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea [pH 6.3]), and eluted with elution buffer (100 mM NaH2PO4, 10 mM Tris-HCl, 8 M urea [pH 4.5]). VirB12 was concentrated using Amicon Ultra-15 (Millipore) concentration tubes and was dialyzed twice against PBS, pH 7.4.
Detection of VirB12-specific IgG in serum samples.
The presence of antibody specific for the recombinant B. abortus VirB12 protein in the sera of mice, goats, and cattle was determined by an indirect ELISA. Nickel-nitrilotriacetic acid HisSorb plates from Qiagen (Valencia, CA) were coated with 100 ng of His-VirB12 per well in carbonate buffer (pH 9.6), and plates were incubated at 4°C overnight. After a wash with PBS and 0.05% Tween 20 (PBS-T), mouse serum samples (1:100), goat serum samples (1:16,000), or bovine serum samples (1:16,000) were diluted in PBS-B for mouse samples (PBS, 0.05% Tween 20, and 2% bovine serum albumin) or PBS-G for goat and bovine samples (PBS, 0.05% Tween 20, and 2% gelatin) and were incubated at 37°C for 1 h. After a wash with PBS-T, the reactivity was measured using horseradish peroxidase-conjugated anti-mouse (1:1,000; BD Pharmingen), anti-goat (1:5,000; Jackson ImmunoResearch), or anti-bovine (1:5,000; Jackson ImmunoResearch) immunoglobulin G (IgG) by incubating the plates at 37°C for 1 h. The reaction was developed with Sigma Fast o-phenylenediamine dihydrochloride tablet sets. The resulting color was read at 450 nm with an ELISA microplate reader (Bio-Rad model 680). Data points are the averages of duplicate dilutions, and each measurement was performed twice.
Detection of VirB1-specific, VirB5-specific, and VirB11-specific IgG.
The presence of antibody specific for the recombinant proteins VirB1, VirB5, and VirB11 of Brucella abortus in the sera of mice, goats, and cattle was determined by ELISA. MaxiSorp plates from Qiagen (Valencia, CA) were coated with 100 ng of VirB1, VirB5, or VirB11 per well in carbonate buffer (pH 9.6), and plates were incubated at 4°C overnight. ELISA was performed as described above. Data points are the averages of duplicate dilutions, and each measurement was performed twice.
Detection of Brucella-specific IgG.
The presence of antibody specific for whole-cell antigen of B. abortus in the sera of mice, goats, and cattle was determined by ELISA. MaxiSorp plates from Qiagen (Valencia, CA) were coated with 100 μl formalin-killed whole B. abortus (1 μg/ml) in carbonate buffer (pH 9.6), and plates were incubated at 4°C overnight. Mouse serum samples were diluted 1:100, while goat and cattle samples were measured at a dilution of 1:16,000. ELISAs were performed as described above. Data points are the averages of duplicate dilutions, and each measurement was performed twice.
Brucellosis card test.
To check the serology status of the goat and cattle samples, the brucella card test (obtained from National Veterinary Service Laboratories, Ames, IA) was performed according to the manufacturers' recommendations.
Statistical analysis.
For determination of statistical significance between experimental groups and naïve groups, Student's t test was performed on the data. A P value of <0.05 was considered significant.
RESULTS
B. abortus-infected mice develop antibody responses specific for VirB12 but not for VirB1, VirB5, or VirB11.
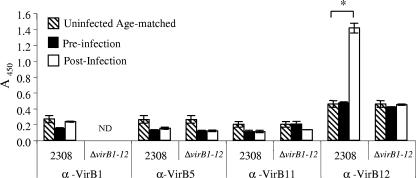
To determine whether VirB1, VirB5, VirB11, and VirB12 can serve as serological markers of infection with Brucella spp., we assayed the antibody responses of infected mice for IgG specific for VirB1, VirB5, VirB11, and VirB12. Serum samples were obtained from mice infected with either B. abortus 2308 or the ΔvirB1-12 mutant (a mutant strain carrying a complete deletion of the virB locus) or from age-matched uninfected controls. We have previously shown that for B. abortus-infected mice, the level of IgG specific for VirB12 increased over that for naïve mice starting at 3 weeks after infection (with the highest response at weeks 8 to 10), while that for mice infected with a ΔvirB12 mutant (in which only virB12 was deleted) did not differ from the preinoculation level (31). The results in Fig. 1 show that at week 9 after infection, levels of IgG-specific for VirB1, VirB5, and VirB11 in preinfection samples or samples from naïve age-matched mice did not differ from those for mice infected with either 2308 or the ΔvirB1-12 mutant. When mice were infected with the ΔvirB1-12 mutant, levels of VirB12-specific IgG did not differ from those in preinfection samples or age-matched naïve mice. VirB12-specific IgG levels did, however, increase in mice infected with B. abortus 2308 (wild type) over those in naïve mice, as previously described (31). These results indicate that VirB12 elicits an antibody response during infection of mice, while VirB1, VirB5, and VirB11 do not.
FIG. 1.
Detection of anti-VirB antibodies in sera from mice experimentally infected with wild-type Brucella abortus 2308 or its ΔvirB1-12 isogenic mutant. Ten to 20 mice were infected intraperitoneally with 2308 or a ΔvirB1-12 mutant. Blood was collected before infection and 9 weeks after infection. Ten age-matched mice were treated with PBS, and blood was collected 9 weeks later. Antibodies specific for VirB1, VirB5, VirB11, and VirB12 (α-VirB1, α-VirB5, α-VirB11, and α-VirB12) were detected by ELISA. Data are presented as the A450 of a 1:100 dilution of serum. Each bar indicates the average ± standard deviation of triplicate samples. ND, not determined. Asterisk indicates statistical significance, determined by Student's t test. A P value of <0.05 was considered significant.
VirB12, but not VirB1, VirB5, or VirB11, is immunogenic in goats.
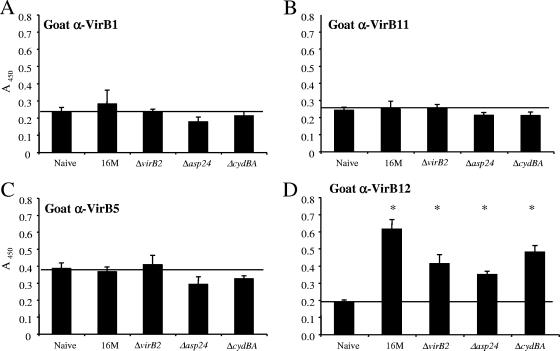
To determine whether VirB1, VirB5, VirB11, and VirB12 elicited antibody responses in a natural host for Brucella, we obtained sera from 12 goats experimentally infected with B. melitensis 16M or its isogenic mutant BMΔasp24, BMΔvirB2 (nonpolar), or BMΔcydBA (12) and sera from 12 naïve goats. The levels of IgG specific for VirB1 (Fig. 2A), VirB11 (Fig. 2B), or VirB5 (Fig. 2C) determined by ELISA did not differ between naïve goats and goats infected with either wild-type B. melitensis 16M or any of the mutant strains. In contrast, for all four groups of animals infected with B. melitensis strains, the levels of IgG-specific for VirB12 (Fig. 2D) increased significantly above those measured for naïve goats. These results indicate that VirB12 is expressed in vivo and generates a host response during infection of goats, while VirB1, VirB5, and VirB11 do not appear to elicit antibody responses.
FIG. 2.
Analysis of anti-VirB (α-VirB) antibodies in sera from 12 goats experimentally infected with wild-type B. melitensis 16M or one of its isogenic mutants BMΔvirB2, BMΔasp24, or BMΔcydBA and from 12 uninfected controls. Blood was collected 8 weeks after infection, and levels of IgG specific for VirB1, VirB5, VirB11, and VirB12 in serum were determined by ELISA. Data are presented as the A450 of a 1:16,000 dilution of serum. Each bar indicates the average ± standard deviation for three goats per infected group assayed in triplicate. The uninfected group contains 12 goats. The horizontal line in each graph represents the background reactivity of uninfected goats. Asterisks indicate statistically significant differences between groups of infected and naïve goats, determined by Student's t test. A P value of <0.05 was considered significant.
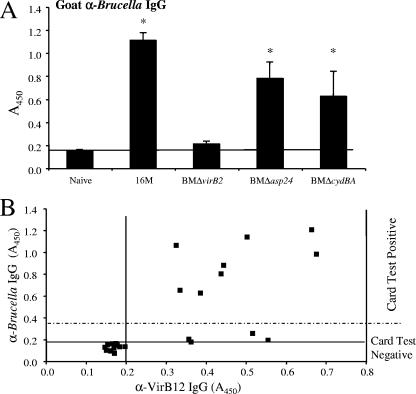
To correlate serological responses to VirB12 with responses to whole-cell antigen, we performed ELISA on the goat serum samples using whole-cell Brucella antigen (Fig. 3A). The serology response to Brucella IgG was confirmed with the brucellosis card test. Goats infected with B. melitensis ΔvirB2 (nonpolar) did not exhibit IgG responses to whole-cell Brucella antigen, while for goats infected with 16M or the Δasp24 mutant and for two of the goats infected with the ΔcydBA mutant, anti-Brucella IgG levels increased over those for naïve controls, in agreement with previous results reported by Kahl-McDonagh et al. (12).
FIG. 3.
(A) Brucella-specific (α-Brucella) IgG in sera from 12 goats experimentally infected with wild-type Brucella melitensis 16M or its isogenic mutant BMΔvirB2, BMΔasp24, or BMΔcydBA and from 12 uninfected controls. Blood was collected 8 weeks after infection, and levels of Brucella-specific IgG in serum samples were determined by ELISA and the brucellosis card test. Data are presented as the A450 of a 1:16,000 dilution of serum. Each bar indicates the average ± standard deviation for 3 goats per infected group or 12 goats per uninfected group, assayed in triplicate. Asterisks indicate that differences between groups of infected and naïve goats, determined by Student's t test, are statistically significant. A P value of <0.05 was considered significant. (B) Correlation between Brucella-specific IgG and VirB12-specific (α-VirB12) IgG in goats. Each dot represents both the level of Brucella-specific IgG and the level of Brucella-specific anti-VirB12 for one goat. Horizontal and vertical lines represent the background reactivities for uninfected goats (A450, 0.2). Data points above the dashed line represent goats with positive card test results, while points below this line represent animals with negative card test results.
The correlation between levels of IgG specific for whole-cell Brucella antigen and VirB12-specific IgG is shown in Fig. 3B. Three goats infected with B. melitensis ΔvirB2 and one goat infected with B. melitensis ΔcydBA did not develop high levels of Brucella-specific IgG antibody but produced high levels of anti-VirB12 antibody. The remaining infected goats generated both a high Brucella-specific IgG response and a high VirB12-specific response. For all 12 infected goats, positive antibody responses to VirB12 were observed.
Cattle seropositive for Brucella also develop an immune response to VirB12.
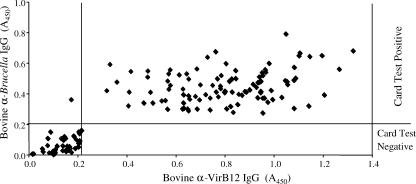
To explore the potential of VirB12 as a serological marker of infection in cattle, we measured VirB12-specific IgG by ELISA in 145 cattle samples of known B. abortus serology (Fig. 4). We obtained 42 seronegative and 103 seropositive samples. In serum samples from 102 cattle, IgG specific for VirB12 was detected, with a mean A450 of 0.792 ± 0.221. For the 43 cattle samples showing no response to VirB12, the mean A450 was 0.061 ± 0.046. The correlation between IgG responses specific for Brucella whole-cell antigen and for VirB12 is shown in Fig. 4. One hundred two samples (70.3%) were serologically positive for both Brucella and VirB12. Forty-two samples (29%) were serologically negative for both whole-cell antigen and VirB12. One animal (0.7%) showed a seropositive result for whole Brucella antigen, but no VirB12-specific IgG was detected. Overall, serological results for whole-cell antigen and VirB12 were concordant for 99% of samples.
FIG. 4.
Analysis of anti-VirB12 (α-VirB12) IgG and Brucella-specific (α-Brucella) IgG in sera from cattle infected with Brucella abortus. The lines represent the background reactivities for uninfected and unvaccinated cattle, as determined by ELISA and the brucellosis card test. Data are presented as the A450 of a 1:16,000 dilution of serum.
DISCUSSION
This study was undertaken to determine whether individual proteins encoded by the virB locus, which assemble to form a secretion apparatus at the surface of the bacterium, are immunogenic and whether serological responses to these proteins can be used for detection of brucellosis.
We purified recombinant VirB1, VirB5, VirB11, and VirB12 antigens and evaluated their potential use for the serological diagnosis of brucellosis by ELISA. Evidence of VirB12 expression (Fig. 1) was detected in mice infected with B. abortus: an IgG response specific for VirB12 was elicited after infection with B. abortus 2308 but not after infection with a mutant lacking the entire virB locus. The cell surface localization of VirB12 may contribute to its immunogenicity, as well as the fact that it is a predicted lipoprotein. VirB1, VirB5, and VirB11 also assemble in the membrane of the bacterium (16, 28), but we could not find evidence of specific antibody response against these proteins in the mouse or the goat model. One possible explanation for this result that we cannot exclude is that the recombinant VirB1, VirB5, and VirB11 antigens used for indirect ELISA were not in their native conformations, which would negatively impact the performance of our detection assay. However, a lack of immunogenicity of VirB1 and VirB5 could benefit Brucella during infection, since in the closely related bacterium Agrobacterium tumefaciens, these two proteins have been shown to be part of a pilus structure that protrudes from the bacterial surface and is therefore exposed to the immune system (15).
The BMΔasp24, BMΔvirB2, and BMΔcydBA mutants used in our study have been assessed as vaccine candidates in the goat and mouse models of infection (12, 13). Both the BMΔasp24 and BMΔvirB2 mutants have shown promise as safe vaccine candidates in mouse and goat models. BMΔasp24 was able to colonize the maternal tissues but not the fetal tissues and cause seroconversion in goats, while BMΔvirB2 was not capable of colonizing maternal or fetal tissues and did not cause seroconversion. The BMΔcydBA mutant was as virulent in the pregnant goat model as 16M (12).
Goats infected with B. melitensis 16M or the BMΔasp24, BMΔvirB2, or BMΔcydBA mutant elicited a response specific for VirB12, but not for VirB1, VirB5, or VirB11. BMΔvirB2 is a nonpolar mutation of VirB2; thus, VirB3 to VirB12 are expected to be expressed during infection (8). Goats infected with BMΔvirB2 did not seroconvert to whole-cell Brucella antigen (Fig. 3) (12) but exhibited a strong VirB12 response. One of the goals for the development of vaccines and diagnostic tools for brucellosis is a test that can distinguish between vaccinated and naturally infected animals. Our results show the potential of using VirB12 for such a goal, since BMΔvirB2 was found to be a safe vaccine with no seroconversion by using the brucellosis card test and whole-cell ELISA but elicited an elevated VirB12 response when an ELISA with recombinant purified VirB12 was used to assay infection.
A second goal of the present study was to assess the potential diagnostic utility of VirB12 in natural hosts of Brucella. We obtained 145 cattle serum samples of known serology (42 seronegative [29%] and 103 seropositive [71%]). Serology status was checked by both the brucellosis card test and whole-cell Brucella antigen ELISA.
As shown in Fig. 4, antibodies to VirB12 were detected in 70.3% (102 of 145) of bovine serum samples tested, while 29.7% of the samples did not give a response to VirB12. One of the 145 cattle samples was serologically positive for Brucella but negative for antibodies specific to VirB12. Serological tests for brucellosis may yield false-positive results for cattle vaccinated with B. abortus S19 or exposed to gram-negative bacteria with LPS O-chains similar to those of Brucella, such as Yersinia enterocolitica O:9. The cross-reactivity between Y. enterocolitica O:9 and Brucella is due to a strong similarity of the LPS O-chains (21, 33). We do not know if this animal showed serological reactivity to Brucella due to cross-reactivity with Y. enterocolitica O:9 or other bacteria, such as E. coli O157:H7, a frequent colonizer of cattle. Since we did not detect seroreactivity to VirB12, either this animal may have been infected and did not respond to VirB12 or it had a serological cross-reaction to whole cells elicited by bacteria other than Brucella. Based on the information at hand, we are unable to distinguish between these two possibilities.
In summary, this study shows that antibodies to B. abortus VirB12 can be identified in mice, goats, and cattle. One hundred percent of the experimentally infected mice and goats generated specific responses to VirB12, including those infected with mutants shown to be promising vaccine candidates (12, 13). These results encourage further investigation with patient samples to determine whether detection of humoral responses to VirB12-specific immune responses can be used for the diagnosis of human brucellosis.
Acknowledgments
We thank C. Baron for providing pT7-7Strep::VirB11.
This work was supported by PHS award U54 AI057156 to L.G.A., T.F., and R.M.T. and by PHS award AI050553 to R.M.T.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Araj, G. F. 1999. Human brucellosis: a classical infectious disease with persistent diagnostic challenges. Clin. Lab. Sci. 12:207-212. [PubMed] [Google Scholar]
- 2.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Lavigne, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. Type IV secretion and Brucella virulence. Vet. Microbiol. 90:341-348. [DOI] [PubMed] [Google Scholar]
- 3.Boschiroli, M. L., S. Ouahrani-Bettache, V. Foulongne, S. Michaux-Charachon, G. Bourg, A. Allardet-Servent, C. Cazevieille, J. P. Liautard, M. Ramuz, and D. O'Callaghan. 2002. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 99:1544-1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cassataro, J., K. Pasquevich, L. Bruno, J. C. Wallach, C. A. Fossati, and P. C. Baldi. 2004. Antibody reactivity to Omp31 from Brucella melitensis in human and animal infections by smooth and rough brucellae. Clin. Diagn. Lab. Immunol. 11:111-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celli, J., C. de Chastellier, D. M. Franchini, J. Pizarro-Cerda, E. Moreno, and J. P. Gorvel. 2003. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 198:545-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comerci, D. J., M. J. Martinez-Lorenzo, R. Sieira, J. P. Gorvel, and R. A. Ugalde. 2001. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 3:159-168. [DOI] [PubMed] [Google Scholar]
- 7.Delrue, R. M., M. Martinez-Lorenzo, P. Lestrate, I. Danese, V. Bielarz, P. Mertens, X. De Bolle, A. Tibor, J. P. Gorvel, and J. J. Letesson. 2001. Identification of Brucella spp. genes involved in intracellular trafficking. Cell. Microbiol. 3:487-497. [DOI] [PubMed] [Google Scholar]
- 8.den Hartigh, A. B., Y. H. Sun, D. Sondervan, N. Heuvelmans, M. O. Reinders, T. A. Ficht, and R. M. Tsolis. 2004. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect. Immun. 72:5143-5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Felek, S., H. Huang, and Y. Rikihisa. 2003. Sequence and expression analysis of virB9 of the type IV secretion system of Ehrlichia canis strains in ticks, dogs, and cultured cells. Infect. Immun. 71:6063-6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hong, P. C., R. M. Tsolis, and T. A. Ficht. 2000. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 68:4102-4107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Höppner, C., A. Carle, D. Sivanesan, S. Hoeppner, and C. Baron. 2005. The putative lytic transglycosylase VirB1 from Brucella suis interacts with the type IV secretion system core components VirB8, VirB9 and VirB11. Microbiology 151:3469-3482. [DOI] [PubMed] [Google Scholar]
- 12.Kahl-McDonagh, M. M., P. H. Elzer, S. D. Hagius, J. V. Walker, Q. L. Perry, C. M. Seabury, A. B. den Hartigh, R. M. Tsolis, L. G. Adams, D. S. Davis, and T. A. Ficht. 2006. Evaluation of novel Brucella melitensis unmarked deletion mutants for safety and efficacy in the goat model of brucellosis. Vaccine 24:5169-5177. [DOI] [PubMed] [Google Scholar]
- 13.Kahl-McDonagh, M. M., and T. A. Ficht. 2006. Evaluation of protection afforded by Brucella abortus and Brucella melitensis unmarked deletion mutants exhibiting different rates of clearance in BALB/c mice. Infect. Immun. 74:4048-4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim, S., M. Watarai, Y. Kondo, J. Erdenebaatar, S. Makino, and T. Shirahata. 2003. Isolation and characterization of mini-Tn5Km2 insertion mutants of Brucella abortus deficient in internalization and intracellular growth in HeLa cells. Infect. Immun. 71:3020-3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai, E.-M., and C. I. Kado. 2000. The T-pilus of Agrobacterium tumefaciens. Trends Microbiol. 8:361-369. [DOI] [PubMed] [Google Scholar]
- 16.Lai, E. M., and C. I. Kado. 1998. Processed VirB2 is the major subunit of the promiscuous pilus of Agrobacterium tumefaciens. J. Bacteriol. 180:2711-2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Letesson, J. J., A. Tibor, G. van Eynde, V. Wansard, V. Weynants, P. Denoel, and E. Saman. 1997. Humoral immune responses of Brucella-infected cattle, sheep, and goats to eight purified recombinant Brucella proteins in an indirect enzyme-linked immunosorbent assay. Clin. Diagn. Lab. Immunol. 4:556-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Limet, J. N., A. Cloeckaert, G. Bezard, J. Van Broeck, and G. Dubray. 1993. Antibody response to the 89-kDa outer membrane protein of Brucella in bovine brucellosis. J. Med. Microbiol. 39:403-407. [DOI] [PubMed] [Google Scholar]
- 19.Lindler, L. E., T. L. Hadfield, B. D. Tall, N. J. Snellings, F. A. Rubin, L. L. Van De Verg, D. Hoover, and R. L. Warren. 1996. Cloning of a Brucella melitensis group 3 antigen gene encoding Omp28, a protein recognized by the humoral immune response during human brucellosis. Infect. Immun. 64:2490-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopez, J. E., G. H. Palmer, K. A. Brayton, M. J. Dark, S. E. Leach, and W. C. Brown. 2007. Immunogenicity of Anaplasma marginale type IV secretion system proteins in a protective outer membrane vaccine. Infect. Immun. 75:2333-2342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mittal, K. R., I. R. Tizard, and D. A. Barnum. 1985. Serological cross-reactions between Brucella abortus and Yersinia enterocolitica O:9. Int. J. Zoonoses 12:219-227. [PubMed] [Google Scholar]
- 22.Nielsen, K., P. Smith, J. Widdison, D. Gall, L. Kelly, W. Kelly, and P. Nicoletti. 2004. Serological relationship between cattle exposed to Brucella abortus, Yersinia enterocolitica O:9 and Escherichia coli O157:H7. Vet. Microbiol. 100:25-30. [DOI] [PubMed] [Google Scholar]
- 23.O'Callaghan, D., C. Cazevieille, A. Allardet-Servent, M. L. Boschiroli, G. Bourg, V. Foulongne, P. Frutos, Y. Kulakov, and M. Ramuz. 1999. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 33:1210-1220. [DOI] [PubMed] [Google Scholar]
- 24.Pugh, G. W., Jr., L. B. Tabatabai, B. J. Bricker, J. E. Mayfield, M. Phillips, E. S. Zehr, and C. A. Belzer. 1990. Immunogenicity of Brucella-extracted and recombinant protein vaccines in CD-1 and BALB/c mice. Am. J. Vet. Res. 51:1413-1420. [PubMed] [Google Scholar]
- 25.Rolán, H. G., and R. M. Tsolis. 2007. Mice lacking components of adaptive immunity show increased Brucella abortus virB mutant colonization. Infect. Immun. 75:2965-2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rossetti, O. L., A. I. Arese, M. L. Boschiroli, and S. L. Cravero. 1996. Cloning of Brucella abortus gene and characterization of expressed 26-kilodalton periplasmic protein: potential use for diagnosis. J. Clin. Microbiol. 34:165-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roux, C. M., H. G. Rolán, R. L. Santos, P. D. Beremand, T. L. Thomas, L. G. Adams, and R. M. Tsolis. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell. Microbiol. 9:1851-1869. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Eisenlohr, H., N. Domke, C. Angerer, G. Wanner, P. C. Zambryski, and C. Baron. 1999. Vir proteins stabilize VirB5 and mediate its association with the T pilus of Agrobacterium tumefaciens. J. Bacteriol. 181:7485-7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Studier, F. W., and B. A. Moffatt. 1986. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J. Mol. Biol. 189:113-130. [DOI] [PubMed] [Google Scholar]
- 30.Sun, Y. H., A. B. den Hartigh, R. L. Santos, L. G. Adams, and R. M. Tsolis. 2002. virB-mediated survival of Brucella abortus in mice and macrophages is independent of a functional inducible nitric oxide synthase or NADPH oxidase in macrophages. Infect. Immun. 70:4826-4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun, Y. H., H. G. Rolán, A. B. den Hartigh, D. Sondervan, and R. M. Tsolis. 2005. Brucella abortus virB12 is expressed during infection but is not an essential component of the type IV secretion system. Infect. Immun. 73:6048-6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Watarai, M., S. Makino, and T. Shirahata. 2002. An essential virulence protein of Brucella abortus, VirB4, requires an intact nucleoside-triphosphate-binding domain. Microbiology 148:1439-1446. [DOI] [PubMed] [Google Scholar]
- 33.Weynants, V., A. Tibor, P. A. Denoel, C. Saegerman, J. Godfroid, P. Thiange, and J. J. Letesson. 1996. Infection of cattle with Yersinia enterocolitica O:9 a cause of the false positive serological reactions in bovine brucellosis diagnostic tests. Vet. Microbiol. 48:101-112. [DOI] [PubMed] [Google Scholar]
- 34.Young, E. 2000. Brucella species, p. 2386-2393. In G. Mandel, J. Bennett, and R. Dolin (ed.), Mandell, Douglas, and Bennett's principles and practice of infectious diseases, vol. 2. Churchill Livingstone, Philadelphia, PA. [Google Scholar]