Abstract
The channel-forming peptide NC-1130 was generated based on the amino acid sequence of the M2 segment of the spinal cord α-subunit of the glycine receptor and has been proposed as a therapeutic agent for anion channelopathies such as cystic fibrosis. Lysine adduction and amino acid substitutions at positions T19R and S22W of the peptide improved its performance as an ion channel. However, these modifications generated an altered self, potentially making this NC-1130 peptide immunogenic, which could preclude the repeated use of NC-1130 as a therapeutic agent. To measure the ability of NC-1130 to induce an immune response, it was administered nasally with or without cholera toxin (CT). The NC-1130 peptide, when given alone without adjuvant, induced very little peptide-specific immunity based on analyses of peptide-specific antibodies by enzyme-linked immunosorbent assay and enzyme-linked immunospot assay, induction of cytokine production, and delayed-type hypersensitivity (DTH) responses. The administration of NC-1130 with the mucosal adjuvant CT induced peptide-specific immunoglobulin G (IgG) antibodies and DTH responses and a Th2-dominant cytokine response. The coadministration of the strong mucosal adjuvant CT induced a systemic NC-1130-specific IgG response but not a mucosal peptide-specific antibody response. The lack of peptide-specific immunity and specifically mucosal immunity should allow repeated NC-1130 peptide applications to epithelial surfaces to correct anion channelopathies.
A 22-residue peptide (peptide NC-1130 [KKKKPARVGLLITTVLTMRTQW]) derived from the transmembrane (M2) segment of the spinal cord glycine receptor α1-subunit (M2GlyR) spontaneously forms anion channels across epithelial monolayers (21). These de novo anion channels have the potential to correct ion transport deficiencies. Ion transport deficiencies have been implicated in a number of human diseases, which include cystic fibrosis (CF) and Alzheimer's dementia. To optimize the performance of the M2GlyR-derived peptide as an ion channel, four lysine residues were added at the amino-terminal end; this improved water solubility, decreased aggregation, increased short-circuit current, and oriented the peptides properly within the cell membrane (26). Extensive biophysical, physiological, and chemical analyses have been performed to characterize this and related peptides for their abilities to bind to and insert across phospholipid bilayers, undergo supramolecular assembly, and display de novo anion transport capabilities (1-5, 10, 16, 18, 31). Two amino acid substitutions at positions T19R and S22W within the transmembrane segment that further increased anion transport and reduced the peptide concentration required for optimal ion transport rates compared with the wild-type sequence were defined. In order for these self-derived peptides to function as therapeutic agents to correct ion channel deficiencies, it would require repeated applications over an extended period of time. If these altered-self peptides would induce an immune response, it would reduce its usefulness for correcting these deficiencies. No information on the ability of peptide NC-1130 to induce an immune response is available. In experiments performed previously, we have made unpublished observations with regard to antibody and delayed-type hypersensitivity (DTH) responses to this peptide when contaminated with lipopolysaccharide (LPS). The LPS-rich peptide preparation that we used, when administered nasally with cholera toxin (CT) as a mucosal adjuvant, induced considerable systemic peptide-specific immune responses (our unpublished observations). Since a considerable amount of LPS (152 endotoxin units/ml in a 1.0-mg/ml peptide solution) was detected in this initial peptide preparation, an LPS-free peptide was synthesized to exclude contributions of LPS to the induction of these immune responses to NC-1130. LPS functions as a mucosal adjuvant, enhances Th1-mediated immune responses, and can potentially enhance peptide-specific immune responses (7, 14, 17). Other mucosal adjuvants, such as CT, differentially induce Th2 (9, 11, 15, 22) responses. To determine the ability of the channel-forming peptide (CFP) NC-1130 to induce immunity following nasal application and separate the contribution of the peptide as an antigen from the adjuvant effect of LPS, we measured the ability of the LPS-free peptide to induce peptide-specific immunity in the host after repeated nasal applications. We hypothesized that this peptide will be unable to generate a significant immune response to the altered-self peptide NC-1130 due to a lack of T-helper and/or B-cell epitopes. By including a strong mucosal adjuvant in the NC-1130 immunization protocol, we will create a scenario that will be optimal for the induction of immune responses to this peptide and would represent a worst-case scenario.
MATERIALS AND METHODS
Mice.
Specific-pathogen-free female C57BL/6 mice were purchased from Harlan (Indianapolis, IN) at 5 to 6 weeks of age and were maintained at the Auburn University School of Veterinary Medicine animal facility. The mice were kept under pathogen-free conditions in microisolators and were fed sterile food and water ad libitum. The mice were screened for pathogens on a regular basis, and none were detected. The mice were used between 8 and 12 weeks of age. The 22-residue peptide NC-1130 derived from the transmembrane (M2) segment of the spinal cord glycine receptor α1-subunit (M2GlyR) used in these studies is 100% homologous between mice and men. All animal protocols were approved by the Institutional Animal Care and Use Committee of Auburn University.
Nasal immunization protocol.
The all-l stereoisomer of the 22-mer peptide NC-1130 (KKKKPARVGLGITTVLTMRTQW) was used for nasal immunizations. This peptide was commercially synthesized under LPS-free conditions (Anaspec, San Jose, CA). The mice were nasally immunized with 5, 20, or 100 μg of peptide NC-1130 either with or without 1.0 μg of CT (List Biological Laboratories, Inc., Campbell, CA) as a mucosal adjuvant in a total volume of 10 μl given 5.0 μl per nare. The immunization was repeated twice 1 week apart. Nasal washes and plasma samples were collected 1 week after the last immunization, as previously described (30). LPS-rich preparations of 1.0 mg/ml peptide NC-1130 contained 152 endotoxin units/ml of LPS (Cambrex Bio Science Inc., Walkersville, MD). No LPS was detected in the LPS-free NC-1130 preparation.
Antibody and cytokine ELISA.
Antibody levels in plasma were measured by enzyme-linked immunosorbent assay (ELISA) using streptavidin-coated Xenopore plates. In order to detect CFP NC-1130-specific immune responses, Xenopore plates were coated with 100 μl biotinylated NC-1130 (3 μg/ml) blocked with 200 μl of phosphate-buffered saline (PBS)-1% bovine serum albumin-Tween 20 (0.05%) (PBS-T) for 1.0 h at room temperature. Plasma or nasal washes were serially diluted twofold in PBS-T to determine endpoint titers. Endpoint titers were defined as the highest dilution whose optical density reading at 415 nm was at least 0.100 above background. The peptide-specific antibodies were detected by using goat anti-mouse immunoglobulin G (IgG), IgA, and IgM conjugated to horseradish peroxidase (HRP) (Southern Biotechnology Associates, Inc., Birmingham, AL) at a concentration of 0.5 μg/ml. Between each step, the plates were washed five times with PBS containing Tween 20. The final washes before the color development were done in PBS alone. Color development was performed at room temperature using the peroxidase substrate and was measured by determining the absorbance at 415 nm using Powerwave XS (BioTek Instruments, Inc., Winooski, VT).
Antibody enzyme-linked immunospot (ELISPOT) assays.
Lymphocytes were isolated from the spleen and cervical lymph nodes (CLN) 6 days after the last immunization as previously described (6, 29), and antigen-specific antibody-secreting cells were measured using nitrocellulose-backed 96-well microtiter plates (Millipore Corp., Bedford, MA) according to similar approaches described previously (28). The plates were coated with 3 μg/ml of peptide NC-1130 overnight at 4°C and blocked with complete RPMI 1640-based culture medium for 1.0 h at 37°C. The lymphocytes were loaded at 5 × 106, 5 × 105, and 5 × 104 cells/ml and incubated for 12 h at 37°C in 5% CO2. The plates were washed five times with PBS-Tween 20 and incubated with goat anti-mouse IgG, IgM, and IgA conjugated to HRP at 0.5 μg/ml (Southern Biotechnology Associates) at 4°C overnight. The plates were washed with PBS-Tween 20 (three times), followed by washing with PBS (three times), and developed with a peroxidase substrate, 3-amino-ethylcarbazole (Moss Inc., Pasadena, CA).
Biotin-conjugated monoclonal antibodies to murine IgG2a and IgG1 (Pharmingen, BD Biosciences, San Jose, CA) were used at 0.5 μg/ml overnight at 4°C, followed by a 1.0-h incubation with streptavidin-HRP (0.5 μg/ml) at room temperature and finally the addition of substrate to detect IgG2a- and IgG1-secreting plasma cells specific for the peptide. All steps of the ELISPOT assays were preceded by washing the plates as outlined above. The spots were enumerated using a dissection microscope (Fisher Scientific).
Cytokine ELISPOT assay.
The cytokine ELISPOT assay was performed as previously reported (27, 29). In short, spleen lymphocytes were isolated 3 days after the last immunization and loaded at various concentrations onto 96-well ELISPOT plates coated with capture antibodies for the murine cytokines interleukin-4 (IL-4), IL-6, IL-10, and gamma interferon (IFN-γ) (Pharmingen). Lymphocytes were loaded at 5 × 106, 5 × 105, and 5 × 104 cells/ml and incubated for 16 h at 37°C in 5% CO2. The cytokine-secreting cells were detected with biotinylated, anticytokine monoclonal antibodies followed by anti-biotin-HRP (0.5 μg/ml). The spots were developed using peroxidase substrate and enumerated as described above.
Proliferation assay.
Antigen-specific proliferation of splenic lymphocytes was accomplished by incubating 5 × 106 lymphocytes with 10 μg/ml CFP NC-1130 for 3 days in RPMI 1640 medium supplemented with 10% fetal calf serum and other nutrients as described previously (29). The cells were pulsed with 0.5 μCi [3H]thymidine (New England Nuclear) for 18 h and harvested and washed on 0.26-mm-thick glass fiber filters using a cell harvester (Inotech Biosystems International, Inc., Basel, Switzerland). The incorporated radioactivity was measured on a 1214 RackBeta liquid scintillation counter (LKB Wallac, San Francisco, CA) using 2.0 ml biodegradable Scintiverse (Fisher Scientific) scintillation fluid.
DTH.
To measure DTH responses, peptide-induced ear swelling was used as described previously (23). The mice were immunized as outlined above. Two to three weeks after the last immunization, a total of 15 μg NC-1130 was injected into the right ear in 10 μl sterile PBS, and 10 μl sterile PBS without peptide was injected into the left ear as a negative control in mice nasally immunized with the peptide. The ear swelling was measured 24 h after the injection using a thickness gauge (catalog no. 7326; Mitutoyo Corp., Japan). Peptide-specific ear swelling was determined by subtracting the swelling of the PBS-injected ear from that of the CFP NC-1130-injected ear.
RESULTS
Peptide-specific antibody responses.
C57BL/6 mice were immunized nasally with various amounts of peptide NC-1130, 5, 20, and 100 μg, with or without 1.0 μg CT as a mucosal adjuvant. The mice were given three immunizations 1 week apart. Controls for these experiments included naïve mice and CT-only controls.
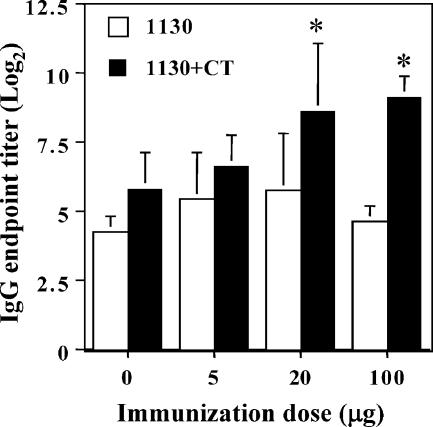
Antibodies were measured in plasma (Fig. 1) and nasal washes (data not shown) collected 1 week after the final immunization. Antibody levels were measured by ELISAs in plasma using streptavidin-Xenopore plates coated with biotinylated NC-1130 (3 μg/ml). No peptide-specific IgA antibodies were detectable in the plasma samples of any of the immunized mice, including those immunized with CT. Furthermore, no significant increase in the NC-1130-reactive IgM antibody titer was observed in any of the groups over that observed in their negative controls (data not shown). No increase in NC-1130-specific IgG antibodies was noted when mice were immunized with peptide alone, even when a high dose of peptide (100 μg) was used (Fig. 1). The only statistically significant NC-1130-specific IgG antibody titer increase over controls was observed when the higher doses of peptide were used (20 μg and 100 μg) in combination with CT. Thus, it can be concluded that peptide NC-1130, when administered repeatedly without adjuvant to a mucosal surface, i.e., the nasal tract, is unable to induce peptide-specific antibodies and required high concentrations of peptide with additional help from an adjuvant to induce IgG antibodies. Even under these conditions, no increase in IgM or IgA antibody levels in plasma was noted. Furthermore, NC-1130-specific antibodies of neither IgG, IgM, nor IgA isotypes were detected in the nasal washes (data not shown).
FIG. 1.
CFP NC-1130-specific IgG antibodies in murine plasma following nasal exposure. Mice were nasally exposed three times at weekly intervals with 5, 20, or 100 μg LPS-free peptide NC-1130 with either 1.0 μg CT (black bars) or no CT (white bars). The 0-μg peptide group involved naïve mice (white bars) or mice immunized three times with CT alone (black bars). Indicated are the means and 1 standard deviation of five mice per group. Statistically significant differences (P < 0.05) between the CT control and peptide plus CT groups are indicated by asterisks.
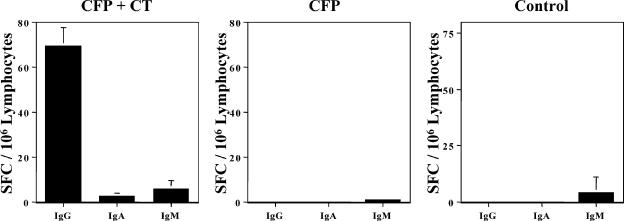
To assess the ability of NC-1130 after nasal application to induce NC-1130-specific antibody-secreting cells, CLN were isolated 6 days after the last application of NC-1130 and analyzed for the presence of IgG, IgA, and IgM antibody-secreting cells. When 100 μg of peptide was applied in conjunction with the mucosal adjuvant CT, an IgG response was observed, while no or a very limited number of IgA and IgM antibody-secreting cells was detected. However, no IgG spot-forming cells were observed in the CLN when NC-1130 was applied alone, and these mice displayed the same CFP-specific antibody-secreting cell profile as naïve controls (Fig. 2). These observations are consistent with the ELISA data and could indicate a lack of T-cell help to generate peptide-specific antibody-secreting cells, requiring a mucosal adjuvant to provide help.
FIG. 2.
CFP NC-1130-specific antibody-secreting cells in the CLN. Mice were nasally immunized weekly three times. CLN-derived lymphocytes from control mice, mice immunized with 100 μg NC-1130 alone, or mice immunized with CFP NC-1130 plus CT were analyzed by ELISPOT assay for numbers of IgG, IgA, and IgM antibody SFC 6 days after the last immunization. Indicated are the means plus 1 standard deviation of SFC per 106 lymphocytes.
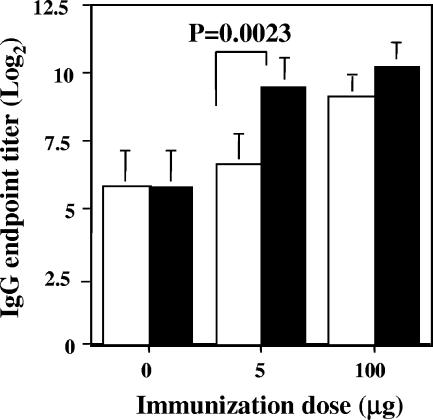
In order to determine any differences between LPS-free and LPS-rich peptide NC-1130 batches, a direct comparison was made for these peptide preparations to induce IgG antibodies. The LPS-rich peptide batch did not induce IgA or a significant increase in IgM peptide-specific antibodies over that seen in controls but did induce IgG antibodies when administered with CT, as is reported for the LPS-free peptides. The inductions of IgG NC-1130-specific antibodies by LPS-rich and LPS-free peptide when administered with and without CT were compared. As illustrated in Fig. 3, the presence of LPS enables the mice to generate a significantly higher IgG antibody response in mice immunized with the lowest dose of 5.0 μg peptide plus CT, representing the lowest therapeutic dose. Thus, the presence of LPS further enhances the peptide-specific humoral immune response, and therefore, it will be important to eliminate LPS from the preparations when these peptides are administered as therapeutic agents for anion channel deficiencies. When higher doses of peptide given with CT were used, the contribution of LPS becomes less important and no longer was significantly higher in the LPS-rich peptide batch. No humoral immune responses to the peptide were detected in nasal washes for either peptide preparation (data not shown).
FIG. 3.
Comparison of LPS-rich and LPS-free peptide NC-1130 preparations for their abilities to induce peptide NC-1130-specific IgG antibody responses. Mice were exposed nasally with the indicated peptide doses and 1.0 μg of CT. Both LPS-free (white bars) and LPS-rich (black bars) peptide preparations were used. The control groups are mice immunized with CT alone. Indicated are the means plus 1 standard deviation of five mice per group. Statistically significant differences are indicated in the figure.
Peptide-specific T-cell responses.
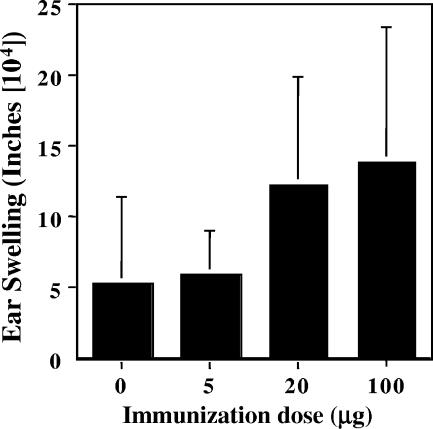
The DTH responses were measured as described above. The LPS-free peptide induced a limited DTH response, as is illustrated in Fig. 4, and the DTH response was not elevated significantly over that observed in the controls in any of the groups. Adding CT made the DTH response more variable. Nevertheless, in the low-dose immunization group (5 μg), even with the presence of CT during immunizations, no significant increase of the DTH response over that observed in control mice was observed (Fig. 4). Thus, immunization in the absence of LPS in the peptide preparations and during the ear injections induced low but not significantly elevated DTH responses to the peptide.
FIG. 4.
DTH responses to LPS-free peptide NC-1130-immunized mice. Ear swelling was measured 24 h after injecting 15 μg peptide in the right ear pinna and PBS in the left ear as a control. C57BL/6 mice were immunized with 0, 5, 20, or 100 μg peptide nasally three times prior to testing for DTH responses. Indicated are the observed mean ear swelling values plus 1 standard deviation for five mice per group. The ear swelling units are expressed in inches (104).
To further assess T-cell responses, LPS-free NC-1130 was tested in a proliferation assay measuring [3H]thymidine incorporation. Basically, lymphocytes isolated from spleens of immunized mice (100 μg peptide with or without CT) were analyzed for proliferative responses after in vitro incubation for 3 days with or without peptide NC-1130 followed by an 18-h [3H]thymidine (0.5 μCi/well) pulse. Incubation of peptide concentrations higher than 20 μg/ml caused a significant decrease in proliferation compared to that of unstimulated controls. Thus, all in vitro stimulations were performed at concentrations of 10 μg/ml of peptide NC-1130. Two groups of mice were tested. The rate of in vitro proliferation of lymphocytes for the unstimulated controls was 4,936 ± 1,790 cpm, while the rates for mice stimulated in vitro with 10 μg/ml of NC-1130 were 5,061 ± 1,911 cpm for those treated with 100 μg peptide nasally three times (n = 5) and 12,446 ± 2,674 cpm for those treated with 100 μg peptide plus 1.0 μg CT nasally three times (n = 6). No increase in proliferative responses by the lymphocytes of NC-1130-immunized mice when exposed in vitro to peptide was observed. These data indicate that no memory T cells are responding to in vitro exposure to peptide NC-1130, or alternatively, no peptide-specific T cells are present in the spleen after repeated immunizations. Clearly, the presence of CT enhances background proliferative responses considerably. The inability to stimulate lymphocytes in vitro precludes the use of several in vitro approaches to further investigate this.
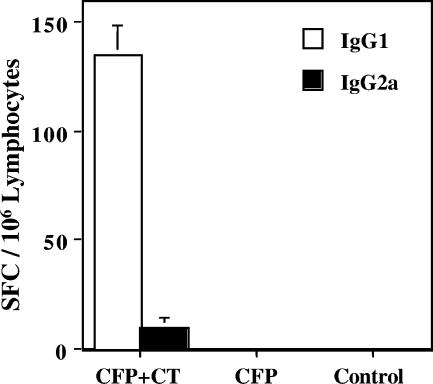
To assess the relative contribution of Th1 and Th2 helper activity for generating a NC-1130 peptide-specific antibody response, splenic lymphocytes were analyzed for IgG1 and IgG2a peptide-specific antibody-secreting cells 6 days after the last nasal application. As illustrated in Fig. 5, only IgG1 and limited IgG2a peptide-specific antibody-secreting cells were observed when the peptide was applied with the mucosal adjuvant CT. Th1 cells mediate their responses predominantly through IFN-γ, which preferentially induces IgG2a (12, 24), while Th2 cells mediate their responses through IL-4 to enhance IgG1 responses (13). The levels of IgG1 spot-forming cells (SFC) were significantly higher than those of IgG2a SFC, consistent with the Th2-dominant immune responses observed with CT. No antibody-secreting cells were detected in the control group or when peptides were administered without adjuvant, which is consistent with a lack of T-helper activity to generate antibody production.
FIG. 5.
Peptide NC-1130-specific IgG1 and IgG2a SFC in the spleen following nasal challenge. C57BL/6 mice were nasally immunized three times with 100 μg of peptide NC-1130 with 1.0 μg of CT. Six days after the last immunization, lymphocytes were isolated from the spleen and analyzed for peptide NC-1130-specific IgG1 and IgG2a antibody-secreting cells. Indicated are the means plus 1 standard deviation.
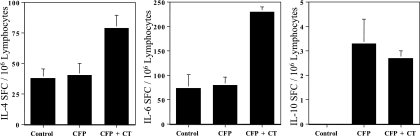
To measure the ability of CFP NC-1130 to activate cytokine production in T cells, we performed a cytokine ELISPOT assay and measured IFN-γ, IL-4, IL-6, and IL-10 SFC in the spleens of mice nasally immunized with 100 μg peptide with or without the presence of the adjuvant CT. The last of a total of three immunizations was performed without CT as adjuvant in order to prevent measuring CT-specific immune responses. The numbers of IL-4 SFC in control mice were consistently somewhat higher than anticipated, which may be due, at least in part, to our recent change of mouse supplier. The numbers of IL-6 and IL-10 SFC in negative controls were as expected. Statistical analyses of the data indicated a significant increase of the Th2 cytokines IL-4 and IL-6 when NC-1130 was administered in the presence of CT compared to NC-1130 administered alone (P values were 0.032 and 0.0003, respectively). This CT-driven increase was not observed for IL-10 (P = 0.609) production (Fig. 6). Thus, although levels of IL-10-secreting cells were low, the NC-1130 group increased IL-10 secretion as much as the group of mice given NC-1130 plus CT and were both significantly elevated compared to the control group. The NC-1130 peptide-alone group did not significantly increase levels of IL-4- and IL-6-secreting cells compared to those of controls. No IFN-γ SFCs were detected in any of the groups (data not shown).
FIG. 6.
Cytokine-secreting cells in the spleen following nasal application of LPS-free peptide NC-1130. Mice were nasally immunized every week two times with 100 μg peptide NC-1130 with or without CT as a mucosal adjuvant. The last nasal application was performed with peptide alone to exclude the induction of cytokine-secreting cells by the adjuvant CT. Splenic lymphocytes were isolated 3 days after the last application of peptide and were analyzed for the Th2 cytokines IL-4, IL-6, and IL-10 and the Th1 cytokine IFN-γ. No IFN-γ-secreting cells were detected (data not shown). Indicated are the means plus 1 standard deviation.
DISCUSSION
LPS-free CFP NC-1130, the amino acid sequence of which was derived from the spinal cord transmembrane (M2) segment of the glycine receptor α1-subunit (M2GlyR), did not induce a significant immune response when applied nasally without the presence of a mucosal adjuvant. This conclusion is based on the inability of the 22-mer peptide NC-1130 to induce detectable IgA, IgM, or IgG antibodies in plasma or nasal washes. The induction of IgG, but not IgA or IgM antibodies, occurred only when mice were immunized in the presence of a strong adjuvant, i.e., CT. It is not clear whether antibodies in the systemic compartment, observed with NC-1130 applications with CT, would inhibit or prevent the intercalation of this CFP into epithelial layers and prevent its therapeutic effects, something which we hope to address in future studies. Furthermore, no proliferative responses by lymphocytes were observed in peptide-immunized mice following exposure to the peptide in vitro. This was true when the mice were immunized with peptide alone or with peptide and CT as an adjuvant. The LPS-free peptides do induce a weak DTH response, which increased with the dose of the immunizing peptide; however, the DTH responses were not significantly higher than those observed in naïve mice. This DTH response was absent in the group immunized with 5 μg NC-1130 peptide. When the LPS-containing NC-1130 peptide preparation was used to immunize the mice, a significant increase in the DTH response was observed. This increase was even observed in the group with the lowest NC-1130 peptide immunization, 5 μg. An increase in the DTH response of up to ninefold was observed in these studies (data not shown). Thus, adjuvants like CT and LPS enhanced the CFP immunogenicity. In the absence of LPS and CT, no significantly elevated DTH response was observed over those observed in naïve controls.
Besides the peptide-specific proliferative response and the DTH response, the cytokine ELISPOT assay also confirmed the limited ability of NC-1130 to stimulate the immune system. The observation that Th2 cytokine production, DTH responses, and peptide-specific antibody responses are elevated in the presence of CT indicated not only that these peptides possess B-cell epitopes to which the immune system can respond but also that without adjuvant, these peptide-specific B cells do not receive sufficient T-cell help to generate a robust antibody response. It could be hypothesized that the limiting factor in NC-1130-specific immunity is a lack of peptides presented in the context of major histocompatibility complex (MHC) class II to activate T-helper cells and provide help in generating an antibody response to NC-1130. Whether or not a similar lack of NC-1130 peptide-specific immunity would be observed in humans is supported by our findings using the Los Alamos Database, which includes an HLA binding motif scanner in the HIV Molecular Immunology Database (http://hiv-web.lanl.gov/content/immunology/motif_scan/). This scanner can analyze peptides or proteins for associations with MHC classes I and II. When NC-1130 was analyzed using this database, two peptide motifs of peptide NC-1130 could interact with MHC class II that contained an altered self, which includes the four adducted Lys residues and the Trp and Arg substitutions. The motif found in amino acids 1 through 9 from the amino terminus of NC-1130, containing the lysine adduction, could associate with HLA DQA1*0201/DPB1*0401, and the motif in the Arg substitution may associate with DRB1*0801 (peptide residues 15 to 19) or 1501 (peptide residues 10 to 19). These findings are consistent with the interpretation that in humans, as in mice, limited T-helper activity could be expected for peptide NC-1130.
In previous studies, single-amino-acid alterations of peptides led to changes in proliferation, cytotoxic responses, and cytokine profiles induced upon T-cell receptor (TCR)-peptide-MHC interactions. These altered-self peptides may display an altered avidity in the TCR-peptide-MHC complex. This can translate into a change in TCR signal and the resulting T-cell response (8, 19, 25). The fact that peptide NC-1130 did not induce a noticeable immune response when administered alone would argue that this mechanism may not play a major role in our system. Furthermore, this indicates that peptide NC-1130 is perceived mainly as self when administered alone and consequently induces no significant immune response. In order to overcome this lack of responsiveness to peptide NC-1130, induction of a self-reactive immune response would be required, as has been tried for prion proteins, the causative agent of transmissible spongiform encephalopathy (20). In the latter case, the use of an appropriate adjuvant enabled investigators to break immune tolerance to prion proteins. At first glance, the transmembrane M2 segment of brain M2GlyR seems to follow a similar scenario.
The central nervous system is considered to be a somewhat immune-privileged site, which, under normal conditions, cannot be reached by self-reactive T and B cells, which lacks lymphatic drainage, and which is deficient in antigen presentations (32). Upon the induction of a systemic immune response to the altered M2 peptide NC-1130 using CT as an adjuvant, no negative consequences attributable to autoimmune reactions in the central nervous system were observed in the mice. The mice behaved normally throughout the experiments, and no influence on the motility of the mice was observed following the induction of NC-1130 immunity.
It is beneficial from a therapeutic point of view that no mucosally secreted antibodies could be detected in the nasal washes even when peptide NC-1130 was administered in the presence of CT adjuvant. This indicates that the incorporation of NC-1130 peptides into cell membranes would not be inhibited by the induction of mucosal antibodies. In addition, it would argue that peptide NC-1130 induced different B-cell responses in the systemic versus mucosal compartment upon nasal administration with CT.
The lowest CFP dose (5 μg) used for nasal application in our study would constitute a therapeutic dose to induce de novo chloride transport in membranes. Thus, treatment with this peptide at low therapeutic doses may be possible without generating detrimental mucosal or systemic immune responses in the recipient, based on our observations in mice. This indicates that repeated administration of this therapeutic peptide to correct deficiencies in chloride transport, such as has been documented for CF, would be worthwhile to test. Our results for the usefulness of NC-1130 as a therapeutic agent are encouraging. However, additional studies will be required to better understand the implication for humans, even though the M2GlyR transmembrane sequence used to design peptide NC-1130 is the same for humans as it is for mouse.
Acknowledgments
We thank Chelsea Mathis for her technical assistance.
This research was supported by PHS 1R44 DK60321-01 and an Auburn College of Veterinary Medicine AH&DR grant.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Broughman, J. R., K. Mitchell, T. Iwamoto, B. D. Schultz, and J. M. Tomich. 2001. Amino-terminal modification of a channel-forming peptide increases capacity for epithelia anion secretion. Am. J. Physiol. 280:C451-C458. [DOI] [PubMed] [Google Scholar]
- 2.Broughman, J. R., L. P. Shank, T. Iwamoto, O. Prakash, B. D. Schultz, J. M. Tomich, and K. E. Mitchell. 2002. Structural implications of placing cationic residues at either the NH2- or COOH-terminus in a pore-forming synthetic peptide. J. Membr. Biol. 190:93-103. [DOI] [PubMed] [Google Scholar]
- 3.Broughman, J. R., L. P. Shank, W. Takeguchi, T. Iwamoto, K. E. Mitchell, B. D. Schultz, and J. M. Tomich. 2002. Distinct structural elements that direct solution aggregation and membrane assembly in the channel forming peptide M2GlyR. Biochemistry 41:7350-7358. [DOI] [PubMed] [Google Scholar]
- 4.Broughman, J. R., R. Brandt, C. Hastings, T. Iwamoto, J. M. Tomich, and B. D. Schultz. 2004. Anion channel-forming peptide modulates transepithelial electrical conductance and solute permeability. Am. J. Physiol. 286:C1312-C1323. [DOI] [PubMed] [Google Scholar]
- 5.Cook, G. A., O. Prakash, K. Zhang, L. P. Shank, A. Robbins, Y.-X. Gong, T. Iwamoto, B. D. Schultz, and J. M. Tomich. 2004. Activity and structural comparisons of solution associating and monomeric channel-forming peptides derived from the glycine receptor M2 segment. Biophys. J. 86:1424-1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czerkinsky, C. C., L.-A. Nilsson, H. Nygren, O. Ouchterlony, and A. Tarkowski. 1983. A solid-phase enzyme-linked immunospot (ELISPOT) assay for enumeration of specific antibody-secreting cells. J. Immunol. Methods 65:109-121. [DOI] [PubMed] [Google Scholar]
- 7.Eisenbarth, S. C., D. A. Piggott, J. W. Huleatt, I. Visintin, C. A. Herrick, and K. Bottomly. 2002. Lipopolysaccharide-enhanced, Toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J. Exp. Med. 196:1645-1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faith, A., C. A. Akdis, M. Akdis, A. Joss, D. Wymann, and K. Blaser. 1999. An altered peptide ligand specifically inhibits Th2 cytokine synthesis by abrogating TCR signaling. J. Immunol. 162:1836-1842. [PubMed] [Google Scholar]
- 9.Hodge, L. M., M. Marinaro, H. P. Jones, J. R. McGhee, H. Kiyono, and J. W. Simecka. 2001. Immunoglobulin A (IgA) responses and IgE-associated inflammation along the respiratory tract after mucosal but not systemic immunization. Infect. Immun. 69:2328-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnston, J. M., G. A. Cook, J. M. Tomich, and M. S. Samson. 2006. Conformation and environment of channel forming peptides: a simulation study. Biophys. J. 90:1855-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jones, H. P., L. M. Hodge, K. Fujihashi, H. Kiyono, J. R. McGhee, and J. W. Simecka. 2001. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J. Immunol. 167:4518-4526. [DOI] [PubMed] [Google Scholar]
- 12.Kaufmann, S. H. 1995. Immunity to intracellular microbial pathogens. Immunol. Today 16:338-342. [DOI] [PubMed] [Google Scholar]
- 13.Kopf, M., G. L. Gross, M. Bachmann, M. C. Lamers, H. Bluethmann, and G. Kohler. 1993. Disruption of the murine IL-4 gene blocks Th2 cytokine responses. Nature 362:245-248. [DOI] [PubMed] [Google Scholar]
- 14.Kuipers, H., D. Hijdra, V. C. De Vries, H. Hammad, J. B. Prins, A. J. Coyle, H. C. Hoogsteden, and B. N. Lambrecht. 2003. Lipopolysaccharide-induced suppression of airway Th2 responses does not require IL-12 production by dendritic cells. J. Immunol. 171:3645-3654. [DOI] [PubMed] [Google Scholar]
- 15.Lavelle, E. C., G. Grant, A. Pusztai, U. Pfuller, and D. T. O'Hagan. 2002. Mucosal immunogenicity of plant lectins in mice. Immunology 99:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mitchell, K. E., J. M. Tomich, T. Iwamoto, and L. C. Freeman. 2000. A synthetic peptide based on a glycine-gated chloride channel induces a novel chloride conductance in isolated epithelial cells. Biochim. Biophys. Acta 1466:47-60. [DOI] [PubMed] [Google Scholar]
- 17.Pulendran, B., P. Kumar, C. W. Cutler, M. Mohamadzadeh, T. Van Dyke, and J. Banchereau. 2001. Lipopolysaccharides from distinct pathogens induce different classes of immune responses in vivo. J. Immunol. 167:5067-5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy, L. G., T. Iwamoto, J. M. Tomich, and M. Montal. 1993. Synthetic peptides and four-helix bundle proteins as model systems for the pore-forming structure of channel proteins. III. Transmembrane segment M2 of the brain glycine receptor channel is a plausible candidate for the pore-lining structure. J. Biol. Chem. 268:14608-14615. [PubMed] [Google Scholar]
- 19.Rogers, P. R., and M. Croft. 1999. Peptide dose, affinity, and time of differentiation can contribute to the Th1/Th2 cytokine balance. J. Immunol. 163:1205-1213. [PubMed] [Google Scholar]
- 20.Rosset, M. B., C. Ballerini, S. Gregoire, P. Metharom, C. Carnaud, and P. Aucouturier. 2004. Breaking immune tolerance to the prion protein using prion protein peptides plus oligodeoxynucleotide-CpG in mice. J. Immunol. 172:5168-5174. [DOI] [PubMed] [Google Scholar]
- 21.Shank, L. P., J. R. Broughman, R. M. Brandt, A. S. Robbins, W. Takeguchi, G. A. Cook, L. Hahn, G. Radke, T. Iwamoto, B. D. Schultz, and J. M. Tomich. 2006. Redesigning channel-forming peptides: amino acid substitutions in channel-forming peptides that enhance rates of supramolecular assembly and raise ion transport activity. Biophys. J. 90:2138-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simecka, J. W., R. J. Jackson, H. Kiyono, and J. R. McGhee. 2000. Mucosally induced immunoglobulin E-associated inflammation in the respiratory tract. Infect. Immun. 68:672-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Staats, H. F., W. G. Nichols, and T. J. Palker. 1996. Mucosal immunity to HIV-1: systemic and vaginal antibody responses after intranasal immunization with the HIV-1 C4/V3 peptide T1SP10 MN(A). J. Immunol. 157:462-472. [PubMed] [Google Scholar]
- 24.Stevens, T. L., A. Bossie, V. M. Sanders, R. Fernandez-Botran, R. L. Coffman, T. R. Mosmann, and E. S. Vitetta. 1988. Regulation of antibody isotype secretion by subsets of antigen-specific helper T cells. Nature 334:255-258. [DOI] [PubMed] [Google Scholar]
- 25.Tao, X., C. Grant, S. Constant, and K. Bottomly. 1997. Induction of IL-4-producing CD4+ T cells by antigenic peptides altered for TCR binding. J. Immunol. 158:4237-4444. [PubMed] [Google Scholar]
- 26.Tomich, J. M., D. Wallace, K. Henderson, K. E. Mitchell, G. Radke, R. Brandt, C. A. Ambler, A. J. Scott, J. Grantham, and T. Iwamoto. 1998. Aqueous solubilization of transmembrane peptide sequences with retention of membrane insertion and function. Biophys. J. 74:256-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.VanCott, J. L., H. F. Staats, D. W. Pascual, M. Roberts, S. N. Chatfield, M. Yamamoto, M. Coste, P. B. Carter, H. Kiyono, and J. R. McGhee. 1996. Regulation of mucosal and systemic antibody responses by T helper cell subsets, macrophages, and derived cytokines following oral immunization with live recombinant Salmonella. J. Immunol. 156:1504-1514. [PubMed] [Google Scholar]
- 28.van Ginkel, F. W., C.-J. Liu, J. W. Simecka, J.-Y. Dong, T. Greenway, R. A. Frizzell, H. Kiyono, J. R. McGhee, and D. W. Pascual. 1995. Intratracheal gene delivery with adenoviral vector induces elevated systemic IgG and mucosal IgA antibodies to adenovirus and β-galactosidase. Hum. Gene Ther. 6:895-903. [DOI] [PubMed] [Google Scholar]
- 29.van Ginkel, F. W., J. R. McGhee, C.-J. Liu, J. W. Simecka, M. Yamamoto, R. A. Frizzell, E. J. Sorscher, H. Kiyono, and D. W. Pascual. 1997. Adenoviral gene delivery elicits distinct pulmonary-associated T helper cell responses to the vector and its transgene. J. Immunol. 159:685-693. [PubMed] [Google Scholar]
- 30.van Ginkel, F. W., R. J. Jackson, H. L. Vu, N. Yoshino, Y. Hagiwara, D. J. Metzger, K. Fujihashi, T. D. Connell, M. Martin, and J. R. McGhee. 2005. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace, D. P., J. M. Tomich, T. Iwamoto, K. Henderson, J. J. Grantham, and L. P. A. Sullivan. 1997. A synthetic peptide derived from the glycine-gated Cl-channel generates Cl- and fluid secretion by epithelial monolayers. Am. J. Physiol. 272:C1672-C1679. [DOI] [PubMed] [Google Scholar]
- 32.Wekerle, H. 2006. Breaking ignorance: the case of the brain. Curr. Top. Microbiol. Immunol. 305:25-50. [DOI] [PubMed] [Google Scholar]