Abstract
The first commercial indirect immunofluorescence assay (IFA) using Euroimmun Biochip technology was evaluated for the serodiagnosis of immunoglobulin G (IgG) and IgM antibodies against yellow fever virus (YFV) and was compared with the plaque reduction neutralization test (PRNT), which is currently the gold standard test for YFV. An overall correlation between the tests of 98.7% was established based on the analysis of 150 sera from individuals after vaccination with the 17D yellow fever vaccine. The sensitivity and specificity, calculated using the 150 sera from vaccinees and 150 sera from healthy blood donors, were 95% and 95%, respectively, for the IgG IFA and 94% and 97% for the IgM IFA. Antibody titers found in the PRNT correlated poorly with the IgM and IgG titers detected by IFA. The analysis of preexisting heterologous flaviviral immunity revealed the presence of antibodies reactive with YFV, tick-borne encephalitis virus, West Nile virus, Japanese encephalitis virus, and dengue virus serotypes 1 to 4 in 20 out of the 150 vaccinees. The indirect IFA showed that nine of these individuals with previous flaviviral exposure who received 17D vaccine failed to produce detectable IgM antibodies. Despite this preexisting immunity, all vaccinees developed protective immunity as detected by PRNT and anti-YFV IgG antibodies as detected by IFA. The high specificity and sensitivity of the IFA make it a useful tool for rapid diagnosis of yellow fever during outbreaks, for epidemiological studies, and for serosurveillance after vaccination.
Yellow fever (YF) is one of the well-known diseases in the areas of Africa and South America where it is endemic. Even though the live attenuated 17D vaccine strain provides very efficient and long-lasting protection against the disease, missing vaccination coverage causes regular outbreaks with high numbers of cases and deaths (20). Because little attention is paid to this deadly disease, several cases of infections in unvaccinated travelers visiting areas of endemicity occurred, some of them with fatal outcome (1, 2). Additionally, in recent years serious side effects after YF vaccination became apparent, which require further thorough analysis (4, 9). So far, 17 cases of viscerotropic disease and 17 fatal cases have been identified, and most of them have been only poorly analyzed. Currently, no commercial serological diagnostic test exists for detection of anti-YF virus (anti-YFV) immunoglobulin M (IgM) or IgG. Tests commonly used are in-house assays such as enzyme-linked immunosorbent assays (ELISA), immunofluorescence assays (IFA), and plaque reduction neutralization tests (PRNT). For the analysis of the protective immune response after vaccination, the PRNT is currently the gold standard (7, 13, 16). Nevertheless, this test is laborious and takes several days, and it is not being performed in many diagnostic laboratories.
IgM and IgG antibody levels determined by IFA were evaluated as additional markers for the presence of antibodies in sera from individuals vaccinated against YFV. The results of the IFA were then compared with those obtained with the PRNT.
MATERIALS AND METHODS
Serum samples were collected during a randomized controlled vaccination study conducted by Berna Biotech, a vaccine manufacturer located in Switzerland. All details regarding the study design have been published previously (13). In brief, the study group comprised 72 men between 18 and 57 years (mean age, 35) and 78 women between 18 and 59 years (mean age, 38). Serum was taken from all vaccinees before vaccination on day 0 and at 28 days postvaccination. Seventy-two vaccinees received Flavimun manufactured by the Berna Biotech AG, 40 vaccinees received RKI-YFV from the Robert Koch Institute, and 38 vaccinees received Stamaril from Sanofi Pasteur. Serum samples were stored at −20°C until use. One hundred fifty blood donor sera from the blood collection facility in Luebeck, Germany, were used as negative controls. The IFA was also analyzed with 20 human sera with antinucleus antibodies and 60 human sera positive for anti-human immunodeficiency virus, anti-hepatitis B virus (anti-HBV), and anti-HCV for unspecific reactivities. For the indirect IFA two Biochips, one coated with YFV-infected cells and the other with noninfected cells, were fixed into the reaction fields of a microscope slide (Fig. 1). In contrast to conventional production methods, the cells were not applied directly to microscope slides but initially were applied to 0.15-mm-thick glass slides (18). After fixation and gamma irradiation, these were cut mechanically into millimeter-sized fragments (Biochips). The Biochips were then glued into the reaction fields of microscope slides using automated assembly equipment. The miniature size of the Biochip substrates means that the reaction fields of the slides can be supplemented with further Biochip substrates if desired. In this way, different antibodies can be determined in parallel and a patient antibody profile obtained with a single incubation.
FIG. 1.
Immunofluorescence slide with YFV-infected cells on the left Biochip and uninfected control cell on the right Biochip.
The Biochip slides were incubated using the Titerplane technique (18), which is performed as follows. Samples or labeled sera are applied to the reaction fields of a reagent tray. The slide is then placed into the recesses of the reagent tray, allowing all Biochips to come into contact with the drops and the reactions to commence simultaneously. With this technique the fluids are confined in a closed space, so there is no need to use a conventional humidity chamber. The position and height of the drops are exactly defined by the geometry of the system. In this way, many samples can be incubated next to each other simultaneously under identical conditions.
For IgG or IgM antibody detection, diluted patient samples are incubated separately. For IgM detection, samples are pretreated with an RF absorbent, which removes IgG antibodies and rheumatic factors from the serum. In the second incubation step, fluorescein isothiocyanate-conjugated anti-human IgG or IgM binds to the human antibody.
Fourteen preimmune sera reacted in the YFV IgG IFA with a titer of ≥1:100 and were therefore analyzed further for other flavivirus-specific antibodies. In brief, sera were diluted in dilution buffer at 1:10, 1:32, 1:100, 1:320, 1:1,000, 1:3,200, and 1:10,000 before being pipetted onto Biochip Mosaic slides coated with antigens from YFV strain 17D, tick-borne encephalitis virus (TBEV) strain K32, West Nile virus (WNV) strain NY, a Japanese encephalitis virus (JEV) strain, or all four dengue virus serotypes 1 to 4 (DENV 1 to 4) on separate reaction fields. After 30 min of incubation at 20°C, the slides were washed with washing buffer before the incubation with the fluorescein isothiocyanate-conjugated secondary detection antibody was started. Finally, after 30 min, the slides were washed again and covered by a cover slide before being analyzed using a fluorescence microscope at a wavelength of 488 nm. Titers of ≥1:100 for IgG and IgM were considered positive. The 50% YFV PRNT, validated according to ICH Q2 R1, was performed as described previously (13). Titers of ≥1:10 were considered positive. The sensitivity and specificity of YFV IFA (IgG and IgM) were calculated with the sera of the 150 vaccinees and the 150 healthy blood donors. The test system's specificity describes how often negative results were found in a group of patient samples considered to react negative. To calculate the specificity, the number of correctly found negative samples is divided by the sum that consists of the number of correctly found negative samples added to the number of false-positive samples. Analysis of the data was performed by Daso GmbH Basel Switzerland based on standard software for data of clinical trials (SAS version 8.2).
RESULTS
All vaccinees developed a strong and protective humoral immune response, defined as a PRNT titer of ≥1:10, on day 28 postvaccination (13) (Table 1). A higher neutralizing titer in the PRNT was found in the male group; this phenomenon was also seen for the IgM and IgG responses in the IFA, with geometric mean of 127 in males compared to 80 in the female group for IgM and 677 compared to 432 for the IgG titer. These finding are confirmed by the 95% confidence interval found in the gender groups.
TABLE 1.
Analysis of antibody titers in serum samples at day 28 postvaccination as detected by PRNT and IFA
| Sex (age range, yr) | Vaccine | No. of sera | Reciprocal titera by:
|
|||||
|---|---|---|---|---|---|---|---|---|
| PRNT
|
IFA
|
|||||||
| IgM
|
IgG
|
|||||||
| GMT | 95% CL | GMT | 95% CL | GMT | 95% CL | |||
| Male (18-57) | Flavimun | 31 | 917.8 | 585-1,439 | 128.9 | 91-182 | 526.1 | 315-880 |
| RKI-YFV | 21 | 824.8 | 478-1,425 | 123.3 | 81-187 | 632.8 | 339-1,182 | |
| Stamaril | 20 | 558.0 | 319-977 | 129.0 | 84-198 | 1,076 | 567-2,041 | |
| Total | 72 | 774.8 | 577-1,041 | 127.3 | 102-160 | 677.2 | 483-949 | |
| Female (18-59) | Flavimun | 41 | 634.0 | 450-894 | 81.3 | 54-122 | 254.9 | 149-435 |
| RKI-YFV | 19 | 718.7 | 434-1,191 | 73.9 | 41-134 | 456.3 | 208-1,001 | |
| Stamaril | 18 | 467.5 | 278-786 | 85.8 | 47-158 | 1,349 | 602-3,024 | |
| Total | 78 | 609.3 | 475-782 | 80.5 | 60-108 | 431.5 | 293-635 | |
| Both (18-59) | Flavimun | 72 | 743.5 | 564-980 | 99.2 | 76-130 | 348.3 | 240-506 |
| RKI-YFV | 40 | 772.6 | 533-1,119 | 96.7 | 67-140 | 541.8 | 328-895 | |
| Stamaril | 38 | 513.1 | 351-750 | 106.4 | 73-155 | 1,198 | 716-2,003 | |
| Total | 150 | 683.8 | 565-828 | 100.3 | 83-121 | 535.7 | 414-694 | |
GMT, geometric mean titer; CL, confidence limits.
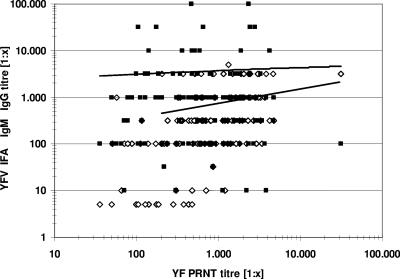
The overall comparison of the titer values as measured by PRNT and IFA showed only a very low comparability, as demonstrated in the graphical plot (Fig. 2). An overall correlation of 98.7% between the tests was established based on the analysis of 150 sera from individuals after vaccination with the 17D yellow fever vaccine. Titers for IgM were in general lower than titers for IgG (Table 2). Nearly all vaccinees (94.6%) developed a high IgG antibody response, while 90.7% developed a detectable IgM response. Of those eight vaccinees showing a negative IgG response in anti-YF IFA titers, six nevertheless showed a strong IgM immune response with titers of between 1:100 and >1:1,000, a finding that may be related to late seroconversion in these individuals (data not shown). Two IgG-negative vaccinees who showed extremely low (and therefore treated as negative) IgM titers of 1:10 and 1:32 developed at the same time protective PRNT titers of 1:305 and 1:871. Interestingly, seven of the eight IgG nonresponders or low responders were female. One male subject had a negative IgG response but developed a high IgM titer of 1:1,000.
FIG. 2.
Scatter plot comparing the results of the PRNT and IFA IgM (⋄) and IgG (▪) titers. The lines are based on a calculation of the logarithmic trends: upper line, IFA IgG titer versus PRNT titer; lower line, IFA IgM titer versus PRNT titer. Titers of 0 were treated as 1:5 to allow a logarithmic presentation.
TABLE 2.
Reactivity of vaccinee sera (n = 150) before and after vaccination with 17D
| Virus, method, and titer | Serum reactivity on day:
|
|||
|---|---|---|---|---|
| 0
|
28
|
|||
| No. (%) positive | Reciprocal titer range | No. (%) positive | Reciprocal titer range | |
| YFV | ||||
| PRNT | ||||
| <1:10 (negative) | 140 (93.3) | 0-10 | 0 | |
| ≥1:10 (positive) | 10 (6.7) | 17-50 | 150 (100.0) | 36-31,200 |
| IgG IFA | ||||
| <1:100 (negative) | 136 (90.7) | 0-32 | 8 (5.3) | 0-32 |
| ≥1:100 (positive) | 14 (9.3) | 100-3,200 | 142 (94.6) | 100-100,000 |
| IgM IFA | ||||
| <1:10 (negative) | NDc | 14 (9.3) | 0 | |
| ≥1:10 (positive) | ND | 136 (90.7) | 10->1,000 | |
| Flavivirusa IgG IFA, positive | 11 (7.3) | 32-3,200 | 0b | |
IgG IFA reactive against YFV, TBEV, WNV, JEV, or DENV 1 to 4.
Twenty representative sera negative against other flaviviruses on day 0 were tested on day 28 for reactivity against other flaviviruses.
ND, not done.
The analysis of the preimmune sera by IFA revealed the existence of anti-YFV antibodies or the presence of antibodies directed against some other flaviviruses (Table 3). The 10 preimmune sera with a detectable antibody titer in the YFV PRNT showed low titers of between 1:17 and 1:50; this finding could be confirmed in seven cases by YFV IgG IFA with titers of ≥1:100, while three cases were evaluated as negative in the IFA. These preexisting anti-YFV neutralizing antibodies were associated with a missing IgM immune response in 6 out of the 10 vaccinees. However, this did not influence the formation of high titers of neutralizing antibodies and/or IgG antibodies in these vaccinees. Even though the vaccinees were interviewed regarding former vaccination against other flaviviruses such as TBEV or JEV and travel history, five of them (no. 104, 111, 137, 099, and 157) had a dominant reactivity with TBEV, which might have been caused by undetected and unknown TBE infection or a forgotten vaccination against TBE. Three other vaccinees (no. 123, 016, and 017) showed an extremely high reactivity against several flaviviruses and predominantly against DENV 1 to 4, which was likely related to an undetected and unknown DENV infection from previous travel activities. The same might also be true for one vaccinee (no. 043) with a titer against JEV. In this group (no. 123, 016, and 017) with preexisting antibodies against DENV, no IgM immune response against YFV was detectable. In one vaccinee (no. 092) antibodies reactive only with YFV could be detected in preimmune sera by IFA, without any cross-reactivity to other flaviviruses. The reason for this reactivity remains unclear. In the samples from the healthy blood donors, specific IgG was found in 5% with IFA, and IgM was found in 3%. The sensitivity and specificity, calculated with the sera of the 150 vaccinees and 150 healthy blood donors, were 95% and 95%, respectively, for IgG and 94% and 97%, respectively, for IgM. No YFV-specific reactivity could be detected with the 20 antinucleus antisera and the 60 anti-human immunodeficiency virus, anti-HBV, and anti-HCV antisera (data not shown).
TABLE 3.
Analysis of vaccinee sera reactive against flaviviruses prior to and after vaccination with 17D
| Vaccinee no. | Sexa | Age (yr) | Reciprocal titer on day:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0b
|
28
|
||||||||||
| YFV PRNTc | IgG IFAd
|
YFV PRNT | IFA
|
||||||||
| Anti-YFV | Anti-TBEV | Anti-WNV | Anti-JEV | Anti-DENV (serotype[s]) | Anti-YFV IgM | Anti-YFV IgG | |||||
| 46 | F | 52 | 50 | 1,000 | Neg | 10 | 100 | 100 (2) | 100 | Neg | 3,200 |
| 141 | M | 38 | 50 | Neg | Neg | Neg | Neg | Neg | 310 | 100 | 1,000 |
| 94 | F | 38 | 48 | 320 | Neg | Neg | Neg | Neg | 128 | Neg | 3,200 |
| 85 | M | 42 | 34 | 100 | Neg | Neg | Neg | Neg | 138 | 100 | 3,200 |
| 79 | F | 53 | 33 | 100 | Neg | Neg | Neg | Neg | 190 | Neg | 1,000 |
| 70 | F | 56 | 26 | Neg | Neg | Neg | Neg | Neg | 4,275 | 320 | 10,000 |
| 98 | F | 49 | 19 | 100 | Neg | Neg | Neg | Neg | 78 | 100 | 1,000 |
| 135 | M | 21 | 20 | Neg | Neg | Neg | Neg | Neg | 186 | Neg | 3,200 |
| 44 | M | 40 | 19 | 100 | Neg | Neg | Neg | Neg | 142 | Neg | 10,000 |
| 15 | F | 25 | 17 | 100 | Neg | Neg | Neg | Neg | 280 | Neg | 3,200 |
| 92 | F | 30 | <10 | 100 | Neg | Neg | Neg | Neg | 768 | 100 | 100 |
| 43 | M | 33 | <10 | Neg | Neg | Neg | 100 | Neg | 2,804 | 1,000 | 32,000 |
| 123 | F | 21 | <10 | 320 | 10 | 32 | 100 | 1,000 (1-4) | 478 | Neg | 3,200 |
| 16 | M | 51 | <10 | 1,000 | 1,000 | 1,000 | 1,000 | 1,000 (1-4) | 106 | Neg | 32,000 |
| 17 | F | 29 | <10 | 3,200 | 1,000 | 1,000 | 3,200 | 3,200 (1-4) | 175 | Neg | 32,000 |
| 104 | F | 20 | <10 | 1,000 | 1,000 | 32 | 100 | 100 (1-4) | 465 | 320 | 100,000 |
| 111 | M | 35 | <10 | Neg | 1,000 | Neg | 10 | Neg | 2,432 | 1,000 | 1,000 |
| 137 | F | 26 | <10 | 100 | 1,000 | 10 | 32 | 100 (2) | 592 | 100 | 10,000 |
| 99 | F | 51 | <10 | 100 | 320 | 32 | 32 | 100 (1-3) | 563 | 100 | 3,200 |
| 157 | F | 21 | <10 | Neg | 320 | 10 | 32 | Neg | 827 | 100 | 3,200 |
F, female; M, male.
Day of vaccination. Boldface indicates sera with high reactivity against other flaviviruses in IFAs specific for antibodies against TBEV, WNV, JEV, and DENV 1 to 4.
<10, no neutralizing antibodies detectable at a dilution of 1:10 in PRNT.
Neg, no reactivity detectable in IFA at a dilution of 1:10.
DISCUSSION
Several methods for detection of antibodies against YFV have been developed, such as the hemagglutination inhibition test, PRNT, IFA, and ELISA (3, 10). Historically, the hemagglutination inhibition test was the first assay used to detect antibodies against YFV, followed by the neutralization assay (5, 14). So far, ELISA and IFA are performed only as in-house tests and might not always meet the high quality standards required today. In this study, we evaluated the ability of a commercial IFA to detect antibodies against YFV in a well-described group of YFV vaccinees before and after administration of the 17D vaccine. The sensitivity and specificity of the IFA were then compared with those of the PRNT. The IFA allows the easy detection of nonspecific reactions because of the use of uninfected cell controls on the same spot in the Biochip; this is an advantage for sera coming from regions where YF is endemic, which usually have a high background of nonspecific reactions. This allows the discrimination of antibodies which are directed against cell structures such as the cell nucleolus or mitochondria, which we also find in patients with rheumatic disorders. In malaria patients we often found a stimulation of a multireactive antibody response, causing a nonspecific reaction in serological assays. For the processing of low numbers of samples, which is usually the case in countries where the disease is not endemic, the commercial IFA would have an advantage over an ELISA because of its rigorous validation and the savings in time and money that would be needed to develop an in-house assay.
The IFA could be a useful tool for the diagnosis of YFV infection during outbreaks. For early diagnosis in the acute phase of infection, virus detection by cultivation and/or PCR is the most common technique (20, 2). However, as soon as specific antibodies are generated, 3 to 7 days after the onset of disease, IgM and/or IgG antibodies can be detected by serological assays such as ELISA, IFA, or immunoblotting. The chance of obtaining false-positive results due to antibodies cross-reacting with similar epitopes found in other flaviviruses such as Saint Louis encephalitis virus, DENV, WNV, or TBEV, resulting from natural infection or vaccination, is evident. To differentiate between a specific and a cross-reactive immune response, a fourfold increase of titer in two consecutive patient sera is mandatory to conclude that a result is positive with this assay. According to the WHO, a single IgM-positive test is considered a presumptive positive, which should be confirmed by assays such as RT-PCR and/or virus isolation, as well as PRNT, to discriminate potentially cross-reactive antibodies.
The indirect YFV IFA showed nearly the same sensitivity as the PRNT while being much faster to perform. Nevertheless, the differing titers in the PRNT and IFA show more of a trend than a correlation. This is in agreement with a previous finding comparing PRNT and “in-house” IFA results for WNV as another member of the flavivirus family (11, 12). The PRNT is also more a functional assay, measuring neutralization activities mediated by IgM and IgG antibodies, while the IFA detects all IgM or IgG antibodies reactive with the fixed YF antigen presented on the slides.
In cases without preexisting YFV immunity, the IFA for IgM and IgG antibodies provided clear diagnostic results. YFV vaccinees with preexisting heterologous flaviviral immunity due to previous exposure to other flavivirus antigens developed broadly cross-reactive IgG antibodies, as described by others (6). IgM antibodies were highly specific in cases of primary vaccination as well as in individuals with preexisting flavivirus antibodies but were detectable in only 11 out of 20 vaccinees with antiflavivirus antibodies (data not shown). In contrast to earlier findings where almost all individuals who received 17D vaccine without previous flaviviral exposure failed to develop antibodies detectable by the indirect IFA (10), we found a 94.6% IgG antibody response in the vaccinees. The reason for this contradiction remains unclear, but the use of a less-qualified in-house IFA seems most likely. It can be concluded that the detection of IgG antibodies by IFA is a good marker for the presence of an antibody response after YFV vaccination, but only in the absence of cross-reactive flavivirus antibodies or if a fourfold increase in titer can be demonstrated in consecutive sera. Therefore, a serious analysis of vaccination and travel history is a necessary prerequisite for good flavivirus serology. Although a neutralization assay is the only reliable assay for assessing protective immunity, the IFA can be used as a suitable test to analyze the antibody response after vaccination instead of performing a time-consuming PRNT. Assessing IgM and IgG specific humoral immunity via the IFA provides a feasible tool in addition to the PRNT as the gold standard in the diagnosis of YFV. In individuals with a history of previous flavivirus infections, it seemed that the YFV vaccination induced a booster reaction resulting in extreme rises of IgG titers (subjects 43, 44, 16, 17, 104, and 137) compared to the case for the flavivirus-naïve vaccinees, probably due to increased levels of flavivirus cross-reactive antibodies. This could be seen in a threefold higher mean for the IgG titer, 6,135, in this flavivirus-preimmune group compared to the total mean of 1,836. The IFA also confirmed the finding obtained by the PRNT of a gender-specific immune response. The reason for the stronger immune response in men than in women after YFV vaccination remains unclear even though the phenomenon is well known (15, 17).
The assay described here is the first commercial IFA using Biochip technology to detect anti-YFV IgM and IgG antibodies. The test is simple, has a rapid turnaround, and should prove especially useful for screening of numerous serum samples, such as would be required in on-site epidemiological investigations of YFV outbreaks.
Acknowledgments
We thank Petra Kreher, Susanne Kass, and Sabine Lederer for excellent technical work. We thank Sonja Basta, Rina Haase, and Pranav Patel for critical reading of the manuscript.
Footnotes
Published ahead of print on 28 November 2007.
REFERENCES
- 1.Bae, H. G., C. Drosten, P. Emmerich, R. Colebunders, P. Hantson, S. Pest, H. Schmitz, M. A. Warnat, and M. Niedrig. 2005. Analysis of two imported cases of yellow fever infection from Ivory Coast and The Gambia to Germany and Belgium. J. Clin. Virol. 33:274-280. [DOI] [PubMed] [Google Scholar]
- 2.Colebunders, R., J. L. Mariage, B. Coche, B. Pirenne, P. Honoré, S. Kempinaire, P. Hantson, A. Van Gompel, E. Bottieau, M. Niedrig, M. Van Esbroeck, M. Parent, R. Bailey, C. Drosten, and H. Schmitz. 2002. A Belgian traveller who acquired yellow fever in The Gambia. Clin. Infect. Dis. 35:e113-e116. [DOI] [PubMed] [Google Scholar]
- 3.Deubel, V., V. Mouly, J. J. Salaun, C. Adam, M. M. Diop, and J. P. Digoutte. 1983. Comparison of the enzyme-linked immunosorbent assay (ELISA) with standard tests used to detect yellow fever virus antibodies. Am. J. Trop. Med. Hyg. 32:565-568. [DOI] [PubMed] [Google Scholar]
- 4.Doblas, A., C. Domingo, H. G. Bae, C. L. Bohorquez, F. de Ory, M. Niedrig, D. Mora, F. J. Carrasco, and A. Tenorio. 2006. Yellow fever vaccine-associated viscerotropic disease and death. J. Clin. Virol. 36:156-158. [DOI] [PubMed] [Google Scholar]
- 5.Groot, H., and R. B. Riberiro. 1962. Neutralizing and haemagglutination-inhibiting antibodies to yellow fever 17 years after vaccination with 17D vaccine. Bull. W. H. O. 27:699-707. [PMC free article] [PubMed] [Google Scholar]
- 6.Holzmann, H., M. Kundi, K. Stiasny, J. Clement, P. McKenna, C. Kunz, and F. X. Heinz. 1996. Correlation between ELISA, hemagglutination inhibition, and neutralization tests after vaccination against tick-borne encephalitis. J. Med. Virol. 48:102-107. [DOI] [PubMed] [Google Scholar]
- 7.Lang, J., J. Zuckerman, P. Clarke, P. Barrett, C. Kirkpatrick, and C. Blondeau. 1999. Comparison of the immunogenicity and safety of two 17D yellow fever vaccines. Am. J. Trop. Med. Hyg. 60:1045-1050. [DOI] [PubMed] [Google Scholar]
- 8.Reference deleted.
- 9.Martin, M., T. F. Tsai, B. Cropp, G. J. Chang, D. A. Holmes, J. Tseng, W. Shieh, S. R. Zaki, I. Al-Sanouri, A. F. Cutrona, G. Ray, L. H. Weld, and M. S. Cetron. 2001. Fever and multisystem organ failure associated with 17D-204 yellow fever vaccination: a report of four cases. Lancet 358:98-104. [DOI] [PubMed] [Google Scholar]
- 10.Monath, T. P., C. B. Cropp, D. J. Muth, and C. H. Calisher. 1981. Indirect fluorescent antibody test for the diagnosis of yellow fever. Trans. R. Soc. Trop. Med. Hyg. 75:282-286. [DOI] [PubMed] [Google Scholar]
- 11.Niedrig, M., M. Lademann, P. Emmerich, and M. Lafrenz. 1999. Assessment of IgG antibodies directed against yellow fever virus after vaccination with 17D by different assays: neutralization test, haemagglutination inhibition test, immunofluorescence assay and ELISA. Trop. Med. Int. Health 4:867-871. [DOI] [PubMed] [Google Scholar]
- 12.Niedrig, M., K. Sonnenberg, K. Steinhagen, and J. T. Paweska. 2007. Comparison of ELISA and immunoassays for measurement of IgG and IgM antibody to West Nile virus in human sera against virus neutralization. J. Virol. Methods 139:103-105. [DOI] [PubMed] [Google Scholar]
- 13.Pfister, M., O. Kürsteiner, H. Hilfiker, D. Favre, P. Durrer, A. Ennaji, J. L'Age-Stehr, A. Kaufhold, and C. Herzog. 2005. Immunogenicity and safety of BERNA-YF compared with two other 17D yellow fever vaccines in a phase 3 clinical trial. Am. J. Trop. Med. Hyg. 72:339-346. [PubMed] [Google Scholar]
- 14.Porterfield, J. S. 1954. The haemagglutination-inhibition test in the diagnosis of yellow fever in man. Trans. R. Soc. Trop. Med. Hyg. 48:261-266. [DOI] [PubMed] [Google Scholar]
- 15.Posma, E., H. Moes, M. J. Heineman, and M. M. Faas. 2004. The effect of testosterone on cytokine production in the specific and non-specific immune response. Am. J. Reprod. Immunol. 52:237-243. [DOI] [PubMed] [Google Scholar]
- 16.Reinhardt, B., R. Jaspert, M. Niedrig, C. Kostner, and J. L'age-Stehr. 1998. Analysis of yellow fever virus 17D viremia after immunization with 17D vaccine and follow-up of various immunological parameters. J. Med. Virol. 56:159-167. [DOI] [PubMed] [Google Scholar]
- 17.Shohat, T., M. S. Green, O. Nakar, A. Ballin, P. Duvdevani, A. Cohen, and M. Shohat. 2000. Gender differences in the reactogenicity of measles-mumps-rubella vaccine. Isr. Med. Assoc. J. 2:192-195. [PubMed] [Google Scholar]
- 18.Stöcker, W. 1985. Rationelle Histochemie mit einer neuen Mikroanalysemethode. Acta Histochem. (Jena) 31(Suppl.):269-281. [PubMed] [Google Scholar]
- 19.Reference deleted.
- 20.World Health Organization. 2006. Yellow fever situation in Africa and South America, 2005. Wkly. Epidem. Rec. 81:317-324. [PubMed] [Google Scholar]