Abstract
The combined adjuvant effect of ginsenoside Rg1 and aluminum hydroxide (alum) on immune responses to ovalbumin (OVA) in mice was investigated. BALB/c mice were subcutaneously (s.c.) inoculated twice with OVA alone or in combination with Rg1, alum, or Rg1 plus alum. Samples were collected 2 weeks after the boosting for the measurement of anti-OVA immunoglobulin G (IgG) isotypes in sera and gamma interferon (IFN-γ) and interleukin-5 (IL-5) produced in singular splenocyte cultures. Delayed-type hypersensitivity (DTH) responses were measured in mice immunized as described above. After 10 days, the mice were injected s.c. with OVA at the footpads. Thereafter, the thickness of the footpads was measured once daily for 5 days. The results indicated that alum enhanced mainly Th2 (IgG1 and IL-5) responses (P < 0.05), while Rg1 enhanced both Th1 (IgG1 and IL-5) and Th2 (IgG2a, IFN-γ, and DTH) responses (P < 0.05). The highest immune responses were found in the mice injected with OVA solution containing both alum and Rg1. In addition, the hemolytic activity of Rg1 was much lower than that of Quil A. Therefore, Rg1 deserves further studies in order to tailor desired immune responses when a mixed Th1/Th2 immune response is needed.
Adjuvants are important components in vaccine formulations. Many adjuvants have been licensed for use in animal vaccines, but for humans, where safety is a primary concern, aluminum hydroxide (alum) remains the most widely used adjuvant (8, 19). Alum effectively enhances humoral immune responses to vaccine antigens and appears to skew immune responses toward Th2 type (9). A major limitation of alum in vaccine applications is its failure to induce Th1-type immune responses (9). For this reason, alum alone is not a rational choice as an adjuvant for vaccines where a Th1 or mixed Th1/Th2 response is required for protection. This has led to additional research for alternative adjuvants.
Ginseng (GS), the root of Panax ginseng C. A. Meyer (Araliaceae), has been utilized as traditional medicine in China for at least 3,500 years (10). Our previous studies have shown that an extract from GS has stimulatory effects on neutrophils and lymphocytes isolated from bovine peripheral blood and milk in vitro (2, 12). A recent investigation has revealed that GS extract has adjuvant properties and acts synergistically with alum to improve immune responses. For example, Rivera et al. (21, 22) reported a synergistic effect of GS and alum on the immune responses induced by porcine parvovirus antigens in guinea pigs. Rivera et al. (20) also found that a supplement of GS in an alum-adjuvanted bivalent vaccine improved immune responses in pigs to vaccination against porcine parvovirus and Erysipelothrix rhusiopathiae. Hu et al. (11) observed increased antibody responses and blood lymphocyte proliferation in dairy cattle elicited by vaccination with Staphylococcus aureus mastitis vaccine mixed with an Rb1 fraction of ginsenosides. Isolation and characterization of the chemical constituents in GS have shown that its active immune components are ginsenosides, which are saponins chemically related to triterpenoid glycosides of the dammarane series (15). At present, more than 30 ginsenosides have been identified in the root of P. ginseng (15). The adjuvant activities of ginsenosides with different molecular structures are dependent mainly on the side sugar chains attached to their dammarane skeleton. After an investigation of ginsenosides Rg3, Rd, Rc, Rb1, Rb2, Rg1, Re, and Rg2 for their adjuvant effects on the immune responses to ovalbumin (OVA) in mice, we recently found that Rg1 has a stronger adjuvant potency than the others (26). Chemical analysis has shown that Rg1 is found not only in the root but also in the stems and leaves of P. ginseng (28). This discovery has greatly decreased the cost of Rg1 production. Because of its adjuvant property, safety, and relatively low cost, Rg1 deserves further study of its effects on host immunity. The research presented here was designed to evaluate the combined adjuvant effects of Rg1 and alum for their modulation of the Th1/Th2 immune response to OVA in mice by measuring specific immunoglobulin G (IgG) isotypes and delayed-type hypersensitivity (DTH) as well as the production of gamma interferon (IFN-γ) and interleukin-5 (IL-5).
MATERIALS AND METHODS
Mice.
Six-week-old female BALB/c mice were purchased from the Shanghai Experimental Animal Center (Shanghai, China), housed in wire cages at 20 to 22°C, with 50% ± 10% humidity, and allowed standard chow and water in the Experimental Animal Center (Zhejiang Chinese Medical University, China). All procedures related to the animals and their care conformed to the ethical guidelines of Zhejiang Chinese Medical University.
Chemicals.
Ginsenoside Rg1 extracted from the root of Panax ginseng C.A. Meyer was from Hongjiu Ginseng Industry Co. Ltd. (Jilin, China). The Rg1 was a white powder with a purity of 98%; a melting point of 194 to 196.5°C; a molecular formula of C42H72O14; an infrared spectrum (KBr)/cm of 3400, 1620; and a molecular structure as shown in Fig. 1. Alum was purchased from Zhejiang Wanma Pharmaceutical Co. Ltd. OVA (grade V) was purchased from Sigma Chemical Co. Rg1 or OVA was dissolved in physiological saline solution and sterilized by passing it through a 0.22-μm filter before its use. The endotoxin level in each of the solutions was less than 0.5 endotoxin unit/ml as determined by a gel clot Limulus amebocyte lysate assay (Zhanjiang A&C Biological Ltd., Zhanjiang, China).
FIG. 1.
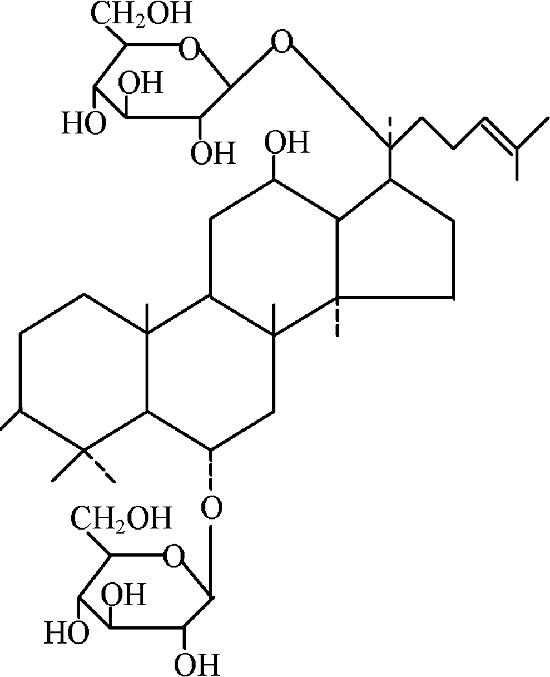
Chemical structure of ginsenoside Rg1 (C42H72O14; molecular weight, 801.02).
Immunization.
Twenty-five BALB/c mice were randomly divided into five groups with each consisting of five animals. The mice were subcutaneously (s.c.) injected with 0.89% saline (200 μl) or 0.89% saline (200 μl) containing OVA (10 μg) alone or with Rg1 (50 μg), alum (200 μg), or Rg1 (50 μg) plus alum (200 μg). A booster injection was administered after 3 weeks. Samples were collected 2 weeks after the booster injection for measurement of OVA-specific IgG isotypes in sera and IFN-γ and IL-5 produced in splenocyte cultures. All animals were anesthetized with ether prior to blood and spleen sample collection. Individual serum samples were stored at −20°C until used.
Measurement of OVA-specific IgG and subclasses.
An indirect enzyme-linked immunosorbent assay (ELISA) was conducted to measure serum OVA-specific IgG and the subclasses, as previously described by Xiao et al. (31). Briefly, flat-bottom 96-well microtiter plates (Gong Dong Medical Plastic Factory, Zhejiang, China) were coated with OVA diluted in 0.05 M carbonate buffer (5 μg/ml) and incubated overnight at 4°C. After being washed with 0.01 M phosphate buffer solution containing 0.05% of Tween 20 (PBST), the plates were blocked with 5% fetal calf serum in phosphate-buffered saline and incubated for 2 h at room temperature. To measure IgG or the subclasses, 100 μl of diluted serum samples (1:500) was added and the plates were incubated for 1 h at 37°C. After being washed, 100 μl of horseradish-peroxidase-conjugated rabbit anti-mouse IgG, IgG1, or IgG2a (Santa Cruz Biotechnology, Inc., CA) diluted in PBST (1:5,000) was added to the plates and incubated for 1 h at 37°C. After being washed again, 100 μl of substrate solution (1 mg of 3,3′,5,5′-tetramethylbenzidine in 10 ml of 0.1 M citrate-phosphate buffer, pH 5.0) was added to each well and further incubated at room temperature for 10 min. The reaction was stopped by adding 50 μl of 2 M H2SO4 to each well. The optical density at 450 nm (OD450) was read by an automatic ELISA plate reader (Dialab, GmbH, Austria).
Measurement of IL-5 and IFN-γ.
Spleen samples were collected from the immunized BALB/c mice under aseptic conditions. Single-cell suspensions (2.5 × 106 cells/ml) were prepared, and 500 μl/well (24-well plates; Costar) was mixed with an equal volume of OVA solution (100 μg/ml) or medium (RPMI 1640 supplemented with 2 mM l-glutamine, 50 mM 2-mercaptoethanol, 50 μg/ml gentamicin sulfate, and 10% fetal calf serum). The culture supernatants were collected 72 h after the incubation. The concentrations of IL-5 and IFN-γ in the supernatants were determined by a capture ELISA (R&D Systems Inc., Minneapolis, MN) and calculated by interpolation of the cytokine standard curves.
DTH reaction.
Fifteen BALB/c mice were randomly divided into five groups with each consisting of three animals. Each of the mice was immunized s.c. with 0.89% saline or 0.89% saline containing 10 μg of OVA alone or with Rg1 (50 μg), alum (200 μg), or Rg1 (50 μg) plus alum (200 μg). After 10 days, each mouse was injected s.c. with 40 μl of OVA (10 μg) at both footpads of the hind limbs. The thickness of the footpads was measured every day within 5 days after the injection, and DTH responses were expressed as the percentages of the increase of swelling of six individual footpads at the injection site. The variation among individuals was <20%.
Hemolytic assay.
A hemolytic assay was performed mainly as described by Xiao et al. (30). Briefly, red blood cells (RBC) from a healthy New Zealand rabbit (Experimental Animal Center of Zhejiang Traditional Chinese Medicine College) were washed three times with physiological saline at 180 × g for 10 min and adjusted to 0.5%. The RBC suspension was incubated with equal volumes of saline solution containing Rg1 or Quil A (Desert King Chile Ltd., Santiago, Chile) at concentrations ranging from 0.5 μg/ml to 1.0 mg/ml at 37°C for 30 min. Thereafter, samples were centrifuged at 1,000 × g for 10 min, and the OD450 value of the supernatant was measured using an automatic ELISA plate reader (Dialab, GmbH, Austria).
Statistical analysis.
Data are expressed as means ± standard deviations. Bonferroni's test was used to compare the mean values of antibody levels, footpad swelling, and cytokine concentrations between groups. P values of less than 0.05 were considered to be statistically significant.
RESULTS
OVA-specific IgG, IgG1, and IgG2a.
Fig. 2 shows IgG, IgG1, and IgG2a levels in sera collected from mice after immunization. The highest serum IgG, IgG1, and IgG2a responses were recorded for mice immunized with OVA containing Rg1 plus alum, although these parameters were significantly higher in mice immunized with OVA containing Rg1 or alum (P < 0.05) than in the control group. In addition, the sequence of the increasing OD values was (Rg1 plus alum)>alum>Rg1 for the IgG1 response and (Rg1 plus alum)>Rg1>alum for the IgG2a response.
FIG. 2.
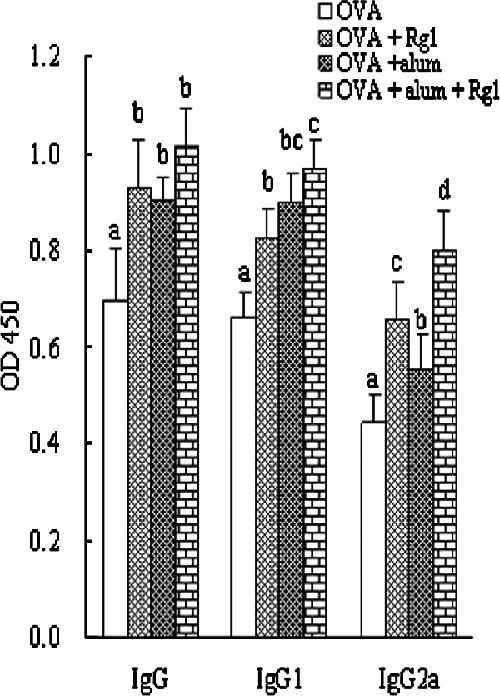
OVA-specific IgG, IgG1, and IgG2a antibody responses in BALB/c mice immunized s.c. with OVA (10 μg) in saline (200 pl) alone or with OVA (10 μg) in saline (200 pl) containing alum (200 μg), Rg1 (50 μg), or alum (200 μg) plus Rg1 (50 μg) on days 1 and 21. Serum was collected 2 weeks after the boosting. Serum OVA-specific IgG, IgG1, and IgG2a antibody levels were measured by an indirect ELISA. The values are presented as means ± standard deviations (n = 5). Values with different letters showed significant difference (P < 0.05).
DTH response.
Significantly higher DTH responses were recorded for the Rg1-plus-alum group and the Rg1 group than for the control (OVA) group (P < 0.05) within 3 days after intrafootpad injection, as shown in Fig. 3. But alum did not increase or only weakly increased (on day 3) the DTH reaction compared with the OVA group.
FIG. 3.

Effects of Rg1 and/or alum on induction of OVA-specific DTH responses. DTH reactions were carried out 10 days after the mice were immunized with OVA alone or in combination with Rg1, alum, or Rg1 plus alum.
IL-5 and IFN-γ in splenocyte cultures.
As shown in Fig. 4, significantly higher production levels of both IL-5 and IFN-γ were found in the cell cultures of the mice immunized with OVA containing Rg1 or Rg1 plus alum than in those of the control group (P < 0.05). In contrast, alum enhanced the production of only IL-5 (P < 0.05), not IFN-γ.
FIG. 4.
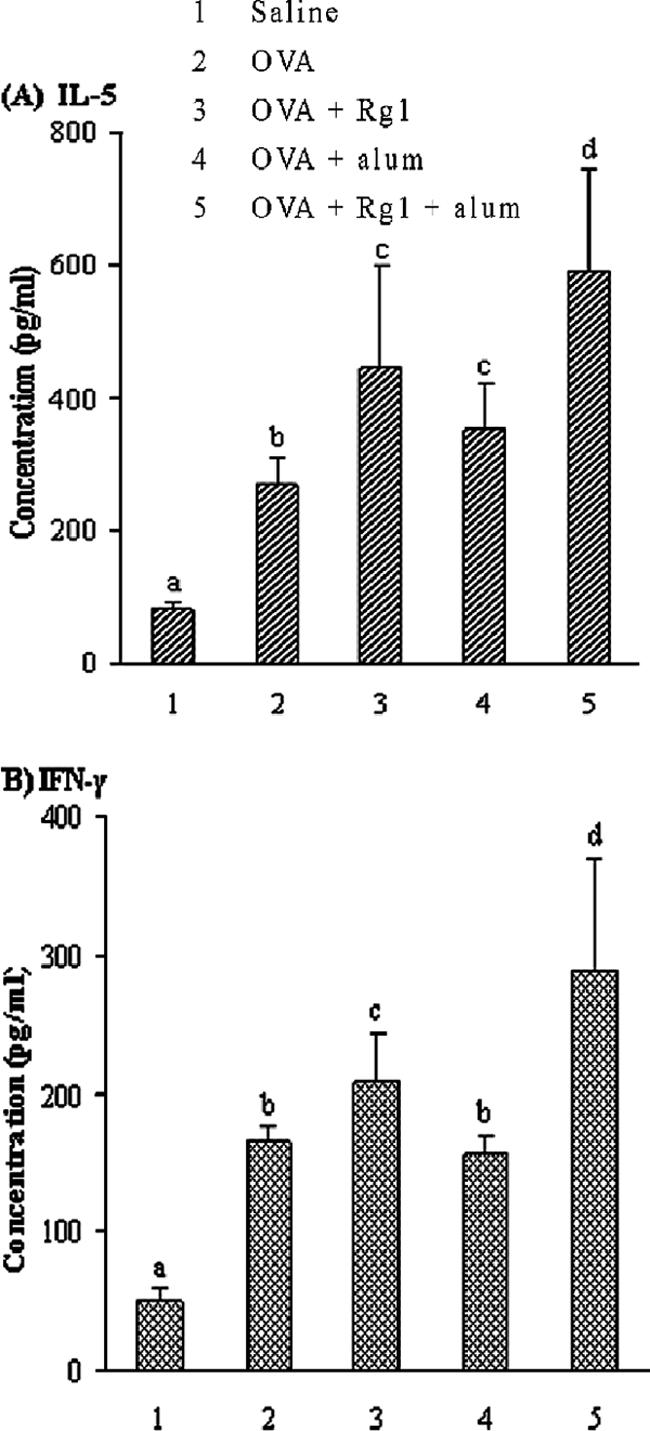
IL-5 (A) and IFN-γ (B) in the supernatants of cultured splenocytes stimulated with OVA (100 μg/ml). BALB/c mice were immunized s.c. with OVA (10 μg) alone in saline (200 pl) or OVA in saline (200 pl) containing alum (200 μg), Rg1 (50 μg), or alum (200 μg) plus Rg1 (50 μg) on days 1 and 21. Splenocytes were prepared and cultured with or without OVA (100 μg/ml) in RPMI 1640 after the mice were scarified 2 weeks after the last immunization. The culture supernatants were harvested after 72 h of incubation of the splenocytes with OVA. The values are presented as means ± standard deviations (n = 5). Values with different letters showed significant difference (P < 0.05).
Hemolytic activity.
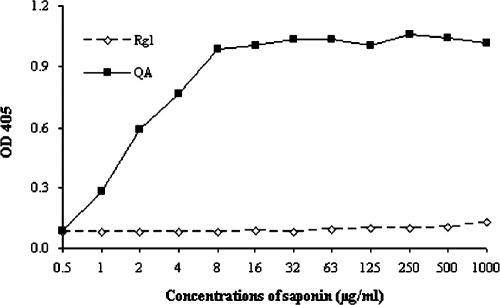
Compared with Quil A, the hemolytic activity of Rg1 was extremely low (Fig. 5), and only slight hemolysis was observed at concentrations of more than 1 mg/ml.
FIG. 5.
Hemolytic activities of Rg1 and Quil A. After the incubation of the RBC suspension with equal volumes of saline solution containing Rg1 or Quil A (QA) at 37°C for 30 min, samples were centrifuged at 1,000 × g for 10 min, and OD values of the supernatants were measured at 405 nm.
DISCUSSION
Immunity to different infectious agents requires distinct types of immune responses. Defense against intracellular pathogens tends to involve Th1-type immune responses dominated by the production of IFN-γ, IgG2a antibodies, DTH, and cytotoxic T lymphocytes, while resistance to extracellular pathogens is often associated with humoral responses dominated by high levels of IgG1 and the production of IL-4 and IL-5 (3). One of the major challenges in vaccinology is the development of vaccine formulations that will induce immune responses appropriate for the particular pathogen, since the wrong response could lead to increased pathology and possibly the enhanced spread of the pathogen. Thus, adjuvants can be a valuable tool for tailoring the desired quality of immune responses. Polarized Th1-type immunity can be achieved by the addition of complete Freund's adjuvant and CpG DNA to an antigen (5, 33). In contrast, Th2 antibody responses can be induced by the adjuvant alum or incomplete Freund's adjuvant, as indicated by more IgG1 relative to IgG2a (4, 33). Some adjuvants or their combinations can promote mixed Th1/Th2 responses. For instant, QS 21 or a combination of complete Freund's adjuvant plus incomplete Freund's adjuvant induces antigen-specific IFN-γ (Th1-type) and IL-4 (Th2-type) responses (4), while a liposomal formulation of leishmania antigens results in mixed Th1/Th2 (both serum IgG1/IgG2a) responses (16). Our present study demonstrated that Rg1 administered alone could significantly enhance the production of antigen-specific IgG isotypes and IL-5 and IFN-γ secretions, as well as DTH reactions, suggesting that Rg1 has adjuvant properties to balance Th1 and Th2 immune responses. Alum increased only IgG1 and IL-5 levels, not IFN-γ levels, or slightly improved IgG2a and DTH responses induced by OVA in mice, indicating that this adjuvant polarizes the immune response to the Th2 branch. More importantly, we observed significantly increased IgG1, IgG2a, IL-5, IFN-γ, and DTH responses when Rg1 and alum were used together compared with those when Rg1 or alum was used alone. These results suggest that Rg1 and alum synergistically act as adjuvants to up-regulate Th1/Th2 immune responses.
The increased Th1 and Th2 immune responses reported here might be attributed to a combination of the immunomodulatory effect of Rg1 with the depot effect of alum. To date, the mechanisms by which Rg1 mediates its adjuvant effects are not fully understood. Previous work has showed that Rg1 could induce production of Th1 cytokines in the absence of antigen. Song et al. (25) found that the subcutaneous injection of a GS extract in mice favored the production of tumor necrosis factor alpha and IFN-γ by splenocytes. Lee and Han (14) observed that intraperitoneal injection of Rg1 in mice resulted in predominantly IFN-γ and IL-2 responses. These results indicate that the stimulation of innate immune responses could be a potential mechanism by which Rg1 mediates its potent adjuvant effects.
One of the major hinderances in the development of effective adjuvants is the balance between adjuvancity and toxicity. Since the saponin Quil A from Quillaja saponaria Molina was reported to have an immunological adjuvant effect (13), many attempts have been made to identify the adjuvant activity of mixed or highly purified saponins (24, 34). However, it was claimed that saponins should not be used as adjuvants due to their intrinsic hemolytic properties (23). The effects of saponins on cell membranes have been reviewed by Francis et al. (6). The hemolytic effect of saponins may absorb cells such as dendritic cells to shift to the local place of the antigen, or the permeabilization of cells with saponins may enable the access of antibodies to the cytoplasm (1); however, no correlation was detected between hemolytic activity and adjuvant potential (18). In the present study, Rg1 showed slight hemolytic activity compared with Quil A. Meng et al. (17) analyzed the hemolytic activity of Rg1 by incubating it with a 2% rabbit RBC suspension for 3 h at 37°C and found its minimal hemolytic concentration to be 200 μg/ml. It has been reported that the hemolytic activities of saponins are related to their chemical composition (27). In general, steroid and triterpenoid saponins with a single sugar chain (monodesmosides) have strong hemolytic activity, whereas those with two sugar chains (bidesmosides) have less activity (7, 29). Rg1 is a triterpenoid saponin, having two glucopyranosyl moieties at the positions of C-6 and C-20 (Fig. 1). This fact could explain its diminished hemolytic effect. In addition, Rg1 is safe for injection as a GS extract solution containing Rg1 and has been licensed for intravenous injection in humans in China (32); no local reaction or systemic side effects were found in mice injected with Rg1 plus OVA or Rg1 plus alum plus OVA in our experiments.
In conclusion, Rg1 promoted both Th1 and Th2 immune responses, and most importantly, Rg1 in combination with alum highly up-regulated Th1/Th2 immune responses resulting in a balanced immunity for broader protection. Since alum as a vaccine adjuvant has had a safe record for a long time and a GS preparation containing Rg1 has been licensed for injection in humans, Rg1 and Rg1 in combination with alum deserve further studies for improving the quality of vaccines where mixed Th1/Th2 immune responses are needed.
Acknowledgments
This study was financed by the National Natural Science Foundation of China (NSFC) (project no. 30471273 and 30771592).
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Baumann, E., G. Stoya, A. Volkner, W. Richter, C. Lemke, and W. Linss. 2000. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 102:21-35. [DOI] [PubMed] [Google Scholar]
- 2.Concha, C., S. Hu, and O. Holmberg. 1996. The proliferative responses of cow stripping milk and blood lymphocytes to pokeweed mitogen and ginseng in vitro. Vet. Res. 27:107-115. [PubMed] [Google Scholar]
- 3.Constant, S. L., and K. Bottomly. 1997. Induction of Th1 and Th2 CD4+ T cell responses: the alternative approaches. Annu. Rev. Immunol. 15:297-322. [DOI] [PubMed] [Google Scholar]
- 4.Cribbs, D. H., A. Ghochikyan, V. Vasilevko, M. Tran, I. Petrushina, N. Sadzikava, D. Babikyan, P. Kesslak, T. Kieber-Emmons, C. W. Cotman, and M. G. Agadjanyan. 2003. Adjuvant-dependent modulation of Th1 and Th2 responses to immunization with beta-amyloid. Int. Immunol. 15:505-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis, H. L., R. Weeratna, T. J. Waldschmidt, L. Tygrett, J. Schorr, and A. M. Krieg. 1998. CpG DNA is a potent enhancer of specific immunity in mice immunized with recombinant hepatitis B surface antigen. J. Immunol. 160:870-876. [PubMed] [Google Scholar]
- 6.Francis, G., Z. Kerem, H. P. S. Makkar, and K. Becker. 2002. The biological action of saponins in animal systems: a review. Br. J. Nutr. 88:587-605. [DOI] [PubMed] [Google Scholar]
- 7.Fukuda, K., H. Utsumi, J. Shoji, and A. Hamada. 1985. Saponins can cause the agglutination of phospholipid vesicles. Biochim. Biophys. Acta 820:199-206. [DOI] [PubMed] [Google Scholar]
- 8.Goto, N., H. Kato, J. Maeyama, K. Eto, and S. Yoshihara. 1993. Studies on the toxicities of aluminum hydroxide and calcium phosphate as immunological adjuvants for vaccines. Vaccine 11:914-918. [DOI] [PubMed] [Google Scholar]
- 9.HogenEsch, H. 2002. Mechanisms of stimulation of the immune response by aluminum adjuvants. Vaccine 20(Suppl. 3):S34-S39. [DOI] [PubMed] [Google Scholar]
- 10.Hu, S. 2002. Ph.D. thesis. Immunomodulatory and adjuvant effects of ginseng extracts with emphasis on defence mechanisms of the bovine udder, p. 23-24. Swedish University of Agricultural Sciences, Uppsala.
- 11.Hu, S., C. Concha, F. Lin, and K. P. Waller. 2003. Adjuvant effect of ginseng extracts on the immune responses to immunization against Staphylococcus aureus in dairy cattle. Vet. Immunol. Immunopathol. 91:29-37. [DOI] [PubMed] [Google Scholar]
- 12.Hu, S., C. Concha, R. Cooray, and O. Holmberg. 1995. Ginseng-enhanced oxidative and phagocytic activities of polymorphonuclear leukocytes from bovine peripheral blood and stripping milk. Vet. Res. 26:155-161. [PubMed] [Google Scholar]
- 13.Kensil, C. R., U. Patel, M. Lennick, and D. Marciani. 1991. Separation and characterization of saponins with adjuvant activity from Quillaja saponaria Molina cortex. J. Immunol. 146:431-437. [PubMed] [Google Scholar]
- 14.Lee, J. H., and Y. Han. 2006. Ginsenoside Rg1 helps mice resist to disseminated candidiasis by Th1 type differentiation of CD4+ T cell. Int. Immunopharmacol. 6:1424-1430. [DOI] [PubMed] [Google Scholar]
- 15.Liu, C. X., and R. C. Xiao. 1992. Recent advances on ginseng research in China. J. Ethnopharmacol. 36:27-38. [DOI] [PubMed] [Google Scholar]
- 16.Mazumdar, T., K. Anam, and N. Ali. 2004. A mixed Th1/Th2 response elicited by a liposomal formulation of Leishmania vaccine instructs Th1 responses and resistance to Leishmania donovani in susceptible BALB/c mice. Vaccine 22:1162-1171. [DOI] [PubMed] [Google Scholar]
- 17.Meng, Q., P. Sun, L. L. Wang, X. Y. Ma, and J. Y. Zhao. 1998. Haemolytic activities of the saponins from Panax ginseng and Panax quinquefolium. J. N. Bethune Univ. Med. Sci. 24:135-136. [Google Scholar]
- 18.Oda, K., H. Matsuda, T. Murakami, S. Katayama, T. Ohgitani, and M. Yoshikawa. 2000. Adjuvant and haemolytic activities of 47 saponins derived from medicinal and food plants. Biol. Chem. 381:67-74. [DOI] [PubMed] [Google Scholar]
- 19.Pashine, A., N. M. Valiante, and J. B. Ulmer. 2005. Targeting the innate immune response with improved vaccine adjuvants. Nat. Med. 11(Suppl.):S63-S68. [DOI] [PubMed] [Google Scholar]
- 20.Rivera, E., A. Daggfeldt, and S. Hu. 2003. Ginseng extract in aluminium hydroxide adjuvanted vaccines improves the antibody response of pigs to porcine parvovirus and Erysipelothrix rhusiopathiae. Vet. Immunol. Immunopathol. 91:19-27. [DOI] [PubMed] [Google Scholar]
- 21.Rivera, E., F. Ekholm Pettersson, M. Inganäs, S. Paulie, and K. O. Gronvik. 2005. The Rb1 fraction of ginseng elicits a balanced Th1 and Th2 immune response. Vaccine 23:5411-5419. [DOI] [PubMed] [Google Scholar]
- 22.Rivera, E., S. Hu, and C. Concha. 2003. Ginseng and aluminium hydroxide act synergistically as vaccine adjuvants. Vaccine 21:1149-1157. [DOI] [PubMed] [Google Scholar]
- 23.Santos, W. R., R. R. Bernardo, L. M. T. Pecanha, M. Palatnik, J. P. Parente, and C. B. P. de Sousa. 1997. Haemolytic activities of plant saponins and adjuvants. Effect of Periandra mediterranea saponin on the humoral response to the FML antigen of Leishmania donovani. Vaccine 15:1024-1029. [DOI] [PubMed] [Google Scholar]
- 24.Silva, B. P., J. B. Soares, E. P. Souza, M. Palatnik, C. B. Sousa, and J. P. Parente. 2005. Pulcherrimasaponin, from the leaves of Calliandra pulcherrima, as adjuvant for immunization in the murine model of visceral leishmaniasis. Vaccine 23:1061-1071. [DOI] [PubMed] [Google Scholar]
- 25.Song, Z. J., C. Moser, H. Wu, V. Faber, A. Kharazmi, and N. Hoiby. 2003. Cytokine modulating effect of ginseng treatment in a mouse model of Pseudomonas aeruginosa lung infection. J. Cyst. Fibros. 2:112-119. [DOI] [PubMed] [Google Scholar]
- 26.Sun, J. H., S. H. Hu, and X. M. Song. 2007. Adjuvant effects of protopanaxadiol and protopanaxatriol saponins from ginseng roots on the immune responses to ovalbumin in mice. Vaccine 25:1114-1120. [DOI] [PubMed] [Google Scholar]
- 27.Takechi, M., and Y. Tanaka. 1995. Haemolytic time course differences between steroid and triterpenoid saponins. Planta Med. 61:76-77. [DOI] [PubMed] [Google Scholar]
- 28.Wang, T. S. 2001. Extraction and isolation of ginsenosides from stems and leaves of Panax ginseng, p. 692-693. In T. S. Wang (ed.), China ginseng. Liaoning Science and Technology Publishing House, Shenyang, China.
- 29.Woldemichael, G. M., and M. Wink. 2001. Identification and biological activities of triterpenoid saponins from chenopodium quinoa. J. Agric. Food Chem. 49:2327-2332. [DOI] [PubMed] [Google Scholar]
- 30.Xiao, C. W., S. H. Hu, and Z. I. Rajput. 2007. Adjuvant effect of an extract from cochinchina momordica seeds on the immune responses to ovalbumin in mice. Front. Agric. China 1:90-95. [Google Scholar]
- 31.Xiao, C. W., Z. I. Rajput, and S. Hu. 2007. Improvement of a commercial foot-and-mouth disease vaccine by supplement of Quil A. Vaccine 25:4795-4800. [DOI] [PubMed] [Google Scholar]
- 32.Xie, H., Y. Wang, G. L. Wang, L. S. Sheng, and Z. Y. Liu. 2006. Content determination of ginsenoside Rgl and ginsenoside Re in Shenfu injection.W. China J. Pharm. Sci. 21:208-209. (In Chinese.) [Google Scholar]
- 33.Yip, H. C., A. Y. Karulin, M. Tary-Lehmann, M. D. Hesse, H. Radeke, P. S. Heeger, R. P. Trezza, F. P. Heinzel, T. Forsthuber, and P. V. Lehmann. 1999. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J. Immunol. 162:3942-3949. [PubMed] [Google Scholar]
- 34.Yoshikawa, M., T. Morikawa, K. Yashiro, T. Murakami, and H. Matsuda. 2001. Bioactive saponins and glycosides. XIX. Notoginseng (3): immunological adjuvant activity of notoginsenosides and related saponins: structures of notoginsenosides-L, -M, and -N from the roots of Panax notoginseng (Burk.) F. H. Chen. Chem. Pharm. Bull. 49:1452-1456. [DOI] [PubMed] [Google Scholar]