Abstract
Mycobacterium avium subsp. paratuberculosis is a zoonotic pathogen whose association with Crohn's disease in humans is under scrutiny. The objective of this work was to investigate its association with other chronic diseases such as type 1 diabetes mellitus (T1DM), where the involvement of a persistent pathogen such as M. avium subsp. paratuberculosis could be the trigger. For this purpose, 59 diabetic patients and 59 healthy controls were investigated for the presence of antibodies against two recombinant proteins of M. avium subsp. paratuberculosis and the whole-cell lysate. Extremely significant humoral immune responses to recombinant heparin binding hemagglutinin and glycosyl transferase proteins and the whole-cell lysates of M. avium subsp. paratuberculosis bacilli were observed in T1DM patients and compared to those of healthy controls. Finding evidence of M. avium subsp. paratuberculosis involvement in T1DM is perhaps a novel finding that might serve as a foundation stone in establishing an infectious etiology for T1DM.
Immune-related disorders are frequently rampant in both developed and developing countries. It is speculated that such diseases probably reflect and connect to long-term effects of a change in lifestyle and thereby a reduced exposure to certain bacteria that have been inherently associated with human societies during most of mammalian evolution (3, 17). A very important group of bacteria among these organisms is saprophytic mycobacteria, which trigger regulatory immune cell populations (3, 17) such as cytokine-secreting and antigen-presenting cells. These immune cell populations are probably the deterrent to some autoimmune diseases such as type 1 (insulin-dependent) diabetes mellitus (T1DM).
T1DM constitutes interactions of polygenic traits with not-well-known environmental factors, and it is not known what triggers autoimmunity to self-antigens such as those expressed in the pancreatic islets of Langerhans cells (5, 11). Drinking of cow's milk in childhood is assumed to be a risk factor for the development of this disease (17). The role of mycobacterial proteins that cross-react with epitopes of human cell surface molecules has been explored (3, 5).
Human populations that lived hygienic lifestyles and therefore remained “sanitized” for decades might react aggressively to exposure to certain microbial communities such as Mycobacterium avium subsp. paratuberculosis. Due to the expansion of the dairy industry in developed countries as a result of modern animal breeding, the exposure of human populations to M. avium subsp. paratuberculosis has increased. Sardinia, Italy, is one such example, where intensive sheep farming is practiced and the sheep population constitutes more than four times the existing human population of this Mediterranean island.
M. avium subsp. paratuberculosis bacilli have been notoriously known to trigger molecular mimicry (15, 23). It has long been a belief that genetic susceptibilities, epitope homologies, and endemic bacterial load in the environment might support the case for an infectious trigger, such as M. avium subsp. paratuberculosis, to be a causative agent of T1DM in genetically susceptible individuals (3, 8, 11, 17). However, their direct association with T1DM has remained largely elusive. Recent attempts (21) have been directed at the demonstration of clinically significant loads of M. avium subsp. paratuberculosis DNA in the blood of diabetes patients. However, it is essential to unravel the interaction of M. avium subsp. paratuberculosis bacilli with the host immune system to know if they are directly involved in the disease process.
We attempted to test the association of M. avium subsp. paratuberculosis with T1DM in an endemic setting like Sardinia and demonstrate for the first time the presence of clinically significant humoral responses of T1DM patients to recombinant M. avium subsp. paratuberculosis antigens and whole-cell lysates.
MATERIALS AND METHODS
A total of 118 participants, comprised of 59 patients with T1DM and 59 healthy controls, were previously tested for the presence of the M. avium subsp. paratuberculosis-specific IS900 signature using total DNA extracted from peripheral blood mononuclear cells (21). Informed consents from patients, including other necessary clearances, were obtained before blood samples were drawn. Patient details are shown in Table 1.
TABLE 1.
Clinical characteristics and results of IS900 PCR testing for M. avium subsp. paratuberculosis in participantsa
| Group and patient | Sex | Age (yr) | Yr of diagnosis | Family history of diabetes (type) | Seropositivity for:
|
|||
|---|---|---|---|---|---|---|---|---|
| M. avium subsp. paratuberculosis lysate (>0.6) | M. avium subsp. paratuberculosis HBHA (>0.5) | M. avium subsp. paratuberculosis GSD (>0.4) | PCR LIZ/AV (294 bp) | |||||
| Diabetic | ||||||||
| 01 | M | 21 | 1999 | ++ | +++ | − | + | |
| 02 | F | 31 | 1996 | +++ | ++ | + | − | |
| 03 | F | 28 | 1985 | ++ | ++ | − | + | |
| 04 | M | 36 | 1982 | I | ++ | − | + | + |
| 05 | M | 41 | 1989 | II | + | − | − | − |
| 06 | F | 36 | 1997 | +++ | + | − | + | |
| 07 | M | 37 | 1996 | + | +++ | − | + | |
| 08 | M | 26 | 2005 | II | +++ | +++ | + | + |
| 09 | M | 30 | 1996 | I/II | + | ++ | − | + |
| 10 | F | 37 | 1998 | I | + | ++ | − | + |
| 11 | M | 35 | 1985 | I | ++ | +++ | − | + |
| 12 | F | 37 | 1970 | I | + | +++ | − | − |
| 13 | F | 27 | 1989 | I | +++ | +++ | + | − |
| 14 | M | 31 | 1979 | I | +++ | +++ | + | + |
| 15 | M | 40 | 2005 | I | + | − | − | − |
| 16 | M | 18 | 2003 | I | − | − | − | − |
| 17 | M | 38 | 1980 | + | − | − | + | |
| 18 | F | 37 | 2005 | I | + | − | − | + |
| 19 | F | 28 | + | + | − | + | ||
| 20 | M | 26 | 2002 | I | +++ | +++ | + | + |
| 21 | F | 35 | ++ | + | − | + | ||
| 22 | F | 40 | 1996 | II | ++ | − | − | + |
| 23 | F | 28 | 1997 | II | + | +++ | + | + |
| 24 | F | 34 | − | − | − | − | ||
| 25 | M | 41 | 1996 | I | + | ++ | + | + |
| 26 | F | 36 | 1994 | I | − | − | − | + |
| 27 | M | 38 | − | + | − | − | ||
| 28 | F | 39 | 2002 | I | ++ | − | − | − |
| 29 | F | 37 | 1988 | + | ++ | + | − | |
| 30 | F | 32 | 1989 | − | + | − | − | |
| 31 | M | 43 | 1996 | − | − | − | − | |
| 32 | F | 33 | 1994 | I | − | − | − | − |
| 33 | F | 33 | +++ | +++ | ++ | − | ||
| 34 | M | 38 | 2002 | − | − | − | − | |
| 35 | M | 33 | 1988 | − | − | − | − | |
| 36 | M | 32 | 1976 | − | − | − | + | |
| 37 | M | 26 | 1994 | II | − | − | − | + |
| 38 | F | 32 | 1998 | I | + | ++ | + | + |
| 39 | F | 38 | − | ++ | + | + | ||
| 40 | M | 34 | 1989 | − | + | + | + | |
| 41 | M | 22 | 1989 | I | + | + | + | + |
| 42 | M | 41 | 1978 | I | − | − | ++ | − |
| 43 | M | 94 | + | +++ | +++ | + | ||
| 44 | M | 36 | − | ++ | ++ | + | ||
| 45 | M | 27 | 2004 | I | − | +++ | +++ | + |
| 46 | M | 33 | 1995 | I | − | − | + | + |
| 47 FR | M | 28 | 2000 | I | − | + | +++ | + |
| 48 FR | F | 33 | 1983 | − | − | + | + | |
| 49 FR | F | 23 | 1984 | I | − | ++ | + | + |
| 50 FR | M | 44 | 1989 | − | − | − | + | |
| 51 FR | M | 43 | 1986 | II | − | ++ | ++ | + |
| 52 FR | M | 34 | + | +++ | +++ | + | ||
| 53 FR | F | 59 | 1979 | I | − | − | + | + |
| 54 FR | F | 39 | − | − | − | + | ||
| 55 FR | F | 42 | 1967 | I | − | − | − | + |
| 56 FR | F | 39 | 1981 | I | + | ++ | + | + |
| 57 FR | F | 47 | 1966 | II | − | − | − | + |
| 58 FR | M | 57 | 1995 | I | − | − | − | |
| 59 FR | M | 38 | 1973 | I | + | − | +++ | − |
| Control | ||||||||
| 01C | F | 33 | − | − | − | + | ||
| 02C | F | 43 | − | − | − | − | ||
| 03C | M | 25 | − | − | − | − | ||
| 04C | F | 50 | − | − | − | + | ||
| 05C | M | 30 | − | − | − | − | ||
| 06C | F | 29 | − | − | − | + | ||
| 07C | F | 36 | − | − | − | + | ||
| 08C | M | 57 | − | − | − | − | ||
| 09C | F | 67 | − | − | − | + | ||
| 10C | M | 45 | − | − | − | − | ||
| 11C | M | 45 | − | − | − | − | ||
| 12C | M | 53 | − | − | − | − | ||
| 13C | M | 37 | − | − | − | − | ||
| 14C | M | 33 | − | − | − | − | ||
| 15C | M | 63 | − | − | − | − | ||
| 16C | M | 63 | − | − | − | − | ||
| 17C | M | 45 | − | − | − | + | ||
| 18C | F | 60 | − | − | − | − | ||
| 19C | F | 43 | − | − | − | − | ||
| 20C | F | 34 | − | − | − | − | ||
| 21C | F | 25 | − | − | − | − | ||
| 22C | M | 57 | − | − | − | − | ||
| 23C | M | 45 | − | − | − | − | ||
| 24C | F | 26 | − | − | − | − | ||
| 25C | M | 45 | − | − | − | − | ||
| 26C | F | 41 | − | − | − | + | ||
| 27C | M | 37 | − | − | − | + | ||
| 28C | M | 48 | − | − | − | − | ||
| 29C | F | 57 | − | − | − | − | ||
| 30C | M | 31 | − | − | − | − | ||
| 31C | M | 37 | + | + | + | − | ||
| 32C | M | 39 | − | − | − | − | ||
| 33C | M | 28 | − | − | − | − | ||
| 34C | F | 35 | − | − | − | − | ||
| 35C | F | 21 | − | − | − | − | ||
| 36C | F | 21 | − | − | − | − | ||
| 37C | M | 45 | − | − | − | − | ||
| 38C | M | 39 | − | − | + | − | ||
| 39C | M | 46 | − | − | + | − | ||
| 40C | F | 19 | − | − | − | − | ||
| 41C | M | 35 | − | − | − | − | ||
| 42C | M | 49 | − | − | − | − | ||
| 43C | F | 25 | − | − | + | − | ||
| 44C | M | 42 | − | − | − | + | ||
| 45C | F | 61 | − | − | + | + | ||
| 46C | M | 31 | − | − | − | − | ||
| 47C | F | 29 | − | − | − | − | ||
| 48C | M | 53 | − | − | − | − | ||
| 49C | M | 25 | − | − | − | − | ||
| 50C | M | 23 | − | − | − | − | ||
| 51C | M | 28 | − | − | − | + | ||
| 52C | F | 35 | − | − | + | − | ||
| 53C | M | 21 | − | − | + | − | ||
| 54C | M | 28 | − | − | − | − | ||
| 55C | M | 29 | − | − | − | − | ||
| 56C | M | 23 | − | − | − | − | ||
| 57C | F | 42 | + | − | − | + | ||
| 58C | F | 36 | − | − | − | − | ||
| 59C | M | 33 | − | − | − | − | ||
M, male; F, female. Type 1 diabetes is indicated as I, and type II diabetes is indicated as II. ELISA arbitrary values depending on the reading values at OD405 are indicated as follows: for M. avium subsp. paratuberculosis lysate, + indicates a value of 0.6 to 0.8, ++ indicates a value of 0.8 to 1, and +++ indicates a value of >1; for the M. avium subsp. paratuberculosis GSD protein, + indicates a value of 0.4 to 0.6, ++ indicates a value of 0.6 to 0.8, and +++ indicates a value of >0.8; and for M. avium subsp. paratuberculosis HBHA protein, + indicates a value of 0.5 to 0.7, ++ indicates a value of 0.7 to 0.9, and +++ indicates a value of >0.9.
Briefly, blood from patients was centrifuged, and serum supernatants were used for enzyme-linked immunosorbent assay (ELISA); the remaining sera were aliquoted and stored frozen at −20°C for short-term storage (<6 months) and −80°C for long-term storage (>6 months).
M. avium subsp. paratuberculosis ATCC cells were subjected to disruption on ice by using Ultrasonic homogenizers (Bandelin Sonopuls). Six bursts of 1 min each were achieved at 50% power intensity, with a 5-min cooling period between each burst. The lysate was centrifuged at 12,000 × g for 20 min to remove unbroken cells and cellular debris. The supernatant was decanted and transferred into a fresh tube. The total protein concentration was determined by the spectrophotometric estimation of the optical density at 280 nm (OD280). Forward primer EcoRI-gsd-F (GCGCGAATTCATGACTGCGC CAGTGTTCTCG), containing the EcoRI restriction site (underlined sequence), and reverse primer HindIII-gsd-R (GCGCAAGCTTCTACGGTTCTGCGCTTCG), containing the HindIII restriction site (underlined sequence), were used to amplify the complete M. avium subsp. paratuberculosis gsd gene as previously described (15). Expression vector pMAL-c2 (New England Biolabs) and the PCR product were double digested with EcoRI and HindIII enzymes and purified with the Qiaquick PCR purification kit. The ligation of restricted fragments resulted in an “in-frame” fusion between the malE gene of the vector and the gsd gene. The construct was electroporated into Escherichia coli BL21(DE3) cells (Invitrogen Life Technologies), and positive clones were selected on LB agar plates supplemented with 100 μg/ml of ampicillin (Sigma). Recombinant colonies were confirmed by both EcoRI/HindIII restriction analysis and DNA sequencing. A culture of a selected clone grown overnight was used to inoculate 200 ml of rich broth (10 g/liter tryptone, 5 g/liter yeast extract, 5 g/liter NaCl) containing 100 μg/ml ampicillin and 2 g/liter glucose. Cells were grown at 37°C with shaking until they reached an OD600 of approximately 0.5. Protein expression was induced for 3 h by the addition of 0.3 mM isopropyl-β-d-thiogalactopyranoside (IPTG) (Sigma-Aldrich). Cells were harvested by centrifugation at 4,000 × g for 20 min, resuspended in 20 ml of column buffer (20 mM Tris-HCl, 200 mM NaCl, 1 mM EDTA), and frozen at −20°C. The next day, the sample was placed into an ice-water slurry and sonicated in short pulses of 15 s (for about 2 to 3 min), with 15 s of cooling between each sonication. The lysate was then clarified at 9,000 × g for 30 min, and the supernatant (crude extract) was decanted and diluted 1:5 with column buffer. Since the recombinant fusion protein contained the maltose binding protein (MBP) as the tag, glycosyl transferase (GSD)-MBP was purified from crude extracts by using an amylose resin (New England Biolabs) according to the manufacturer's protocol. Eluted fractions were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Coomassie brilliant blue (Sigma-Aldrich) staining to assess protein yield, purity, and size. The GSD-MBP fusion migrated at the expected molecular mass of 73 kDa. Fractions containing the purified GSD protein were pooled and concentrated by using Centricon centrifugal filter devices (Millipore). Purified protein from the control strain consisted of an MBP fusion of the LacZ alpha peptide.
Recombinant HBHA was previously cloned and expressed in our laboratory (19). High-quality, endotoxin-free purification of the protein of high homogeneity was carried out as described above.
Optimal working dilutions for antigens, serum samples, and conjugated secondary antibody were determined after checkerboard titration. ELISA was performed in 96-well microplates (Nunc-Immuno plate). Purified GSD-MBP, purified HBHA protein, MBP-LacZ control peptide, and M. avium subsp. paratuberculosis crude lysate were diluted in carbonate bicarbonate buffer (Sigma-Aldrich) at a final concentration of 5 μg/ml and used as antigens. Each well was coated with 50 μl of diluted antigen overnight at 4°C. The next day, the unabsorbed antigen was discarded, and wells were blocked with 5% nonfat dried milk (Sigma-Aldrich) at 37°C for 1 h. Plates were washed three times with 200 μl phosphate-buffered saline-Tween (PBS-T) (PBS-0.05% Tween 20) before 100 μl of diluted serum (1:100 in PBS-T) was added to each well.
After 2 h, plates were washed five times with PBS-T and incubated for 1 h with 100 μl of anti-human immunoglobulin G alkaline phosphatase antibody (Sigma-Aldrich) diluted 1:1,000 in PBS-T. Five rounds of washing were performed, and 200 μl of Sigma Fast p-nitrophenyl phosphatase substrate was added to each well. As the color developed, plates were read at 405 nm on a VERSA Tunable Max microplate reader (Molecular Devices).
Glucose levels in the blood of the type 1 diabetic patients ranged between 50 and 400 mg/dl. These variable levels did not affect the ELISA performed (confirmed by the fact that sera with low and high glucose levels correlated very well with positivity to IS900 PCR). Moreover, we tested two T1DM sera (one positive and one negative for M. avium subsp. paratuberculosis) and PBS as a control by ELISA against M. avium subsp. paratuberculosis HBHA protein at different concentrations of glucose (50, 100, 200, 300, and 400 mg/dl). Positivity was not affected by the glucose concentrations (data not shown).
RESULTS
Among the diabetic patients, a total of 29 blood samples out of 46 were previously found to be positive for M. avium subsp. paratuberculosis (63%), whereas only 8 out of the 50 healthy control samples (16%) generated a positive signal as previously reported (21). While a majority of M. avium subsp. paratuberculosis PCR-positive diabetics carried a family history of diabetes or other genetic/autoimmune disorders, 16 PCR-positive individuals with diabetes did not have any history of diabetes or other autoimmune diseases in their family (Table 1).
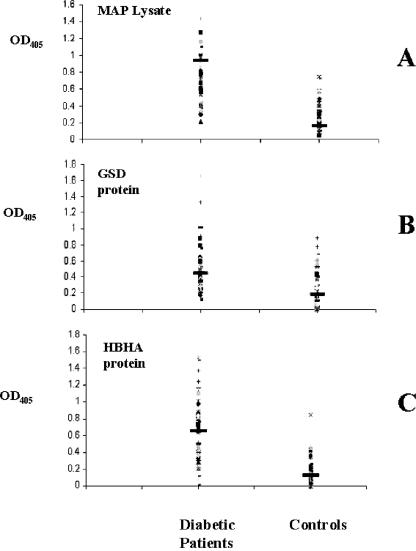
Cloning, expression, and purification of M. avium subsp. paratuberculosis antigens was achieved up to a very high standard and homogeneity. The purified protein fractions were used for ELISA. We observed humoral responses of the diabetics to HBHA, whole-cell lysates, and the M. avium subsp. paratuberculosis GSD protein as an unequivocal signature of the presence of M. avium subsp. paratuberculosis bacilli within these patients (Fig. 1). The HBHA antigen gave strong ELISA titers (cutoff titer value of 0.5) in 55.9% of the diabetic patients and only 1.6% of the controls (chi = 39.7; P < 0.0001). Also, the GSD protein revealed significant differences in ELISA titers of the diabetic (45.7% positivity) and control (11.8% positivity) individuals at the cutoff titer value of 0.4 (chi = 14.9; P < 0.01). The overall humoral responses to the whole-cell lysates of the M. avium subsp. paratuberculosis bacilli were also as significant and are supportive of the infectious evidence as the HBHA and GSD antigens that we analyzed. The lysates revealed significantly high titers in 32 of the 59 patients (54%) compared to controls (3.3%) at a cutoff titer of 0.5 (chi = 34.7; P < 0.0001).
FIG. 1.
Evaluation of serum samples from diabetic patients (left column) and healthy donors (right column) against M. avium subsp. paratuberculosis (MAP) lysate (A), GSD recombinant protein (B), and HBHA recombinant protein (C). Data are presented as values of the OD405 observed following ELISA, as described in the text. Data from a representative experiment out of three are shown. The median value for each group is indicated by a dark solid horizontal line.
The fact that two out of the three BCG-vaccinated diabetic patients were positive by all the three ELISAs may indicate that a cross-reaction among M. avium subsp. paratuberculosis antigens and BCG antigens may occur. Note that one of the patients was negative by IS900 PCR. None of the patients and controls suffered from inflammatory bowel diseases.
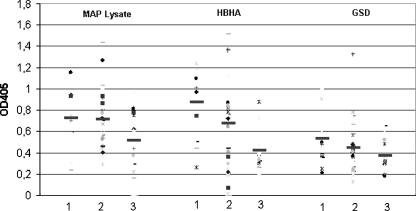
A correlation between the ages of the patients and the presence of antibodies against M. avium subsp. paratuberculosis was found (according to the Student t test), as shown in Table 2. In particular, a stronger antibody response against M. avium subsp. paratuberculosis lysate was found in the first group of T1DM patients (18 to 28 years of age) than in third group (39 to 59 years of age) (Student t = 2.168788; P = 0.039075) and in the second group of T1D patients (29 to 38 years of age) than in the third group (39 to 59 years) (Student's t = 2.373435; P = 0.022274). The same situation was observed for the two antigens tested (HBHA and GSD) (Fig. 2 and Table 2).
TABLE 2.
Correlation between ages of the patients and the presence of antibodies against M. avium subsp. paratuberculosis
| Protein and age group | Correlation for age (yr):
|
Student's t value | P value | ||
|---|---|---|---|---|---|
| 18-28 | 29-38 | 39-59 | |||
| M. avium subsp. paratuberculosis lysate | |||||
| 18-28 yr | 0.728586 | 0.71191 | 0.187714 | 0.852027 | |
| 29-38 yr | 0.728586 | 0.517673 | 2.168788 | 0.039075 | |
| 39-59 yr | 0.71191 | 0.517673 | 2.373435 | 0.022274 | |
| HBHA | |||||
| 18-28 yr | 0.879871 | 0.675797 | 1.463017 | 0.151087 | |
| 29-38 yr | 0.879871 | 0.419484 | 4.440892 | 0.000128 | |
| 39-59 yr | 0.675797 | 0.419484 | 2.101854 | 0.041461 | |
| GSD | |||||
| 18-28 yr | 0.53445 | 0.443817 | 0.886397 | 0.380574 | |
| 29-38 yr | 0.53445 | 0.371363 | 2.215312 | 0.035044 | |
| 39-59 yr | 0.443817 | 0.371363 | 0.800364 | 0.4279 | |
FIG. 2.
Values of T1DM serum antibody titers against the different M. avium subsp. paratuberculosis (MAP) antigens of patients divided by age: 18 to 28 years of age (group 1), 29 to 38 years of age (group 2), and 39 to 59 years of age (group 3). Data are presented as values of OD405 observed following ELISA, as described in the text. Data from a representative experiment out of three are shown. The median value for each group is indicated by a dark solid horizontal line.
A significant difference among the humoral antibody responses to specific M. avium subsp. paratuberculosis antigens and whole-cell lysates, as shown by diabetic patients and the nondiabetic controls (Fig. 1), might strongly signify the involvement of M. avium subsp. paratuberculosis in T1DM.
DISCUSSION
M. avium subsp. paratuberculosis is a pathogen with a broad host range, and it can persistently infect the intestinal tracts of many animals including primates (7, 18). It has been found to persist within the gut in a Ziehl-Neelsen-negative “cell wall-deficient” form (22). These forms can potentially be the source of inflammatory antigens in the host that may direct autoimmune responses. T1DM is thought to develop as a consequence of such autoimmune responses that lead to the destruction of insulin-producing beta cells of the pancreas (11). There has long been speculation on the involvement of an infectious trigger underlying such autoimmune responses; however, no concrete evidence for the same was presented (17). Genetic evidences point to the existence of immune dysfunctions that promote both T1DM and mycobacterial infection (11, 17). Susceptibility factors such as Nramp1 gene polymorphisms (10, 20) have also been linked to such diseases. Vitamin D deficiency has been implicated as being a risk factor for T1DM (3, 11). Interestingly, vitamin D is also implicated in limiting mycobacterial infections by upregulating the expression of an antimicrobial peptide (12). Such studies help link the two diseases, diabetes and Crohn's disease, where M. avium subsp. paratuberculosis could be the common agent, putatively behaving as an environmental trigger of autoimmunity. Our results do not rule out this possibility by demonstrating significant immune responses to M. avium subsp. paratuberculosis antigens. These observations therefore support the infectious trigger hypothesis described previously by Dow (5, 11), although it will certainly be important to dissect out the direct mechanism of the autoimmune responses mediated by the infectious triggers.
T1DM is characterized by elevated levels of T-helper 1 (Th1) responses targeted against several autoantigens including Hsp60, glutamic acid decarboxylase, and insulin. Given this, it becomes conceivable that some molecular mimicry has a role to play (4), especially for epitope homologies between the mycobacterial proteins like Hsp65 and the diabetes antigen glutamic acid decarboxylase (2). Such cross-reactive antigens in a genetically susceptible host might pave the way for the destruction of the islet cells. More recent studies actually firm up this hypothesis by proving that DNA vaccines involving mycobacterial Hsp65 protected NOD mice against diabetes (16). Moreover, seroreactivity against mycobacterial heat shock proteins has also been implicated in host tissue damage due to antibody cross-reactivity against self-antigens. Autoantibodies have been identified in patients with tuberculosis (due to infection with Mycobacterium tuberculosis) (6). Serum reactivity against mycobacterial antigens has also been correlated with human autoimmune diseases including Crohn's disease (13). Moreover, pancreatic antibodies are associated with Crohn's disease (9).
Shared genetic susceptibilities among mycobacterioses and T1DM could be another explanation of our results. This is because the island of Sardinia has the highest incidence of Crohn's disease and other autoimmune diseases such as T1DM, with a very high prevalence of M. avium subsp. paratuberculosis in Sardinian Crohn's disease patients (7, 11, 22). Since M. avium subsp. paratuberculosis is present in almost half of the sheep herds tested in Sardinia, it is supposed to be endemically contaminating water, milk, and animal feed, as reported previously in the United Kingdom (14, 18, 23). High levels of exposure might thus cause enhanced infection rates. Therefore, our setting of Sardinia for such a clinical association study appears to be a legitimate choice. The fact that antibody titers against M. avium subsp. paratuberculosis were higher in young T1DM patients than in older patients may reflect that M. avium subsp. paratuberculosis infection occurs at an early age.
In conclusion, finding immune responses to M. avium subsp. paratuberculosis bacteria in T1DM should indeed be a novel observation that strengthens our thinking regarding an infectious cause for T1DM. These results also have implications for countries like India and United States, which respectively have the highest livestock populations and high incidences of M. avium subsp. paratuberculosis simultaneously with a high incidence of T1DM.
Acknowledgments
Funding for the work was provided by the University of Sassari (60%) and Italian Miur (PRIN 2005).
N.A. thanks the CDFD for providing core and infrastructural support to his laboratory.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Berger, S., J. P. Bannantine, and J. F. Griffin. 2007. Autoreactive antibodies are present in sheep with Johne's disease and cross-react with Mycobacterium avium subsp. paratuberculosis antigens. Microbes Infect. 9:963-970. [DOI] [PubMed] [Google Scholar]
- 2.Child, D. F., C. P. Williams, R. P. Jones, P. R. Hudson, M. Jones, and C. J. Smith. 1995. Heat shock protein studies in type 1 and type 2 diabetes and human islet cell culture. Diabet. Med. 12:595-599. [DOI] [PubMed] [Google Scholar]
- 3.Daneman, D. 2006. Type 1 diabetes. Lancet 367:847-858. [DOI] [PubMed] [Google Scholar]
- 4.Davies, J. M. 1997. Molecular mimicry: can epitope mimicry induce autoimmune disease? Immunol. Cell Biol. 75:113-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dow, C. T. 2006. Paratuberculosis and type I diabetes: is this the trigger? Med. Hypoth. 67:782-785. [DOI] [PubMed] [Google Scholar]
- 6.Flores-Suarez, L. F., J. Cabiedes, A. R. Villa, F. J. van der Woude, and J. Alcocer-Varela. 2003. Prevalence of antineutrophil cytoplasmic autoantibodies in patients with tuberculosis. Rheumatology 42:223-229. [DOI] [PubMed] [Google Scholar]
- 7.Frongia, O., C. Pascutto, G. M. Sechi, M. Soro, and R. M. Angioi. 2001. Genetic and environmental factors for type 1 diabetes: data from the province of Oristano, Sardinia, Italy. Diabet. Care 24:1846-1847. [DOI] [PubMed] [Google Scholar]
- 8.Guarner, F., R. Bourdet-Sicard, P. Brandtzaeg, H. S. Gill, P. McGuirk, W. van Eden, J. Versalovic, J. V. Weinstock, and G. A. Rook. 2006. Mechanisms of disease: the hygiene hypothesis revisited. Nat. Clin. Pract. Gastroenterol. Hepatol. 3:275-284. [DOI] [PubMed] [Google Scholar]
- 9.Joossens, S., S. Vermeire, K. Van Steen, G. Godefridis, G. Claessens, M. Pierik, R. Vlietinck, R. Aerts, P. Rutgeerts, and X. Bossuyt. 2004. Pancreatic autoantibodies in inflammatory bowel disease. Inflamm. Bowel Dis. 10:771-777. [DOI] [PubMed] [Google Scholar]
- 10.Kissler, S., P. Stern, K. Takahashi, K. Hunter, L. B. Peterson, and L. S. Wicker. 2006. In vivo RNA interference demonstrates a role for Nramp1 in modifying susceptibility to type 1 diabetes. Nat. Genet. 38:479-483. [DOI] [PubMed] [Google Scholar]
- 11.Knip, M., R. Veijola, S. M. Virtanen, H. Hyoty, O. Vaarala, and H. K. Akerblom. 2005. Environmental triggers and determinants of type 1 diabetes. Diabetes 54:125-136. [DOI] [PubMed] [Google Scholar]
- 12.Liu, P. T., S. Stenger, H. Li, L. Wenzel, B. H. Tan, S. R. Krutzik, M. T. Ochoa, J. Schauber, K. Wu, C. Meinken, D. L. Kamen, M. Wagner, R. Bals, A. Steinmeyer, U. Zugel, R. L. Gallo, D. Eisenberg, M. Hewison, B. W. Hollis, J. S. Adams, B. R. Bloom, and R. L. Modlin. 2006. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311:1770-1773. [DOI] [PubMed] [Google Scholar]
- 13.Olsen, I., H. G. Wiker, E. Johnson, H. Langeggen, and L. J. Reitan. 2001. Elevated antibody responses in patients with Crohn's disease against a 14-kDa secreted protein purified from Mycobacterium avium subsp. paratuberculosis. Scand. J. Immunol. 53:198-203. [DOI] [PubMed] [Google Scholar]
- 14.Pickup, R. W., G. Rhodes, T. J. Bull, S. Arnott, K. Sidi-Boumedine, M. Hurley, and J. Hermon-Taylor. 2006. Mycobacterium avium subsp. paratuberculosis in lake catchments, in river water abstracted for domestic use, and in effluent from domestic sewage treatment works: diverse opportunities for environmental cycling and human exposure. Appl. Environ. Microbiol. 72:4067-4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polymeros, D., D. P. Bogdanos, R. Day, D. Arioli, D. Vergani, and A. Forbes. 2006. Does cross-reactivity between mycobacterium avium paratuberculosis and human intestinal antigens characterize Crohn's disease? Gastroenterology 131:85-96. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues dos Santos, R., Jr., A. Sartori, V. L. Deperon Bonato, A. A. M. Coelho, A. M. Castelo, C. A. Vilella, R. L. Zollner, and C. Lopes Silva. 2007. Immune modulation induced by tuberculosis DNA vaccine protects non-obese diabetic mice from diabetes progression. Clin. Exp. Immunol. 149:570-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rook, G. A., V. Adams, J. Hunt, R. Palmer, R. Martinelli, and L. R. Brunet. 2004. Mycobacteria and other environmental organisms as immunomodulators for immunoregulatory disorders. Springer Semin. Immunopathol. 25:237-255. [DOI] [PubMed] [Google Scholar]
- 18.Rowe, M. T., and I. R. Grant. 2006. Mycobacterium avium ssp. paratuberculosis and its potential survival tactics. Lett. Appl. Microbiol. 42:305-311. [DOI] [PubMed] [Google Scholar]
- 19.Sechi, L. A., N. Ahmed, G. E. Felis, I. Duprè, C. Cannas, G. Fadda, A. Bua, and S. Zanetti. 2006. Immunogenicity and cytoadherence of recombinant heparin binding haemagglutinin (HBHA) of Mycobacterium avium subsp. paratuberculosis: functional promiscuity or a role in virulence? Vaccine 24:236-243. [DOI] [PubMed] [Google Scholar]
- 20.Sechi, L. A., M. Gazouli, L. E. Sieswerda, P. Molicotti, N. Ahmed, J. Ikonomopoulos, A. M. Scanu, D. Paccagnini, and S. Zanetti. 2006. Relationship between Crohn's disease, infection with Mycobacterium avium subspecies paratuberculosis and SLC11A1 gene polymorphisms in Sardinian patients. World J. Gastroenterol. 12:7161-7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sechi, L. A., D. Paccagnini, S. Salza, A. Pacifico, N. Ahmed, and S. Zanetti. 2008. Mycobacterium avium subsp. paratuberculosis bacteraemia in type-1 diabetes cases: an infectious trigger? Clin. Infect. Dis. 46:148-149. [DOI] [PubMed] [Google Scholar]
- 22.Sechi, L. A., A. M. Scanu, P. Molicotti, S. Cannas, M. Mura, G. Dettori, G. Fadda, and S. Zanetti. 2005. Detection and isolation of Mycobacterium avium subspecies paratuberculosis from intestinal mucosal biopsies of patients with and without Crohn's disease in Sardinia. Am. J. Gastroenterol. 100:1529-1536. [DOI] [PubMed] [Google Scholar]
- 23.van Halteren, A. G., B. O. Roep, S. Gregori, A. Cooke, W. van Eden, G. Kraal, and M. H. Wauben. 2002. Cross-reactive mycobacterial and self hsp60 epitope recognition in I-A(g7) expressing NOD, NOD-asp and Biozzi AB/H mice. J. Autoimmun. 18:139-147. [DOI] [PubMed] [Google Scholar]