Abstract
Tests based on the gamma interferon (IFN-γ) assay (IGA) are used as adjunctive tools for the diagnosis of Mycobacterium tuberculosis infection. Here we compared in-house and commercial whole-blood IGAs to identify a suitable assay for the surveillance of tuberculosis in population studies. The IGAs were selected on the basis of the ease with which they are performed and because they require a small amount of a biological sample and do not require cell purification. Since a “gold standard” for latently M. tuberculosis-infected individuals is not available, the sensitivities and the specificities of the IGAs were determined with samples from patients with clinically diagnosed active tuberculosis and in Mycobacterium bovis BCG-unvaccinated healthy controls. The in-house tests consisted of a bulk assay based on diluted whole blood and a single-cell assay based on IFN-γ intracellular staining. The commercial assays used were the QuantiFERON-TB-Gold (Q-TB) and the Q-TB in-tube tests. When the purified protein derivative was used as the antigen, in-house whole-blood intracellular staining was found to be highly discriminatory between active tuberculosis patients and BCG-vaccinated healthy controls, whereas the other IGAs did not discriminate between the two categories of patients. When M. tuberculosis-specific antigens were used, a very strong agreement between the results of the Q-TB in-tube assay and the clinical diagnosis was observed, while the Q-TB assay, performed according to the manufacturer's instructions, showed a significantly lower performance. Intriguingly, when the test was performed with RD1 proteins instead of peptides, its sensitivity was significantly increased. The in-house assay with diluted whole blood showed an elevated sensitivity and an elevated specificity, and the results agreed with the clinical diagnosis. Considering that the in-house assay uses 1/20 of the sample compared with the amount of sample used in the commercial IGA, it appears to be particularly promising for use in pediatric studies. Overall, the different assays showed different performance characteristics that need to be considered for surveillance of tuberculosis in population studies.
One-third of the world's population is infected with Mycobacterium tuberculosis. Although primary infection leads to active tuberculosis in only a minority of individuals, the others can remain latently M. tuberculosis infected (LTBI) for the duration of their lives (1).
Tuberculosis infection surveillance and control strictly depend on the availability of highly sensitive and specific diagnostic tests. Until recently, the tuberculin skin test (TST) has been the only tool available for the diagnosis of M. tuberculosis infection, particularly in those who are LTBI. However, this test is limited by its low sensitivity and specificity (70% and 66% pooled sensitivity and specificity, respectively) (27). Indeed, false-positive results for individuals vaccinated with M. bovis BCG and those infected by nontuberculous mycobacteria are common (22, 30, 32, 37), implying that high-risk patients who could benefit from preventive chemotherapy might not be treated because of an uncertain diagnosis.
The development of new tools that possibly also allow the more accurate identification of individuals who are LTBI remains a public health priority. New tests that use whole blood or purified lymphocytes and that are based on the gamma interferon (IFN-γ) produced by T cells in response to M. tuberculosis-specific antigens encoded by genes within regions of difference (RDs) have been developed, and some of them (IFN-γ assays [IGAs]) are now commercially available in regulatory agency-approved formats (27, 28). Several studies indicate that these new tests appear to be more accurate than TST, have sensitivities greater than or equal to the sensitivity of TST for the diagnosis of active tuberculosis, provide results that have a better correlation with exposure to M. tuberculosis, and have a higher specificity than TST when samples from healthy controls are tested (2, 7, 11, 12, 14, 20, 25, 27, 29, 31, 32, 35).
The objective of the present study was to identify an IGA that may be used in population studies to detect individuals who are LTBI and that may be used to monitor the contacts of M. tuberculosis-infected individuals. Because a “gold standard” for individuals who are LTBI is not available, we decided to compare the different assays for their sensitivities and specificities with samples from patients with clinically diagnosed active tuberculosis and BCG-unvaccinated healthy controls. The ideal IGA should be easy to perform, should require only a small amount of biological sample and thus may also be used with samples from pediatric subjects, should not require cell purification (e.g., they use whole blood as the biological sample), and should potentially be able to yield information about the presence and the types of memory cells specific for M. tuberculosis. To achieve this goal we developed in-house assays that use whole blood, in bulk or at the single-cell level, taking into account the ideal requirements that are not yet present in the available commercial tests. The specificities and the sensitivities of the selected in-house assays were compared with those of the commercial diagnostic QuantiFERON-TB-Gold assay (Q-TB assay; Cellestis Limited, Carnegie, Victoria, Australia) and the new-generation Q-TB in-tube assay (Cellestis), using as antigens purified protein derivative (PPD) and M. tuberculosis-specific RD proteins as overlapping peptides from Cellestis Limited and using entire proteins from Lionex Diagnostic and Therapeutics GmbH (Braunschweig, Germany).
The selected in-house IGAs consisted of a bulk assay based on diluted whole blood and a single-cell assay based on intracellular staining for IFN-γ.
The tests performed with whole blood were also compared with the classical antigen-specific assays, performed by using isolated peripheral mononuclear cells (PBMCs) as effector cells, i.e., M. tuberculosis-specific antigen-induced lymphocyte proliferation (the PBMC-proliferation assay) and IFN-γ release cytokines (the PBMC-cytokine assay) (3, 4, 5).
To achieve the purpose described above, a pilot study was performed with consecutive patients enrolled at the National Institute for Infectious Diseases L. Spallanzani in Rome, Italy, with a confirmed diagnosis of pulmonary tuberculosis. Age- and sex-matched healthy subjects, who were either vaccinated or nonvaccinated with BCG, served as controls.
MATERIALS AND METHODS
Patient population and healthy donors.
Patients were admitted to the infectious disease and respiratory disease wards at the National Institute for Infectious Diseases L. Spallanzani. Subjects were included in the study if they tested human immunodeficiency virus negative and were not undergoing cortisone therapy. None of them were undergoing antituberculous therapy at the time of enrollment.
Patients underwent clinical and microbiological examinations to confirm or exclude the diagnosis of active tuberculosis. Sequential respiratory samples (three expectorated or two induced sputum samples) were collected over the first 7 days following hospital admission. An acid-fast bacillus smear (Ziehl-Neelsen) and culture (on Lowenstein-Jensen and Bactec 460 media [BD Biosciences Division, Sparks, MD]) were performed with each specimen. Chest X rays were done for each patient.
Non-BCG-vaccinated healthy donors were enrolled from among blood donors (Centro Trasfusionale dell'Università, Rome, Italy). A small population of BCG-vaccinated individuals was also enrolled. The study was approved by an ethical committee, and all patients and controls enrolled were informed and gave written consent. A total of 51 individuals were enrolled in the study, but 7 of these individuals excluded for the following reasons: four patients had a final diagnosis different from active tuberculosis, one patient was under cortisone therapy at the time of IGA, and two controls were healthy contacts of individuals with tuberculosis. Table 1 summarizes the details of the study populations, while Table 2 lists the individual patient/control samples that scored positive/negative against each test/antigen and TST, as appropriate.
TABLE 1.
Epidemiological characteristics of subjects enrolled in this studya
| Characteristic | Active tuberculosis patients | Healthy controls
|
|
|---|---|---|---|
| BCG unvaccinated | BCG vaccinated | ||
| Total no. of participants | 20 | 19 | 5 |
| Mean age (yr) ± SEM | 36 ± 2 | 37 ± 2 | 35 ± 2 |
| No. TST positive/no. tested | 9/12 | 0/7 | 0/1 |
| No. sputum culture positive | 20 | ||
Active tuberculosis patients were classified according to clinical and microbiological presentation criteria as having active disease. The control group represents individuals with no history or evidence of M. tuberculosis infection or exposure.
TABLE 2.
Results for samples from the individual active tuberculosis patients and healthy controls tested by TST and the different IGAs and scored as positive or negative for reactivity to each antigen
| Subjecta | Result by the following assayb
|
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TST | Q-TB in-tube | Q-TB
|
Diluted whole blood
|
Whole-blood intracellular staining
|
PBMC-cytokine
|
PBMC-proliferation
|
||||||||
| PPD | Proteins | Peptides | PPD | Proteins | PPD | Proteins | Peptides | PPD | Proteins | PPD | Proteins | |||
| 7A | ND | ND | + | ND | ND | + | ND | + | ND | ND | + | ND | − | ND |
| 8A | ND | ND | + | ND | ND | + | ND | + | ND | ND | + | ND | + | ND |
| 10A | ND | ND | + | ND | + | + | ND | + | ND | + | + | ND | + | ND |
| 14A | + | − | + | + | − | + | + | + | − | − | ND | ND | + | + |
| 17A | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 25A | + | − | − | − | − | + | + | + | + | − | + | + | + | + |
| 26A | ND | + | + | + | − | + | + | + | + | + | − | + | + | + |
| 28A | ND | + | − | + | − | + | + | + | + | + | − | − | − | − |
| 29A | ND | + | − | + | − | + | + | + | + | + | + | + | + | + |
| 31A | + | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 32A | + | + | + | + | − | + | + | + | − | + | + | + | + | + |
| 37A | ND | + | + | + | + | + | + | + | − | + | ND | ND | ND | ND |
| 39A | − | + | + | + | + | + | + | + | + | + | + | + | + | + |
| 40A | + | + | + | + | + | + | + | + | − | + | ND | ND | ND | ND |
| 41A | − | + | − | + | + | − | + | + | + | + | ND | ND | − | + |
| 42A | + | + | + | + | + | − | + | + | − | − | − | + | − | + |
| 43A | + | + | + | + | + | + | + | + | + | + | ND | ND | − | + |
| 44A | + | + | + | + | − | + | + | + | + | + | ND | ND | ND | + |
| 46A | + | + | − | + | − | + | + | + | + | + | + | + | + | + |
| 47A | ND | − | − | + | − | − | − | + | − | − | − | − | − | − |
| No. of samples positive/no. tested | 9/12 | 14/17 | 14/20 | 16/17 | 9/18 | 17/20 | 16/17 | 20/20 | 11/17 | 14/18 | 10/14 | 9/11 | 11/17 | 13/15 |
| 1B | ND | ND | − | ND | ND | − | ND | − | ND | ND | − | ND | + | ND |
| 2B | ND | ND | − | ND | ND | − | ND | ND | ND | ND | − | ND | + | ND |
| 3B | ND | ND | − | ND | ND | − | ND | − | ND | ND | − | ND | − | ND |
| 4B | ND | ND | + | ND | ND | − | ND | − | ND | ND | − | ND | − | ND |
| 5B | ND | ND | − | ND | ND | − | ND | − | ND | ND | − | ND | − | ND |
| 11B | − | ND | − | ND | − | − | ND | − | ND | − | + | ND | − | ND |
| 18B | ND | − | − | + | ND | − | − | + | + | + | − | − | + | − |
| 22B | − | − | − | − | − | − | − | − | − | − | ND | ND | + | − |
| 24B | − | − | − | − | − | − | − | − | − | − | − | − | − | − |
| 27B | ND | − | − | − | − | − | − | − | − | − | − | − | + | + |
| 30B | − | − | + | − | − | − | − | + | − | − | + | − | + | + |
| 33B | ND | − | + | − | − | − | + | − | − | − | − | − | − | − |
| 34B | − | − | − | − | − | + | − | − | − | − | − | − | − | − |
| 36B | ND | − | − | − | + | − | − | − | − | − | − | + | + | − |
| 38B | ND | − | − | + | − | − | + | − | − | + | − | − | + | − |
| 45B | ND | − | − | − | − | − | − | − | − | − | ND | ND | ND | ND |
| 48B | − | − | − | ND | − | − | − | − | − | ND | − | − | − | − |
| 50B | ND | − | − | ND | − | − | − | + | − | ND | − | + | + | − |
| 51B | − | − | + | ND | − | − | − | − | − | ND | − | − | − | − |
| No. of samples negative/no. tested | 7/7 | 13/13 | 15/19 | 8/10 | 12/13 | 18/19 | 11/13 | 15/18 | 12/13 | 13/14 | 15/17 | 9/11 | 9/18 | 10/12 |
| 6B BCG vaccinated | ND | ND | − | ND | ND | ND | ND | − | ND | ND | ND | ND | + | ND |
| 9B BCG vaccinated | ND | ND | − | ND | − | + | ND | − | ND | ND | + | ND | + | ND |
| 13B BCG vaccinated | ND | ND | + | ND | − | + | ND | + | ND | ND | + | ND | + | ND |
| 15B BCG vaccinated | − | − | − | ND | − | − | − | − | − | ND | ND | ND | − | − |
| 35B BCG vaccinated | ND | ND | ND | ND | ND | ND | ND | − | − | ND | ND | − | ND | ND |
| No. of samples negative/no. tested | 1/1 | 1/1 | 3/4 | 3/3 | 1/3 | 1/1 | 4/5 | 2/2 | 0/2 | 1/1 | 1/4 | 1/1 | ||
A, active tuberculosis patients; B, healthy controls.
The proteins were ESAT-6 and CFP-10, and the peptides were ESAT-6 and CFP-10 overlapping peptides. Positive responses (+) were classified for samples with values greater than or equal to the cutoff, and negative responses (−) were classified for samples with values less than the cutoff. For the ESAT-6 and CFP-10 proteins/peptides, a positive response was determined on the basis of the best-performing antigen. ND, not determined. IFN-γ levels were measured by ELISA and are expressed in IU/ml or by intracellular staining and are expressed as the percentage of positive cells. PBMC proliferation was measured by [3H]thymidine incorporation and is expressed as the SI. The cutoff values for each of the assays were as follows: Q-TB in-tube assay, 0.35 IU/ml; Q-TB assay with PPD, 9.0 IU/ml; Q-TB assay with proteins, 0.2 IU/ml with CFP-10 and 0.08 IU/ml with ESAT-6; Q-TB assay with peptides, 0.35 IU/ml with CFP-10 and ESAT-6; diluted-whole-blood assay and PPD, 9.8 IU/ml; diluted-whole-blood assay and proteins, 0.35 IU/ml with CFP-10 and 0.05 IU/ml with ESAT-6; whole-blood intracellular staining with PPD, 0.15% IFN-γ-positive cells; whole-blood intracellular staining with proteins, 0.05% IFN-γ-positive cells with CFP-10 and ESAT-6; whole-blood intracellular staining with peptides, 0.05% IFN-γ-positive cells with CFP-10 and ESAT-6; PBMC-cytokine assay with PPD, 4.2 IU/ml; PBMC-cytokine assay with proteins, 0.15 IU/ml with CFP-10 and ESAT-6; PBMC-proliferation assay with PPD, 49.0 SI; PBMC-proliferation assay with proteins, 3.00 SI with CFP-10 and ESAT-6.
Peripheral blood was drawn directly into two 5-ml “green-capped” Vacutainer tubes containing sodium heparin and was processed within 3 h from the time of withdrawal.
Reagents and antigens.
PPD from M. tuberculosis was obtained from the Statens Serum Institut (Copenhagen, Denmark) and was used at 10 μg/ml; Staphylococcus enterotoxin B (SEB; 1 μg/ml) and brefeldin A were from Sigma Chemical Co. (St. Louis, MO). M. tuberculosis-specific early secretory antigen target 6 (ESAT-6) and culture filtrate protein 10 (CFP-10) antigens from the RD1 region were from Lionex and Cellestis. The Lionex proteins were used at a predetermined optimal dose of 2 μg/ml, unless specified differently (18), while the Cellestis peptides were used at the doses recommended by the manufacturer.
Whole-blood and PBMC antigen-specific assays.
Four assays with whole blood (the Q-TB assay, the Q-TB in-tube assay, an assay with diluted whole blood, and whole-blood intracellular staining) and two assays with isolated PBMCs (the PBMC-proliferation and PBMC-cytokine assays) were compared. ESAT-6 and CFP-10 (proteins from Lionex and peptides from Cellestis) from the RD1 region and PPD antigens were used. TB7.7 (strain Rv2654) from the RD11 region was an adjunctive M. tuberculosis-specific antigen present in the Q-TB in-tube assay (6).
The SEB superantigen was used as a positive control. With the exception of whole-blood intracellular staining, which was a single-cell assay, all the others were bulk assays based on the secretion of IFN-γ or cell proliferation. The assays also presented differences in the times of culture: 7 h for whole-blood intracellular staining, 24 h for the Q-TB and Q-TB in-tube assays, 48 h for the PBMC-cytokine assay, and 5 days for the diluted-whole-blood assays. Six days of culture were needed to measure proliferation in the PBMC-proliferation assay.
Q-TB and Q-TB in-tube assays.
The Q-TB and Q-TB in-tube assays were produced by Cellestis and are currently increasingly favored for use for the diagnosis of M. tuberculosis infection. The Q-TB assay was approved for use in the United States by the Food and Drug Administration (FDA) in December 2004 (26). A simplified and more specific variant, the Q-TB in-tube test, has recently been developed and was approved for use by FDA in October 2007. Both of these assays use whole blood (0.5 ml/sample in the Q-TB assay and 1 ml/sample in the Q-TB in-tube assay) as the biological specimen, and the tests were performed according to the manufacturer's instructions. Briefly, after 24 h of whole-blood stimulation with the appropriate antigen, the plasma was collected and stored at −20°C. IFN-γ was then measured by a specific enzyme-linked immunosorbent assay (ELISA; Cellestis). In the Q-TB in-tube assay, the stimulation by the antigenic mixture occurs within the tube used to collect the blood. The Q-TB assay was performed with overlapping ESAT-6 and CFP-10 peptides of the entire sequence of the proteins obtained from Cellestis and the entire ESAT-6 and CFP-10 proteins obtained from Lionex.
Data are expressed as IU/ml of IFN-γ released in response to M. tuberculosis-derived antigens by subtracting the background value for the unstimulated cultures or as positive responses. A response was considered positive when the levels of IFN-γ to either ESAT-6 or CFP-10 were above the cutoff. The same was true in the case of the Q-TB in-tube assay, but here it was not possible to distinguish to which antigen the response was positive. The cutoffs for positive responses were indicated by the manufacturer and are in accordance with that determined by receiver operating characteristic (ROC) curve analysis for each antigen, as described below (see also Fig. 1).
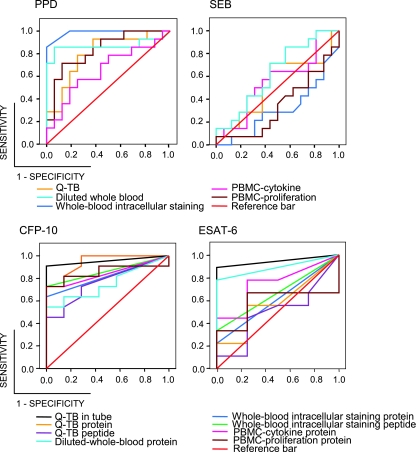
FIG. 1.
ROC curves of IGAs for discriminating patients with active tuberculosis and healthy controls upon stimulation with the PPD antigen, the SEB superantigen, or the CFP-10 or ESAT-6 M. tuberculosis-specific antigens, as indicated. ROC curves were generated for the indicated IGAs for each antigen/superantigen. The number of active tuberculosis patients and controls tested for each IGA is reported in footnote b of Table 2. The appropriate cutoff point was defined to maximize the diagnostic accuracy for active tuberculosis. The calculated cutoff values are reported in Table 2. M. tuberculosis-specific antigens and PPD clearly discriminated between patients (sensitivity) and controls (1 − specificity), while, as expected, the SEB superantigen did not. It also appears to be clear that the best-performing IGAs were the whole-blood intracellular staining assay when PPD was taken into consideration and the Q-TB in-tube assay and the diluted-whole-blood protein assay when M. tuberculosis-specific antigens were considered.
Diluted-whole-blood assay.
The diluted-whole-blood assay was developed in our laboratory and uses diluted whole blood as a biological specimen and an extended culture period of 5 days. Blood was diluted 1/10 in RPMI 1640 (ICN-Flow, Aurora, OH) supplemented with 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (all from HyClone Laboratories, Logan, UT), divided into 0.5-ml aliquots, placed into 5-ml round-bottom polystyrene test tubes, stimulated with the appropriate antigen, and incubated at 37°C in a 5% CO2 humidified atmosphere for 5 days. The samples were then centrifuged, and the supernatants were collected and stored at −20°C until the IFN-γ level was measured by a specific ELISA (Cellestis). Data are expressed as described above for the Q-TB assays. The cutoff for a positive response for each antigen was calculated by using the ROC curve analysis, as described below (see also Fig. 1).
Whole-blood intracellular staining assay.
The whole-blood intracellular staining method visualizes cytokines in antigen-activated T cells at the single-cell level and is based on a short stimulation, the inhibition of secretion, fixation, permeabilization of the cells, and visualization of the intracellular cytokines with specific fluorochrome-labeled antibodies (8, 13). This assay is, in general, more informative than bulk assays because it identifies the effector cell involved in the response (21). Specifically, blood was divided into 300-μl aliquots and placed in 15-ml Falcon polypropylene conical tubes. Costimulatory molecules (monoclonal antibodies [MAbs] CD28 and CD49d [1 μg/ml; BD Biosciences]) and the appropriate stimuli (PPD, SEB, ESAT-6, and CFP-10, each of which was used at 0.2 μg/ml) were added. The tubes were incubated at 37°C in a 5% CO2 atmosphere for 7 h. Brefeldin A (10 μg/ml) was added for the last 5 h of incubation to block cytokine secretion. Lysis of the red blood cells was carried out by incubating the samples in fluorescent-activated cell sorter lysing solution (BD Biosciences) (10 times the blood volume) for 10 min at room temperature (RT). The samples were washed twice in phosphate-buffered saline (PBS) and fixed in 2 ml formalin (2%) for 15 min at RT. PBS-bovine serum albumin (BSA; 0.5%)-NaN3 (0.02%) was then added, and the samples were centrifuged. The cells were stained with anti-human MAbs (extracellular markers [CD3, CD4, CD69] and intracellular IFN-γ; BD Biosciences) and diluted in 5% saponin. The samples were then incubated in the darkness for 15 min at RT. The cells were then washed in PBS-BSA (0.5%)-NaN3 (0.02%), centrifuged, and resuspended in 250 μl of PBS-BSA (0.5%)-NaN3 (0.02%) prior to flow cytometric analysis. Isotype-matched antibodies were used as a negative control. The samples were analyzed with a BD FACSCanto cytometer and BD FACSDiva software (BD Biosciences). A total of 2 × 104 events in the gate for each sample were acquired. Fluorescence data are reported as the percentage of IFN-γ-positive cells determined by using the isotype-matched control MAb or as a positive response. The cutoff for a positive response for each antigen was calculated by using the ROC curve analysis, as described below and as shown in Fig. 1.
PBMC-proliferation and PBMC-cytokine assays.
PBMCs were purified from peripheral blood after separation on a Ficoll gradient (lympholyte H; Cedarlane), as described previously (4). The PBMCs were cultured at 1 × 106/ml in complete medium (RPMI 1640 [ICN-Flow]) supplemented with 5% human serum (Blutspendezentrum, Universitatsspital Basel, Basel, Switzerland), 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 2 mM l-glutamine, 25 mM HEPES, 100 U/ml of penicillin, and 100 μg/ml of streptomycin (all from HyClone), as well as with 0.05 mM 2-mercaptoethanol (Sigma) and PPD, ESAT-6, and CFP-10. The PBMCs were cultured in flat-bottom 96-well plates (Costar; Corning Life Sciences, Lowell, MA) in triplicate. The plates were tested for cell proliferation on day 6 (4). DNA synthesis was evaluated by using [3H]thymidine (Amersham Biosciences, United Kingdom) at 0.5 μCi/well, and incorporation was measured after the last 16 h of culture. The data are shown as the stimulation index (SI; i.e., the counts per minute of the antigen-stimulated cultures divided by the background value for unstimulated cultures) in the PBMC-proliferation assay or as a positive response. In parallel cultures, for the PBMC-cytokine assay, supernatants were collected for IFN-γ detection by ELISA after 48 h (4). The data are expressed as described above for the Q-TB assays. The cutoff for positive responses for each antigen and for each test were calculated by using the ROC curve analysis, as described below (see also Fig. 1).
Statistical analysis.
All data were recorded in a computerized database. The results are reported as the means ± standard errors of the means (SEMs). Statistical analyses were carried out with SPSS (version 13.0; SPSS Inc., Chicago, IL) or Stata (version 8.2; Stata Corp., College Station, TX) software.
For comparison of the overall differences in the results between the active tuberculosis patient group and the control group obtained by each assay, the Mann-Whitney U test for multiple pairwise comparisons was performed. The differences within groups obtained by the different assays were evaluated by the Wilcoxon paired-sample test. P values less than 0.05 were considered to indicate statistical significance, and all reported P values are two sided.
To validate the Cellestis and Lionex antigens and to compare the performance characteristics of the assays selected for use in this study with respect to the performance characteristics of the commercially available Q-TB in-tube assay, a linear regression model was applied and the Pearson correlation coefficient was calculated.
ROC curves were generated for each assay to determine the cutoff point for each antigen. Figure 1 shows the curves obtained by using the IGA and PBMC-proliferation assay results for the active tuberculosis patients and the healthy controls.
The number of patients and controls tested by each IGA are reported in Table 2. The appropriate cutoff point was defined to maximize the diagnostic accuracy for active tuberculosis. The cutoff values for each IGA are reported in footnote b of Table 2.
To assess the agreement between the clinical and the microbiological diagnoses and each different assay, the κ statistics measure was used. This statistic provides values of +1 (perfect agreement) to −1 (complete disagreement) via 0 (no agreement above that expected by chance).
As an indicator of the accuracy of each assay, the diagnostic odds ratio and the 95% confidence interval (CI) were calculated. This diagnostic odds ratio defines the odds of positivity for disease relative to the odds of positivity for nondisease (15), and the high values of a diagnostic odds ratio indicate better discriminatory test performance. A value of 1 means that the test does not discriminate between the controls and the active tuberculosis patients.
RESULTS
Peripheral blood from patients was tested by the four whole-blood assays (the Q-TB and Q-TB in-tube assay, the diluted-whole-blood assay, and the whole-blood intracellular staining assay) and the two PBMC assays (the PBMC-proliferation and PBMC-cytokine assays). The patients were classified according to clinical criteria as having active tuberculosis. The details of the clinical diagnoses and the ages of the subjects are reported in Table 1. Healthy age- and sex-matched individuals and five BCG-vaccinated individuals served as the control groups (Table 1). To allow a ready comparison of each patient across the range of tests performed and rapid interpretation of the data, Table 2 lists the 20 active tuberculosis patients and 24 healthy individuals tested by the diagnostic assays performed and, for each assay, the positive or negative response to each antigen preparation tested (PPD, peptides, and proteins).
Aliquots of blood samples and PBMCs obtained from each subject under study and tested by all methods used in the study were stimulated with SEB to assess the immune competence of the subjects. All samples, regardless of the subject source, generated high levels of IFN-γ or lymphocyte proliferation upon SEB stimulation, and the actual responses did not differ in strength between the active tuberculosis patients and the controls, as was also evident from the ROC curves shown in Fig. 1.
Diagnostic performance of the different assays in response to PPD.
As summarized in Table 2, in order to compare the performance characteristics of the different assays, whole blood and PBMCs isolated from the active tuberculosis patients and the controls (Table 2) were cultured in the presence of PPD and IFN-γ or cell proliferation was evaluated. Higher levels of IFN-γ were produced in patients than in controls, independently of the assay used. Only in the PBMC-proliferation assay, PPD activation was unable to distinguish the infected individuals from the control individuals (Table 3). As expected, BCG-vaccinated individuals presented a response not dissimilar from that of the active tuberculosis patients. Only the whole-blood intracellular staining assay was able to discriminate between the infected and the vaccinated categories of subjects when PPD was used as the antigen, in accordance with data from Cosmi and colleagues (8).
TABLE 3.
IFN-γ or PBMC proliferation induced in response to PPDa
| Subject condition | Mean assay result ± SEM (no. of subjects)
|
||||
|---|---|---|---|---|---|
| Q-TB (IU/ml) | Diluted whole blood (IU/ml) | Whole-blood intracellular staining (% positive cells) | PBMC-cytokine (IU/ml) | PBMC-proliferation (SI) | |
| Active tuberculosis | 40.2 ± 11.5b (20) | 71.4 ± 16.4c (20) | 1.3 ± 0.4d,f (20) | 56.6 ± 22.0e (17) | 164.3 ± 50.5 (17) |
| BCG unvaccinated | 6.4 ± 2.6 (19) | 3.1 ± 1.2 (19) | 0.07 ± 0.02 (18) | 8.8 ± 7.4g (17) | 91.3 ± 25.8 (18) |
| BCG vaccinated | 17.9 ± 11.9 (4) | 33.7 ± 22.8 (3) | 0.2 ± 0.1 (5) | 54.7 ± 30.6 (2) | 200.1 ± 61.5 (4) |
IFN-γ was measured by ELISA and is expressed in IU/ml or was measured by intracellular staining and is expressed as the percentage of positive cells. PBMC proliferation was measured by [3H]thymidine incorporation and is expressed as the SI. The differences between the active tuberculosis patients, unvaccinated individuals, and vaccinated individuals were assessed by the Mann-Whitney U Test.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
P = 0.001 compared with the results for the BCG-unvaccinated controls.
P = 0.008 compared with the results for the BCG-vaccinated controls.
P = 0.046 compared with the results for the BCG-vaccinated controls.
Table 4 shows the results of the statistical analysis for each assay. Even if the Q-TB assay was able to discriminate between the patients with active tuberculosis and the healthy subjects, this test had the lowest sensitivity (70%) and specificity (79%) among the whole-blood assays. The agreement with the clinical diagnosis was moderate (κ = 0.49 ± 0.14; P = 0.002), and the diagnostic capacity (diagnostic odds ratio) of this test was equal to 9.
TABLE 4.
Diagnostic performance characteristics of the different assays in response to PPD antigenic stimulationa
| Assay (cutoff) | Sensitivity
|
Specificity
|
Diagnostic odds ratio (95% CI) | κ-statistic measure ± SE (P value) | ||
|---|---|---|---|---|---|---|
| % (95% CI) | No. of positive responders/total no. of subjects | % (95% CI) | No. of nonresponders/total no. of subjects | |||
| Q-TB (9.0 IU/ml) | 70 (55-80) | 14/20 | 79 (63-90) | 15/19 | 9 (2.1-36.3) | 0.49 ± 0.14 (0.002) |
| Diluted whole blood (9.8 IU/ml) | 85 (72-89) | 17/20 | 95 (81-99) | 18/19 | 102 (11.4-808.0) | 0.80 ± 0.09 (<0.001) |
| Whole-blood intracellular staining (0.15% IFN-γ-positive cells) | 100 (89-100) | 20/20 | 83 (71-83) | 15/18 | Infinity (19.5-infinity) | 0.84 ± 0.09 (<0.001) |
| PBMC-cytokine (4.2 IU/ml) | 71 (53-81) | 10/14 | 88 (73-96) | 15/17 | 19 (3.1-109.4) | 0.60 ± 0.14 (0.001) |
| PBMC-proliferation (49.0 SI) | 65 (48-80) | 11/17 | 50 (34-64) | 9/18 | 2 (0.5-6.9) | 0.15 ± 0.10 (0.380) |
IFN-γ was measured by ELISA and is expressed in IU/ml or was measured by intracellular staining and is expressed as the percentage of positive cells; PBMC proliferation was measured by [3H]thymidine incorporation and is expressed as the SI (shown in Table 3).
The diluted-whole-blood assay was able to discriminate between the patients with active tuberculosis and the controls; indeed, 17 of 20 patients were positive (sensitivity, 85%) and 18 of 19 control subjects were negative (specificity, 95%); the κ measure was good (κ = 0.80 ± 0.09; P < 0.001), and the diagnostic odds ratio value was equal to 102.
The whole-blood intracellular staining test yielded the highest sensitivity, in that all active tuberculosis patients were positive (sensitivity, 100%). However, the specificity of this test was lower than that of the diluted-whole-blood assay, since 3 of 18 individuals with no history or evidence of M. tuberculosis infection or exposure responded to PPD. Overall, the agreement with the clinical diagnosis was very good (κ = 0.84 ± 0.09; P < 0.001), and this assay had an extremely high diagnostic odds ratio for the diagnosis of active tuberculosis.
Concerning the PBMC in vitro assays, the results of the PBMC-cytokine assay were similar to those of the Q-TB assay, while in the PBMC-proliferation assay, only 65% (11/17) of the active tuberculosis patients and 50% (9/18) of the controls responded to this antigen. The agreement of the results of these assays with the clinical diagnosis was the lowest in terms of the κ measure (κ = 0.15 ± 0.1; P = 0.38), and the diagnostic odds ratio value was equal to 2.
In conclusion, these data indicate that the response to PPD in all tests except the PBMC-proliferation assay is associated with active tuberculosis and that the results of the in-house whole-blood intracellular staining assay and the diluted-whole-blood assay correlated with the clinical diagnosis. In accordance with the findings of other studies (reviewed in reference 30), our data confirm that PPD is not able to discriminate between active tuberculosis patients and BCG-vaccinated individuals, with the interesting exception of when PPD is used in the in-house whole-blood intracellular staining assay.
CFP-10 and ESAT-6: use of peptides versus entire proteins.
A more accurate diagnosis of individuals who are LTBI on the basis of the specific responses to the ESAT-6 and CFP-10 antigens has been reported (6, 30, 32). These proteins, encoded by genes located within the RD1 region of the M. tuberculosis genome, are more specific than PPD because they are not shared with BCG strains or most environmental mycobacteria. However, in the CFP-10 and ESAT-6 peptide preparation from Cellestis, thimerosal is present as a preservative. This compound induces apoptotic changes in the membranes of almost all living cells, even at relatively low concentrations (38). Serum proteins in assays with whole blood somehow neutralize the toxic activity. In our hands, the antigens from Cellestis displayed toxicity in diluted whole blood and in isolated PBMC cultures, and it was impossible to use the same antigen preparation in all the assays performed in this study. Therefore, we were obliged to validate the performance characteristics of antigens obtained from two different suppliers. Thus, CFP-10 and ESAT-6 overlapping peptides (Cellestis) and entire proteins (Lionex) were compared for their abilities to elicit specific responses in whole-blood assays, i.e., the Q-TB assay and the whole-blood intracellular staining assay, in active tuberculosis patients and controls.
Concerning the Q-TB assay, entire CFP-10 proteins showed a higher sensitivity and recognized 12 of 14 active tuberculosis patients. The proteins were also found to have a high sensitivity when the data were analyzed with respect to ESAT-6 (10/14 patients) (Table 5). The results obtained by each tested clearly indicate that, with the exception of two active tuberculosis patients, all others showed lower levels of IFN-γ release when the overlapping peptides were used (data not shown).
TABLE 5.
Comparison of IFN-γ production obtained with CFP-10 and ESAT-6 overlapping peptides versus that obtained with entire proteins by Q-TB assay and whole-blood intracellular staining assay
| Subject condition | IFN-γ (IU/ml [mean ± SEM]) by Q-TB assaya
|
% Cells positive for IFN-γ (mean ± SEM) by whole-blood intracellular staining assayb
|
||||||
|---|---|---|---|---|---|---|---|---|
| CFP-10 peptides | CFP-10 proteins | ESAT-6 peptides | ESAT-6 proteins | CFP-10 peptides | CFP-10 proteins | ESAT-6 peptides | ESAT-6 proteins | |
| Active tuberculosis | 7.6 ± 5.6c (6/14)d | 2.6 ± 0.9e (12/14) | 0.4 ± 0.2 (3/14) | 0.5 ± 0.2f (10/14) | 0.20 ± 0.06g (12/17) | 0.14 ± 0.04h (10/17) | 0.09 ± 0.04i (8/17) | 0.05 ± 0.03 (4/17) |
| BCG unvaccinated | 0.02 ± 0.01 (8/8)j | 0.05 ± 0.03 (7/8) | 0.10 ± 0.05 (7/8) | 0.04 ± 0.04 (7/8) | 0.01 ± 0.01 (12/13) | 0.01 ± 0.01 (12/13) | 0.00 ± 0.00 (13/13) | 0.02 ± 0.01 (12/13) |
IFN-γ levels were measured by ELISA. The differences between the two groups of subjects were assessed by the Mann-Whitney U test. The differences between the antigens in the same group of subjects were assessed by the Wilcoxon paired-sample test.
IFN-γ was measured by intracellular staining, as detailed in the Materials and Methods section. The differences between the two groups of subjects were assessed by the Mann-Whitney U test. The differences between the antigens in the same group of subjects were assessed by Wilcoxon paired-sample test.
P = 0.008 compared with the results for the BCG-unvaccinated controls.
For the active tuberculosis patients the values in parentheses represent the number of subjects with a positive response/total number of subjects.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
P = 0.018 compared with the results for the BCG-unvaccinated controls.
P = 0.001 compared with the results for the BCG-unvaccinated controls.
P = 0.004 compared with the results for the BCG-unvaccinated controls.
P = 0.005 compared with the results for the BCG-unvaccinated controls.
For the BCG-unvaccinated subjects the values in parentheses represent the number of subjects with a negative response/total number of subjects.
In the whole-blood intracellular staining assay, the differences in the amounts of IFN-γ induced by the CFP-10 and ESAT-6 peptides and proteins antigens were less evident, both as protein levels and in terms of sensitivity and specificity (Table 5). This point is statistically demonstrated by the regression analysis, and in fact, a good concordance was shown when the CFP-10 antigen in the two forms was taken into account (Pearson coefficient R = 0.796; P < 0.001), indicating that in the whole-blood intracellular staining assay, the use of overlapping peptides and entire proteins gives almost equivalent results in terms of IFN-γ-positive cells.
The two antigenic preparations showed equivalent negative results for the BCG-unvaccinated controls (Table 5).
Diagnostic performance of the different assays in response to M. tuberculosis-specific antigens from genes located within RD segments.
In order to compare the performance characteristics of the different assays for responsiveness to the M. tuberculosis-specific antigens CFP-10 and ESAT-6 in two forms (overlapping peptides and entire proteins), whole blood and isolated PBMCs were cultured in the presence of the antigens, and the level of IFN-γ production or cell proliferation was evaluated, as summarized in Table 2. Table 6 indicates that higher levels of IFN-γ and cell proliferation were detected in patients with active tuberculosis than in BCG-unvaccinated healthy controls, and these differences were statistically significant for all assays.
TABLE 6.
IFN-γ or PBMC proliferation induced in response to M. tuberculosis-specific antigensa
| Subject condition | Mean assay result ± SEM (no. of subjects)
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Q-TB in-tube (IU/ml) | Q-TB(IU/ml)
|
Diluted whole blood with proteins (IU/ml) | Whole-blood intracellular staining (% IFN-γ-positive cells)
|
PBMC-cytokine with proteins (IU/ml) | PBMC-proliferation with proteins (SI) | |||
| Peptides | Proteins | Proteins | Peptides | |||||
| Active tuberculosis | 108.8 ± 91b (17) | 24.1 ± 16.3c (18) | 2.3 ± 0.8d (17) | 30.0 ± 24.1e (17) | 0.1 ± 0.04f (17) | 0.3 ± 0.1g (18) | 10.8 ± 4.8h (11) | 35.1 ± 12.3i (15) |
| BCG unvaccinated | 0.1 ± 0.03 (13) | 0.02 ± 0.01 (13) | 0.08 ± 0.04 (10) | 0.2 ± 0.1 (13) | 0.01 ± 0.01 (13) | 0.01 ± 0.01 (14) | 0.02 ± 0.01 (11) | 1.4 ± 0.21 (12) |
The proteins were ESAT-6 and CFP-10, and the peptides were ESAT-6 and CFP-10 overlapping peptides. IFN-γ was measured by ELISA and is expressed as IU/ml or was measured by intracellular staining and is expressed as the percentage of positive cells. Lymphocyte proliferation was measured by [3H]thymidine incorporation and is expressed as the SI. The differences between the two groups of subjects were assessed by the Mann-Whitney U test.
P = 0.009 compared with the results for the BCG-unvaccinated controls.
P = 0.001 compared with the results for the BCG-unvaccinated controls.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
P = 0.007 compared with the results for the BCG-unvaccinated controls.
P = 0.004 compared with the results for the BCG-unvaccinated controls.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
P = 0.006 compared with the results for the BCG-unvaccinated controls.
P < 0.001 compared with the results for the BCG-unvaccinated controls.
Table 7 shows statistical analysis estimates for each assay. The Q-TB in-tube assay was the best performer in terms of specificity (100%) and sensitivity (82%; i.e., 14/17 subjects). The agreement with the clinical diagnosis was very good (κ = 0.80 ± 0.10; P < 0.001), and the diagnostic odds ratio value was extremely high. According to these data, the Q-TB in-tube assay had the highest diagnostic odds ratio for the diagnosis of active tuberculosis in whole-blood assays.
TABLE 7.
Diagnostic performance of the different assays in response to M. tuberculosis-specific antigenic stimulationa
| Assay (cutoff) | Sensitivity
|
Specificity
|
Diagnostic odds ratio (95% CI) | κ statistic measure ± SE (P value) | ||
|---|---|---|---|---|---|---|
| % (95% CI) | No. of positive responders/total no. of subjects | % (95% CI) | No. of negative responders/total no. of subjects | |||
| Q-TB in tube (0.35 IU/ml) | 82 (72-82) | 14/17 | 100 (57-100) | 13/13 | Infinity (3.5-infinity) | 0.80 ± 0.10 (<0.001) |
| Q-TB, peptides (CFP-10, 0.35 IU/ml; ESAT-6, 0.35 IU/ml) | 50 (36-54) | 9/18 | 92 (73-99) | 12/13 | 12 (1.6-83.9) | 0.39 ± 0.14 (0.013) |
| Q-TB, proteins (CFP-10, 0.2 IU/ml; ESAT-6, 0.08 IU/ml) | 94 (81-99) | 16/17 | 80 (58-88) | 8/10 | 64 (16.0-615.6) | 0.76 ± 0.13 (<0.001) |
| Diluted whole blood, proteins (CFP-10, 0.35 IU/ml; ESAT-6, 0.05 IU/ml) | 94 (80-99) | 16/17 | 85 (67-91) | 11/13 | 88 (8.4-829) | 0.80 ± 0.12 (<0.001) |
| Whole-blood intracellular staining, proteins (CFP-10, 0.05% IFN-γ-positive cells; ESAT-6, 0.05%-γ-positive cells) | 65 (50-70) | 11/17 | 92 (73-99) | 12/13 | 22 (2.8-158.3) | 0.55 ± 0.14 (0.002) |
| Whole-blood intracellular staining, peptides (CFP-10, 0.05% IFN-γ-positive cells; ESAT-6, 0.05% IFN-γ-positive cells) | 78 (64-82) | 14/18 | 93 (75-99) | 13/14 | 46 (5.4-343.8) | 0.69 ± 0.13 (<0.001) |
| PBMC-cytokine, proteins (CFP-10, 0.15 IU/ml; ESAT-6, 0.15 IU/ml) | 82 (62-93) | 9/11 | 82 (62-93) | 9/11 | 20 (2.5-160.9) | 0.64 ± 0.14 (<0.001) |
| PBMC-proliferation, proteins (CFP-10, 3.00 SI; ESAT-6, 3.00 SI) | 87 (71-95) | 13/15 | 83 (64-93) | 10/12 | 33 (4.2-249.2) | 0.70 ± 0.1 (0.001) |
The proteins were ESAT-6 and CFP-10, and the peptides were ESAT-6 and CFP-10 overlapping peptides. The results from the best performer between CFP-10 and ESAT-6 in each subject tested were used for the calculation.
The Q-TB assay performed with overlapping peptides presented a high specificity (92%), but its sensitivity was very low (50%); indeed, it had the lowest sensitivity results among all the assays. The agreement with the clinical diagnosis was fair (κ = 0.39 ± 0.14; P = 0.013), and the diagnostic odds ratio was equal to 12. However, when the Q-TB assay was carried out with the entire proteins, the sensitivity increased to 94%, which was even higher than that of the Q-TB in-tube assay. The agreement was good (κ = 0.76 ± 0.13; P < 0.001), and the diagnostic odds ratio was equal to 64.
The agreement of the results of the diluted-whole-blood assay with the clinical presentation was also very good, as 16 of 17 active tuberculosis patients were positive (sensitivity, 94%), while its specificity was quite high, being 85%, and it recognized 11 of 13 control individuals as negative. The κ measure was very good (κ = 0.80 ± 0.12; P < 0.001), and the diagnostic odds ratio was equal to 88.
The whole-blood intracellular staining test with entire CFP-10 and ESAT-6 proteins gave quite a low sensitivity: only 11/17 patients classified as affected by active tuberculosis were positive (sensitivity, 65%). The specificity was high; only 1/13 controls responded to CFP-10 or ESAT-6 (specificity, 92%). Overall, the agreement with the clinical diagnosis was good (κ = 0.55 ± 0.14; P = 0.002). This assay had a diagnostic odds ratio equal to 22. However, when the whole-blood intracellular staining assay was carried out with the overlapping peptides, even if the differences observed were not statistically significant, the sensitivity increased to 78%, and its specificity did not change, being 93%. The agreement was good (κ = 0.69 ± 0.13; P < 0.001), and the diagnostic odds ratio was equal to 46.
Concerning the PBMC in vitro assays, the performance was very high. The PBMC-cytokine and PBMC-proliferation assays presented results that were similar to or even better than those of the Q-TB in-tube assay. The sensitivity was equal to or greater than 82% for both assays, and the specificity was good and was equal to 82% for the PBMC-cytokine assay. Considering the PBMC-cytokine assay, overall, the agreement of the results with the clinical diagnosis was good (κ = 0.64 ± 0.16; P < 0.001), and the diagnostic odds ratio for the active tuberculosis patients was equal to 20.
In an effort to analyze the concordance of the assays used in this study, we performed regression analysis by taking as the provisional standard the best commercial assay, the Q-TB in-tube assay. Indeed, the only significant regression analysis was obtained when the Q-TB in-tube assay and the diluted-whole-blood assay were compared (Pearson coefficient R = 0.565; P = 0.009); however, the numeric correlation was quite low. Similar results were obtained when the Q-TB in-tube assay was compared with the PBMC-cytokine assay (Pearson coefficient R = 0.702; P = 0.008).
In conclusion, these data indicate that the response to RD antigens in all tests is associated with active tuberculosis and that the Q-TB in-tube assay and the diluted-whole-blood assay results present better diagnostic odds ratio values.
Comparison of TST and IGA.
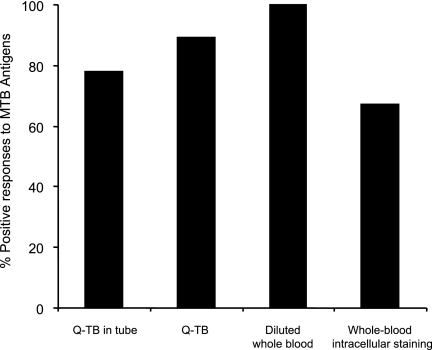
TST was used to test 12 of 20 active tuberculosis patients, and the result was positive for 9. The three TST-negative patients had positive responses by both the in-house and the commercial IGAs. Figure 2 summarizes the concordance of the results obtained by the IGAs when RD antigens were used for the nine TST-positive active tuberculosis patients. Indeed, the best concordance was reached with the diluted-whole-blood assay.
FIG. 2.
Comparison of TST and IGA in active tuberculosis patients. Whole blood from nine TST-positive patients with active tuberculosis was analyzed by the indicated IGAs. Data are reported by taking into account the best performer of the two antigenic formulations (peptides versus proteins). Thus, the results for the proteins from Lionex were reported for the Q-TB assay, while those for the peptides from Cellestis were reported in the whole-blood intracellular staining assay. Bars indicate the percentage of positive responses in active tuberculosis patients by the indicated assay. The cutoffs for positive responses are indicated in footnote b of Table 2.
TST was used to test only 8 of 24 healthy controls, and the results were negative. The concordance of the results of TST with those of the IGAs, when it was determined, was complete (Table 2).
DISCUSSION
The aim of our study was to identify an assay for T cells in whole blood for use in a program of surveillance for individuals who are LTBI. To achieve this goal we developed in-house assays and compared them with commercial tests performed with whole blood. The assays have different requirements for the blood samples used, different throughputs, different complexities, and different abilities to identify the mechanism involved in the immune response in patients with M. tuberculosis infection. Therefore, we compared bulk IGAs with different abilities to activate memory cells (24) with short (24 h) and long (5 to 6 days) culture times and a single-cell assay that allowed the characterization of the specific T cell producing the IFN-γ.
At this stage it is important to evaluate the degree of concordance between the different tests and whether they deliver similar information. The final stage will be to select an assay that may be used in large-scale studies for the diagnosis of M. tuberculosis infection in individuals who are LTBI and that is also suitable for identifying the correlates or surrogates of protection in vaccinated subjects.
No gold standard exists to define subjects who are LTBI; thus, the sensitivities of our assays were determined with samples from active tuberculosis patients, and the specificities were determined with samples from healthy subjects with a low risk of being LTBI. A caveat here is that this choice of study population is not totally satisfactory, as the cell-mediated immune response is often partially compromised in active tuberculosis patients, particularly at the time of diagnosis, or in patients with more advanced disease (17, 36). Overall, one would expect a reduced performance of the IGAs and, therefore, an underestimation of sensitivity because the cell-mediated immunity measured in the assays described here may have failed to some extent in some subjects. Nevertheless, all patients analyzed were fully responsive to superantigenic SEB activation, suggesting that the degree of immunocompromise was minimal, if any, thus indirectly justifying our approach.
The other limitation of this study resides in the low number of subjects studied in each category. Thus, the estimates for sensitivity and specificity might not be fully representative of those that would be obtained with larger populations. Yet, with all this accounted for, our study provides useful information on the diagnostic quality of different assays for the detection of M. tuberculosis infection.
When PPD was used, all IGAs discriminated between the active tuberculosis patients and the healthy subjects, while only the PBMC-proliferation assay was unable to discriminate between them. A possible explanation could reside in the high sensitivity of this assay and, presumably, in its capacity to detect exposure to environmental mycobacteria expressing PPD or a PPD-like antigen. In addition, PBMC proliferation in response to PPD was measured after 7 days of culture; thus, this assay could also detect the expansion of central memory T cells and amplify low responses not otherwise detectable. This interpretation is in accordance with data showing a higher sensitivity of prolonged assays compared with that of short assays (24). As expected, the few BCG-vaccinated subjects tested here were all responsive when PPD was used as the antigen, confirming the strong limitation of the use of PPD for the diagnosis of M. tuberculosis infection in these subjects.
The results obtained by the whole-blood intracellular staining assay with PPD were of interest. This assay was able to identify as positive all the active tuberculosis patients, only 3 of 19 control subjects, and 1 of 5 BCG-vaccinated subjects. It thus appears that because this assay is able to detect only a very intense response, the low response induced by infection due to environmental mycobacteria or by a previous BCG vaccination does not influence the ability of this assay to detect M. tuberculosis. All of these findings confirm previous results (8). Obviously, the possibility of using PPD in the whole-blood intracellular staining assay to identify active tuberculosis and, prospectively, subjects who are LTBI should be validated with an expanded population.
When M. tuberculosis antigens obtained from RD regions were used, an overall agreement between the in-house assays (with diluted whole blood and purified PBMCs) and the commercial bulk assays (the Q-TB and Q-TB in-tube assays) was obtained, confirming the suitability of the use of whole blood as a source of biological material with which adjunctive assays for identifying active tuberculosis may be performed.
The superior sensitivity of the Q-TB in-tube assay for clinical diagnosis could be the consequence of the presence of TB7.7, an M. tuberculosis-specific protein (6). In previous studies, the Q-TB in-tube assay did not always show a better performance than the Q-TB assay (reviewed in reference 27), although in a recent study Detjen and colleagues noted the very high level of performance of this test (9).
Surprising results were obtained with the Q-TB assay; in particular, its low sensitivity was noteworthy. It is not easy to explain why, in our hands, this assay, widely used as an adjunctive test for the diagnosis of M. tuberculosis infection, yielded such a low sensitivity. Menzies and coworkers (27), in their meta-analyses, estimated a pooled sensitivity of 80%, with values ranging from 64% to 100%, depending on the specific study. In our case the limited number of subjects analyzed might have influenced the estimate, suggesting again that a larger population should be studied (10, 27). However, when the Q-TB assay was performed with entire RD1 proteins rather than the peptides, a great improvement in sensitivity was reached. The main difference between the two antigen preparations, besides their structures (overlapping peptides versus entire proteins), is the presence of thimerosal as a preservative in the Cellestis preparation; but it is not clear how the presence of this preservative could influence differently the release of specific IFN-γ levels in this assay but not, for instance, in the whole-blood intracellular staining assay. Differences due to the protein structures of the two antigenic preparations might also be important in the response elicited.
The differences in the sensitivities of the Q-TB assay when it was performed with antigens in the two chemical forms raise general concerns on the interpretation of Q-TB assay results and suggest that more caution be used in the interpretation of IGA results. More than a single assay, including IGAs and TST, should probably be performed to define the M. tuberculosis infection status of a given subject (22).
The in-house bulk assay performed with diluted whole blood yielded an excellent capacity to distinguish between active tuberculosis patients and healthy controls. This assay uses only 1/10 to 1/20 of the blood customarily used in commercial assays; thus, once it is validated in a study with a larger population, this assay could be particularly useful in pediatric studies.
Although bulk assays with purified PBMCs presented high sensitivity and specificity values, they are quite cumbersome to use and require a purification step and also, in the case of the PBMC-proliferation assay, a radioactive tracer; thus, these assays are not particularly suited for use in studies with large populations.
The performance of the in-house single-cell assay (the whole-blood intracellular staining assay) is of interest because it might be able to identify antigen-specific memory T cells for M. tuberculosis. These are heterogeneous cell populations which have been divided into at least two different subsets, defined as central memory and effector memory cells, on the basis of the differential expression of homing receptors (19, 23, 34). Altogether, several reports indicate that the pool of effector cells is expanded during active microbial replication, whereas only memory cells remain detectable after control or eradication of the infectious agents (17, 33).
The analysis of the memory response could be useful for better mapping of the state of M. tuberculosis infection in a given individual. Indeed, Goletti and colleagues (17) analyzed the T effector and central memory cells responding to RD1 antigens in groups of patients with different states of active tuberculosis. The results indicated that the response to the peptides selected from RD1 was mediated by effector memory cells and was significantly associated with patients with mild active tuberculosis. Moreover, in patients with severe active tuberculosis, the absence of both effector and central memory responses to the RD1 peptides was associated with the poor control of the active M. tuberculosis infection, suggesting a protective role of the T effector memory cells (17). By consideration of these results, it is important to improve the performance of this whole-blood intracellular assay by adding, for instance, the TB7.7 antigen to increase its sensitivity.
When the differences observed in this study are compared, it is important to stress that each of the different assays may highlight a different aspect of the M. tuberculosis-specific response. The whole-blood intracellular staining assay and the commercially available Q-TB and Q-TB in-tube assays are short-term culture assays, performed within the first 24 h after blood stimulation, while the diluted-whole-blood and PBMC-proliferation assays can be considered long-term culture assays, since they require 5 or more days. In a recent study, Leyten and coworkers (24) have compared the performance characteristics of TST with those of a panel of IGAs and found a better concordance between TST and IGA cultures that lasted 6 days. Those authors hypothesize that a short-term culture IGA identifies T effector memory cells producing IFN-γ, thus mainly detecting recent or ongoing infection. A prolonged-culture IGA also expands IFN-γ-secreting central-memory T cells and is thus more sensitive for the diagnosis of past latent infections (24). This interpretation is supported by data obtained from patients with hepatitis C virus infection, showing that the depletion of CCR7-positive central-memory T cells affects the antigen-specific response in prolonged but not short-term cultures (16). Here we were able to compare TST and IGAs using only 12 patients with ongoing infections. The results of the diluted-whole-blood assay, which requires prolonged cultures, showed the best concordance with those of TST, giving support in part to the hypothesis of Leyten et al. (24).
In conclusion, in a pilot study we identified two in-house IGAs (the diluted-whole-blood assay and whole-blood intracellular staining assay) and one commercial IGA (the Q-TB in-tube assay) that are worth using with large populations with known exposures to M. tuberculosis. Their predictive values in assessing individuals' M. tuberculosis infection status now need to be better evaluated. Then, once they are further validated, these assays can be used in population studies for monitoring active tuberculosis and LTBI status and risk.
Acknowledgments
This work was supported by grants from the ISS-NIH-USA scientific cooperation agreement (grants 5303 and 28C6) and from the Italian Ministry of Health, Istituto Superiore di Sanità, 6th AIDS project, and the AIFA project (to C.M.A.) and by the Commission of the European Communities, Sixth Framework Programme (contract LSHP-CT-2003-503240, Mucosal Vaccines for Poverty-Related Diseases [MUVAPRED]).
The editorial assistance of Adam Nixon is gratefully acknowledged. We thank Antonio Cassone for constructive discussions, support, and critically reading the manuscript and Sergio Carbonara for critically reading the manuscript.
Footnotes
Published ahead of print on 21 November 2007.
REFERENCES
- 1.Andersen, P., T. M. Doherty, M. Pai, and K. Weldingh. 2007. The prognosis of latent tuberculosis: can disease be predicted? Trends Mol. Med. 13:175-182. [DOI] [PubMed] [Google Scholar]
- 2.Arend, S. M., S. F. Thijsen, E. M. Leyten, J. J. Bouwman, W. P. Franken, B. F. Koster, F. G. Cobelens, A. J. van Houte, and A. W. Bossink. 2007. Comparison of two interferon-gamma assays and tuberculin skin test for tracing tuberculosis contacts. Am. J. Respir. Crit. Care Med. 175:618-627. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello, C. M., G. C. Spagnoli, M. Boccanera, I. Casalinuovo, F. Malavasi, C. U. Casciani, and A. Cassone. 1986. Proliferation of human peripheral blood mononuclear cells induced by Candida albicans and its cell wall fractions. J. Med. Microbiol. 22:195-202. [DOI] [PubMed] [Google Scholar]
- 4.Ausiello, C. M., R. Lande, A. la Sala, F. Urbani, and A. Cassone. 1998. Cell-mediated immune response of healthy adults to Bordetella pertussis vaccine antigens. J. Infect. Dis. 178:466-470. [DOI] [PubMed] [Google Scholar]
- 5.Bloom, B. R. 1971. In vitro methods in cell-mediated immunity in man. N. Engl. J. Med. 284:1212-1213. [DOI] [PubMed] [Google Scholar]
- 6.Brock, I., K. Weldingh, E. M. Leyten, S. M. Arend, P. Ravn, and P. Andersen. 2004. Specific T-cell epitopes for immunoassay-based diagnosis of Mycobacterium tuberculosis infection. J. Clin. Microbiol. 42:2379-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brock, I., K. Weldingh, T. Lillebaek, F. Follmann, and P. Andersen. 2004. Comparison of tuberculin skin test and new specific blood test in tuberculosis contacts. Am. J. Respir. Crit. Care Med. 170:65-69. [DOI] [PubMed] [Google Scholar]
- 8.Cosmi, L., L. Maggi, V. Santarlasci, F. Liotta, F. Frosali, R. Angeli, M. Mazzetti, A. Vultaggio, A. Matucci, E. Maggi, S. Romagnani, and F. Annunziato. 2007. Detection by flow cytometry of ESAT-6- and PPD-specific circulating CD4+ T lymphocytes as a diagnostic tool for tuberculosis. Int. Arch. Allergy Immunol. 143:1-9. [DOI] [PubMed] [Google Scholar]
- 9.Detjen, A. K., T. Keil, S. Roll, B. Hauer, H. Mauch, U. Wahn, and K. Magdorf. 2007. Interferon-gamma release assays improve the diagnosis of tuberculosis and nontuberculous mycobacterial disease in children in a country with a low incidence of tuberculosis. Clin. Infect. Dis. 45:322-328. [DOI] [PubMed] [Google Scholar]
- 10.Dewan, P. K., J. Grinsdale, and L. M. Kawamura. 2007. Low sensitivity of a whole-blood interferon-gamma release assay for detection of active tuberculosis. Clin. Infect. Dis. 44:69-73. [DOI] [PubMed] [Google Scholar]
- 11.Diel, R., M. Ernst, G. Doscher, L. Visuri-Karbe, U. Greinert, S. Niemann, A. Nienhaus, and C. Lange. 2006. Avoiding the effect of BCG vaccination in detecting Mycobacterium tuberculosis infection with a blood test. Eur. Respir. J. 28:16-23. [DOI] [PubMed] [Google Scholar]
- 12.Ewer, K., J. Deeks, L. Alvarez, G. Bryant, S. Waller, P. Andersen, P. Monk, and A. Lalvani. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361:1168-1173. [DOI] [PubMed] [Google Scholar]
- 13.Fedele, G., I. Celestino, F. Spensieri, L. Frasca, M. Nasso, M. Watanabe, M. E. Remoli, E. M. Coccia, F. Altieri, and C. M. Ausiello. 2007. Lipooligosaccharide from Bordetella pertussis induces mature human monocyte-derived dendritic cells and drives a Th2 biased response. Microbes Infect. 9:855-863. [DOI] [PubMed] [Google Scholar]
- 14.Ferrara, G., M. Losi, R. D'Amico, P. Roversi, R. Piro, M. Meacci, B. Meccugni, I. M. Dori, A. Andreani, B. M. Bergamini, C. Mussini, F. Rumpianesi, L. M. Fabbri, and L. Richeldi. 2006. Use in routine clinical practice of two commercial blood tests for diagnosis of infection with Mycobacterium tuberculosis: a prospective study. Lancet 367:1328-1334. [DOI] [PubMed] [Google Scholar]
- 15.Glas, A. S., J. G. Lijmer, M. H. Prins, G. J. Bonsel, and P. M. Bossuyt. 2003. The diagnostic odds ratio: a single indicator of test performance. J. Clin. Epidemiol. 56:1129-1135. [DOI] [PubMed] [Google Scholar]
- 16.Godkin, A. J., H. C. Thomas, and P. J. Openshaw. 2002. Evolution of epitope-specific memory CD4+ T cells after clearance of hepatitis C virus. J. Immunol. 169:2210-2214. [DOI] [PubMed] [Google Scholar]
- 17.Goletti, D., O. Butera, F. Bizzoni, R. Casetti, E. Girardi, and F. Poccia. 2006. Region of difference 1 antigen-specific CD4+ memory T cells correlate with a favorable outcome of tuberculosis. J. Infect. Dis. 194:984-992. [DOI] [PubMed] [Google Scholar]
- 18.Goletti, D., S. Carrara, D. Vincenti, C. Saltini, E. B. Rizzi, V. Schinina, G. Ippolito, M. Amicosante, and E. Girardi. 2006. Accuracy of an immune diagnostic assay based on RD1 selected epitopes for active tuberculosis in a clinical setting: a pilot study. Clin. Microbiol. Infect. 12:544-550. [DOI] [PubMed] [Google Scholar]
- 19.Harari, A., V. Dutoit, C. Cellerai, P. A. Bart, R. A. Du Pasquier, and G. Pantaleo. 2006. Functional signatures of protective antiviral T-cell immunity in human virus infections. Immunol. Rev. 211:236-254. [DOI] [PubMed] [Google Scholar]
- 20.Hill, P. C., R. H. Brookes, I. M. Adetifa, A. Fox, D. Jackson-Sillah, M. D. Lugos, S. A. Donkor, R. J. Marshall, S. R. Howie, T. Corrah, D. J. Jeffries, R. A. Adegbola, and K. P. McAdam. 2006. Comparison of enzyme-linked immunospot assay and tuberculin skin test in healthy children exposed to Mycobacterium tuberculosis. Pediatrics 117:1542-1548. [DOI] [PubMed] [Google Scholar]
- 21.Kern, F., G. LiPira, J. W. Gratama, F. Manca, and M. Roederer. 2005. Measuring Ag-specific immune responses: understanding immunopathogenesis and improving diagnostics in infectious disease, autoimmunity and cancer. Trends Immunol. 26:477-484. [DOI] [PubMed] [Google Scholar]
- 22.Lalvani, A. 2007. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 131:1898-1906. [DOI] [PubMed] [Google Scholar]
- 23.Lanzavecchia, A., and F. Sallusto. 2005. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 17:326-332. [DOI] [PubMed] [Google Scholar]
- 24.Leyten, E. M., S. M. Arend, C. Prins, F. G. Cobelens, T. H. Ottenhoff, and J. T. van Dissel. 2007. Discrepancy between Mycobacterium tuberculosis-specific gamma interferon release assays using short versus prolonged in vitro incubation. Clin. Vaccine Immunol. 14:880-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahomed, H., E. J. Hughes, T. Hawkridge, D. Minnies, E. Simon, F. Little, W. A. Hanekom, L. Geiter, and G. D. Hussey. 2006. Comparison of Mantoux skin test with three generations of a whole blood IFN-gamma assay for tuberculosis infection. Int. J. Tuberc. Lung Dis. 10:310-316. [PubMed] [Google Scholar]
- 26.Mazurek, G. H., J. Jereb, P. Lobue, M. F. Iademarco, B. Metchock, and A. Vernon. 2005. Guidelines for using the QuantiFERON-TB Gold test for detecting Mycobacterium tuberculosis infection, United States. MMWR Morb. Mortal. Wkly. Rep. Recommend. Rep. 54:49-55. [PubMed] [Google Scholar]
- 27.Menzies, D., M. Pai, and G. Comstock. 2007. Meta-analysis: new tests for the diagnosis of latent tuberculosis infection: areas of uncertainty and recommendations for research. Ann. Intern. Med. 146:340-354. [DOI] [PubMed] [Google Scholar]
- 28.National Collaborating Centre for Chronic Conditions. 2006. Tuberculosis: clinical diagnosis and management of tuberculosis, and measures for its prevention and control. Royal College of Physicians, London, United Kingdom. [PubMed]
- 29.Pai, M., K. Gokhale, R. Joshi, S. Dogra, S. Kalantri, D. K. Mendiratta, P. Narang, C. L. Daley, R. M. Granich, G. H. Mazurek, A. L. Reingold, L. W. Riley, and J. M. Colford, Jr. 2005. Mycobacterium tuberculosis infection in health care workers in rural India: comparison of a whole-blood interferon gamma assay with tuberculin skin testing. JAMA 293:2746-2755. [DOI] [PubMed] [Google Scholar]
- 30.Pai, M., S. Kalantri, and K. Dheda. 2006. New tools and emerging technologies for the diagnosis of tuberculosis. Part I. Latent tuberculosis. Expert Rev. Mol. Diagn. 6:413-422. [DOI] [PubMed] [Google Scholar]
- 31.Porsa, E., L. Cheng, M. M. Seale, G. L. Delclos, X. Ma, R. Reich, J. M. Musser, and E. A. Graviss. 2006. Comparison of a new ESAT-6/CFP-10 peptide-based gamma interferon assay and a tuberculin skin test for tuberculosis screening in a moderate-risk population. Clin. Vaccine Immunol. 13:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Richeldi, L. 2006. An update on the diagnosis of tuberculosis infection. Am. J. Respir. Crit. Care Med. 174:736-742. [DOI] [PubMed] [Google Scholar]
- 33.Salerno-Goncalves, R., and M. B. Sztein. 2006. Cell-mediated immunity and the challenges for vaccine development. Trends Microbiol. 14:536-542. [DOI] [PubMed] [Google Scholar]
- 34.Schenal, M., S. Lo Caputo, F. Fasano, F. Vichi, M. Saresella, P. Pierotti, M. L. Villa, F. Mazzotta, D. Trabattoni, and M. Clerici. 2005. Distinct patterns of HIV-specific memory T lymphocytes in HIV-exposed uninfected individuals and in HIV-infected patients. AIDS 19:653-661. [DOI] [PubMed] [Google Scholar]
- 35.Shams, H., S. E. Weis, P. Klucar, A. Lalvani, P. K. Moonan, J. M. Pogoda, K. Ewer, and P. F. Barnes. 2005. Enzyme-linked immunospot and tuberculin skin testing to detect latent tuberculosis infection. Am. J. Respir. Crit. Care Med. 172:1161-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sodhi, A., J. Gong, C. Silva, D. Qian, and P. F. Barnes. 1997. Clinical correlates of interferon gamma production in patients with tuberculosis. Clin. Infect. Dis. 25:617-620. [DOI] [PubMed] [Google Scholar]
- 37.Tissot, F., G. Zanetti, P. Francioli, J. P. Zellweger, and F. Zysset. 2005. Influence of bacille Calmette-Guerin vaccination on size of tuberculin skin test reaction: to what size? Clin. Infect. Dis. 40:211-217. [DOI] [PubMed] [Google Scholar]
- 38.Ueha-Ishibashi, T., Y. Oyama, H. Nakao, C. Umebayashi, S. Hirama, Y. Sakai, S. Ishida, and Y. Okano. 2005. Flow-cytometric analysis on cytotoxic effect of thimerosal, a preservative in vaccines, on lymphocytes dissociated from rat thymic glands. Toxicol. In Vitro 19:191-198. [DOI] [PubMed] [Google Scholar]