Abstract
Enterohemorrhagic Escherichia coli (EHEC) strains are important human food-borne pathogens. EHEC strains elaborate potent Shiga toxins (Stx1, and/or Stx2) implicated in the development of hemorrhagic colitis (HC) or hemolytic-uremic syndrome (HUS). In this report, we evaluated the immunogenicity and protective efficacy of Stx1 subunit B (StxB1) administered by transcutaneous immunization (TCI). Three groups of Dutch Belted rabbits received patches containing StxB1, StxB1 in combination with Escherichia coli heat-labile enterotoxin (LT), or LT alone. An additional group of naïve rabbits served as controls. The protective efficacy following TCI with StxB1 was assessed by challenging rabbits with a virulent Stx1-producing strain, RDEC-H19A, capable of inducing HC and HUS in rabbits. Antibodies specific to StxB1 from serum and bile samples were determined by enzyme-linked immunosorbent assay and toxin neutralization test. Rabbits immunized with StxB1 demonstrated improved weight gain and reduced Stx-induced histopathology. Rabbits receiving StxB or StxB1/LT showed a significant increase in serum immunoglobulin G titers specific to StxB1 as well as toxin neutralization titers. These data demonstrated that the StxB delivered by TCI could induce significant systemic immune responses. Thus, Stx subunit B vaccine delivered by a patch for a high-risk population may be a practical approach to prevent (and/or reduce) Stx-induced pathology.
Enterohemorrhagic Escherichia coli (EHEC) strains are important human food-borne pathogens (19, 21, 25). The clinical manifestations of EHEC infections range from watery diarrhea, or hemorrhagic colitis (HC), to the most severe outcome, the life-threatening hemolytic-uremic syndrome (HUS) (26).
The striking feature of EHEC infection is the production of potent Shiga toxins (Stx1 and/or Stx2) implicated in the development of HUS (13, 27, 28). EHEC strains are a subgroup of Shiga toxin-producing E. coli (STEC). Stx(s) produced by EHEC belongs to a family of bacterial cytotoxins structurally related to those produced by the dysentery bacillus Shigella dysenteriae (16, 27). Both Stx1 and Stx2 are in the class known as AB toxins composed of one A subunit and five identical B subunits (17, 18, 35). The A subunits of both toxins are highly selective N-glycosidases that depurinate a specific adenine residue on the eukaryotic 60S ribosomal subunit, thus blocking protein synthesis and leading to cell death (15, 17, 18, 37). The B subunits bind to receptor molecules on the host cell surface (18, 37). A primary mechanism of Stx-mediated damage is direct Stx cytotoxicity for vascular endothelial and renal epithelial cells (27, 29). StxB subunits selectively bind to GB3 receptors that mediate the endocytosis of StxA subunits. The epithelial cells of the kidneys are rich in GB3 receptors (5).
While there is indirect evidence that human vaccination against STEC may be effective in preventing illness in humans, at present, there are no human vaccines or therapeutics for human STEC infections (19). A successful human vaccine would need to elicit antibodies aimed at either preventing STEC colonization in the intestinal tract or neutralizing Stx to prevent the development of HUS. Although STEC strains are generally susceptible to a variety of antibiotics, retrospective studies have shown that the use of antibiotics negatively alters the outcome of STEC infections, causing an increased incidence of HUS (12, 20, 23, 39). This is likely because the lysis of bacteria by some antibiotics leads to the increased release of toxin as well as to increased toxin synthesis during the induction of lysogenic toxin-producing bacteriophages (12, 23).
In this study, we evaluated the safety and immunogenicity of StxB1 administered by transcutaneous immunization (TCI). To determine the level of protection following immunization, we challenged rabbits with Stx-producing strain RDEC-H19A, a rabbit-enteropathogenic E. coli strain transduced with Stx-converting phage H19A (34). We demonstrated that the StxB1 delivered by TCI induced significant systemic immune responses. In vivo challenge studies showed significant protection from Stx-induced vascular and renal histopathology in StxB1-immunized rabbits. Our results suggest that vaccination with StxB subunits by TCI is a practical approach for the prevention or reduction of Stx-induced pathology.
MATERIALS AND METHODS
Bacterial strain and culture conditions.
Strain RDEC-H19A is an RDEC-1 derivative containing the Stx1-producing phage H19A from human EHEC O26 (34). RDEC-H19A was grown on LB agar or broth supplemented with nalidixic acid (Nal) (50 μg/ml) and tetracycline (Tet) (25 μg/ml).
TCI procedure.
The purified pentameric StxB1 was kindly provided by David Acheson (USDA). The E. coli heat-labile enterotoxin (LT) was prepared from strain HE22/TP/235. Patches for TCI were formulated with 75 μg StxB1, 75 μg StxB1 plus 50 μg LT, or 50 μg LT. A total of 24 8-week-old Dutch Belted rabbits purchased from Covance Research Products (Denver, PA) were segregated into four groups of six rabbits each. Rabbits in groups 1, 2, and 3 received StxB1, StxB1 plus LT, and LT, respectively. Rabbits in group 4 served as controls. Before the application of patches, the shaven skin was abraded (rubbed) with 15 strokes using ECG Prep pads (Marquette Medical Systems) soaked with a pretreatment solution of 10% glycerol in saline. Any excess liquid was blotted, and the immunization site was then marked with a permanent marker. The antigens, prepared as described above, were administered in three doses. In initial TCI at day 0, the patch was applied over the right scapula region. The booster TCIs were applied at day 14 over the left scapula and again at day 28 over the right scapula.
TCI was performed using a semiocclusive patch consisting of a 2- by 2-in. cotton gauze matrix (Kendall) with a 2- by 2-in. polyethylene (Saran Wrap) backing covered by a 4- by 4′-in. Tegaderm dressing (semiocclusive; 3M). At the time of immunization, the antigen preparations were applied in 150 μl of sterile saline and administered as a split dose on the back. To ensure proper patch adherence, patches were covered with a modified Tegaderm overlay. The patch margins were secured with surgical tape and then wrapped by Vetrap and Elastikon (or other similar bandage material). Patches were applied for 18 to 24 h and removed, and the skin was rinsed with warm water. The dosing sites were observed for erythema and edema and scored at patch removal at 20 to 24 h and 44 to 48 h from patch application.
Experimental challenge of rabbits.
Orogastric inoculation of rabbits with 5 × 107 CFU of RDEC-H19A was performed as previously described (2). Rabbits were observed daily for clinical signs of disease. Fecal bacterial shedding of challenge RDEC-H19A bacteria was determined by semiquantitative rectal swab on MacConkey agar supplemented with Nal and Tet to differentiate RDEC-H19A from other E. coli strains present in the normal flora. The individual organisms recovered from rectal swabs were further identified by PCR as described previously (41) using two pairs of primers for stxB1 (stx1bf [5′-ATGAAAAAAACATTATTA]/stx1br [5′-TCAGCGAAAGATCACCTC]) derived from the bacteriophage H19B sequence (GenBank accession no. M16625) and the eae gene (Agin1 [5′-CCAGTATTACTGAGATTAAG]/Agin2 [5′-TCCGGGATTTGAGATGTAAT]) derived from the RDEC-1 locus of enterocyte effacement (GenBank accession no. AF200363) (40). The expected amplification products for the stxB1 and eae genes are 267 bp and 812 bp, respectively.
Rabbits were euthanized 7 days postchallenge according to standard protocols (2). At necropsy, transmural sections from the cecal segments and kidneys were excised and fixed in 10% buffered formalin. Tissues were processed for paraffin sectioning and staining with hematoxylin and eosin or Giemsa, and intestinal tissues were observed for enterobacterial adherence and vascular pathology in the intestine (2, 34). Edema depth was quantitated with an ocular micrometer by measuring the distance from the muscularis mucosa to the muscularis propria and graded from 0 to 4 as previously described (2).
Renal histopathology was observed and graded as described previously (7) for the following parameters: fibrin-like deposits (FLD) in vessels, intraglomerular and cortical congestion, intraglomerular heterophiles, and perivascular edema. FLD was observed for 10 cortex vessels per section at random and expressed as the percentage filled with FLD per vessel in the lumen. Congestion was scored as a percentage of the quantity of blood present per glomeruli or cortex read with a 40× objective lens. Intraglomerular heterophiles was observed with a 40× objective lens and expressed as the number of heterophiles per glomeruli. Perivascular edema was observed in the perivascular connective tissue of interlobular arterioles and arcuate arteries and was scored as follows: 0 for none, 1 for mild perivascular connective tissue edema, 2 for medium perivascular connective tissue edema, 3 for severe perivascular connective tissue edema, and 4 for very severe perivascular connective tissue edema.
Detection of antibodies specific to StxB1.
Serum was collected prior to each immunization (days 0, 13, and 27) or prior to challenge with RDEC-H19A (day 42). Bile was aspirated from the rabbit gall bladder at the time of sacrifice. An enzyme-linked immunosorbent assay (ELISA) was used to determine anti-StxB1 and anti-LT immunoglobulin G (IgG) titers from each serum sample or IgA from bile samples (22, 38). Briefly, microtiter wells were coated with StxB1 or LT (identical to that used in TCI [4 μg/ml]) in bicarbonate buffer (pH 9.6). Serial dilutions of rabbit sera or bile were added, and bound antibodies were detected with horseradish peroxidase-conjugated sheep anti-rabbit IgG or goat anti-rabbit IgA and developed with ABT peroxidase substrate (KPL, Gaithersburg, MD). The ELISA results were reported as ELISA units (EU), which is the dilution equal to an optical density at 405 nm of 1.0 for serum IgG or the actual optical density for IgA. The neutralization assay for Stx1 cytotoxicity was performed as described previously (42).
Statistical analysis.
Values for differences in rabbit weight gain, heterophiles/high-power field (HPF), bacterial adherence, and antibody titers were compared by the Student t test. A nonparametric test (Wilcoxon rank sum) was used to compare scores for fecal bacterial shedding, stool consistency, edema, and microvascular lesions in the tissue compartments between experimental groups.
RESULTS
Reactogenicity.
Rabbits receiving LT alone (five out of six rabbits) or in combination with StxB1 (five out of six rabbits) showed erythema (ranging from light pink to distinct bright red) within 24 h after application of patches, with reactions lasting over 72 h (data not shown). Areas of reaction showed various degrees of edema ranging from slight swelling to defined swelling with a raised border. However, the erythema and edema were rarely seen at the time when booster immunizations were performed. Rabbits receiving StxB1 patches showed neither erythema nor edema on the site of patch application following initial or booster immunizations.
Protection from RDEC-H19A challenge.
Rabbits in all groups exhibited normal weight gain and showed no clinical signs of disease following immunization (data not shown). Following orogastric administration of RDEC-H19A organisms, Nalr/Tetr bacteria were recovered in all rabbits the next day. The heavy fecal bacterial shedding was observed at day 3 postchallenge, and rabbits in all groups shed bacteria to the same extent (Fig. 1A), although one rabbit in the naïve group had transient bacterial shedding. PCR amplification using primers derived from the stxB1 and eae genes of recovered Nalr/Tetr organisms yielded PCR amplicons identical to those seen in RDEC-H19A controls, indicating that these organisms were the challenge RDEC-H19A strains (data not shown).
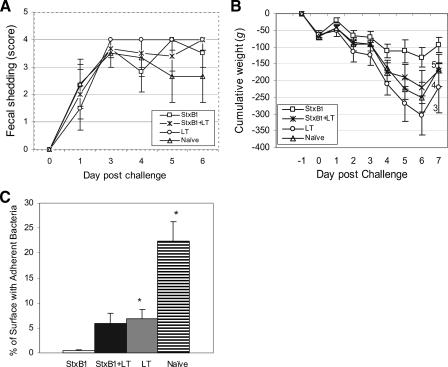
FIG. 1.
Comparisons of fecal bacterial shedding (A), cumulative weight change (numbers indicate the total numbers of animals remaining at the observation time point) (B), and percentage of surface with adherent bacteria (C). Bars indicate standard errors. * indicates statistically significant differences compared to the StxB1 group.
Rabbit weight changes following RDEC-H19A challenge varied among groups. All rabbits gained weight at day 1 postchallenge but started losing body weight at day 2. The most severe weight loss was observed among rabbits receiving LT (−288 ± 48 g) or naïve rabbits (−249 ± 63 g) at day 6 postchallenge (Fig. 1B). Rabbits immunized with StxB1 or StxB1 plus LT lost an average weight of 131 g (±22 g) or 220 g (±52 g), respectively. Statistical analysis indicated significant (P < 0.034) weight loss among rabbits receiving LT or naïve rabbits compared to those receiving StxB1. Mild diarrhea, as evidenced by soft to semiliquid discharge, was seen for a day or two in two of six rabbits receiving StxB1 patches, whereas the remaining animals of this group discharged normal stools. One out of six rabbits receiving StxB1/LT exhibited soft stool, and the remaining five rabbits developed watery diarrhea. All rabbits receiving LT had watery diarrhea, and three of them had bloody diarrhea in late stage. Watery diarrhea occurred among five out of six naïve rabbits, two of which had bloody diarrhea. One naïve rabbit, which showed transient fecal shedding of RDEC-H19A, remained clinically normal postchallenge. Because of severe weight loss and diarrhea, one rabbit receiving StxB1 plus LT, three rabbits receiving LT, and two in the naïve group required earlier euthanasia (Fig. 1B). The Wilcoxon rank sum test indicated that the mean measure of abnormal stool consistency was significantly lower for the StxB1 TCI group (P < 0.01). It is worth noting that no death occurred in the group immunized with StxB1 alone, whereas three of six rabbits in the group that received LT and two of six naïve rabbits died. It appears that StxB1 protects against both weight loss and mortality.
Microscopically, closely adherent RDEC-H19A organisms were seen covering 0.39% of the cecal mucosa among StxB1 TCI rabbits (Fig. 1C). On the other hand, 5.96%, 6.83%, or 22.33% enterobacterial adherence was observed among rabbits receiving StxB1 plus LT, rabbits receiving LT alone, or naïve rabbits, respectively. Significant differences (P < 0.007) in bacterial adherence between the StxB1 group and LT or naïve rabbits were seen. The close bacterial attachment and effacement of microvilli, when observed, are typical attaching and effacing lesions described in our previous studies (34).
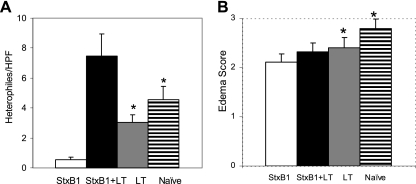
Polymorphonuclear heterophile infiltration was observed in cecal samples among rabbits receiving StxB1 (0.6 ± 2 polymorphonuclear heterophiles/HPF), StxB1 plus LT (7.5 ± 1.5 polymorphonuclear heterophiles/HPF), or LT (3 ± 0.5 polymorphonuclear heterophiles/HPF) or naïve rabbits (4.6 ± 0.9 polymorphonuclear heterophiles/HPF) (Fig. 2A). The degree of inflammatory infiltrate was significantly lower (P < 0.002) in rabbits receiving StxB1 than observed for the remaining three groups. Rabbits receiving StxB1/LT showed the highest number of heterophiles. Submucosal edema observed in the LT or naïve group was significantly more severe than in StxB1-immunized rabbits (Fig. 2B).
FIG. 2.
Comparisons of enumeration of heterophiles per HPF (A) and scoring of edema (B) in cecal samples following challenge with RDEC-H19A. Bars indicate standard errors. * indicates a statistically significant difference compared to the StxB1 group.
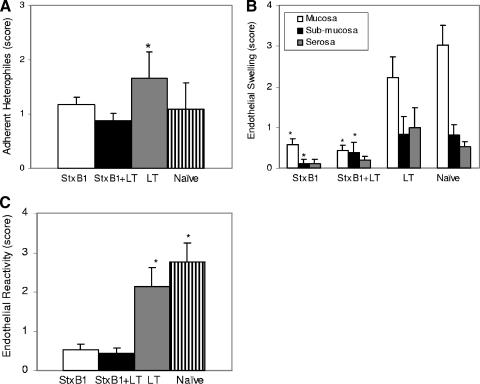
Heterophiles adherent to the microvascular endothelium (Fig. 3A) were more numerous in the mucosa among rabbits receiving LT (P value of <0.01 by Wilcoxon rank sum test) than in those receiving StxB1 or StxB1 plus LT. Microvascular changes, as measured by endothelial swelling (Fig. 3B) and endothelial reactivity (Fig. 3C), were more prominent among rabbits receiving LT or naïve rabbits (P value of <0.01 of Wilcoxon rank sum test) than those receiving StxB1 (alone or in combination with LT).
FIG. 3.
Microvascular changes in mucosal, submucosal, and serosal compartments as measured by scores for adherent heterophiles (A), endothelial swelling expressed as individual parameters (B), and endothelial reactivity (C) among different groups of rabbits. Bars indicate standard errors. * indicates statistically significant differences between rabbits receiving LT or naïve rabbits and those receiving StxB1 (alone or in combination with LT).
Rabbits receiving StxB1 or StxB1/LT showed an apparent reduction in endothelial swelling in the compartments of mucosa, submucosa, or serosa compared with those in the naïve group. However, the differences in these parameters between rabbits receiving StxB1 (alone or in combination with LT) and rabbits receiving LT was statistically different only in the mucosal compartments (P < 0.01) but not in the submucosa and serosa compartments due to a high level of standard deviation (Fig. 3B). Similarly, the endothelial reactivity score was significantly (P < 0.01) reduced among rabbits receiving StxB1 or StxB1/LT compared to those in the remaining groups (Fig. 3C).
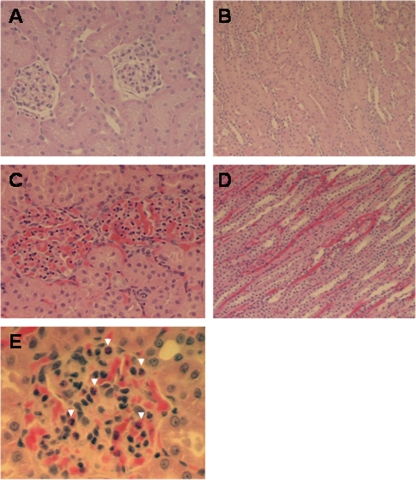
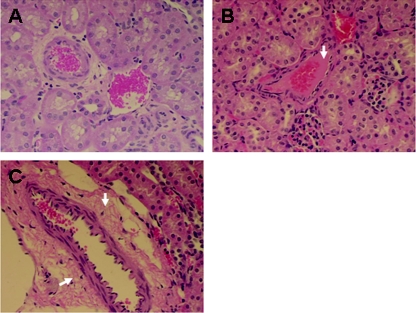
Examination of kidney sections among experimentally challenged rabbits revealed renal lesions associated with HUS, including glomerular heterophile infiltrates, FLD, glomerular congestion, cortical congestion, and perivascular edema (Table 1 and Fig. 4 and 5). The number of heterophiles per glomerular heterophile infiltrate averaged 0.78 in rabbits receiving StxB1 or StxB1/LT, which is significantly less than that observed for rabbits receiving LT or naïve rabbits (P < 0.05), which averaged 1.07 or 1.22, respectively. FLD in vessels was seen in all kidney sections examined, ranging from 0 to 80%. The severity of FLD seen in medium vessels averaged 22 or 10% among rabbits receiving StxB1 or StxB1/LT, respectively, comparable to that of rabbits receiving LT or that of naïve rabbits (Table 1). Mild to severe congestion was seen in the glomeruli and cortex. The average glomerular congestion seen in StxB1 or StxB1/LT rabbits was 17.3% or 16.3%, respectively, similar to that observed for the rabbits immunized with LT (25.5%) or naïve rabbits (19.4%). However, cortical congestion seen among rabbits receiving StxB1 or StxB1/LT averaged 5.3 or 5.0%, respectively, significantly less (P < 0.001) severe than what was observed for rabbits immunized with LT (10.9%) or naïve rabbits (12.2%). The occurrence of perivascular edema was seen in only one rabbit receiving either StxB1 (average, 0.06) or StxB1/LT (average, 0.03). However, perivascular edema was seen among three of six LT-immunized rabbits (average, 0.2) or four of five naïve rabbits (average, 0.38). Statistical analysis indicates significant differences (P < 0.04) between StxB1 or StxB1/LT groups and those receiving LT or naïve rabbits.
TABLE 1.
Comparison of renal lesions among rabbit groupsa
| Group | Mean number (SE) of renal lesions
|
||||
|---|---|---|---|---|---|
| Glomerular heterophiles | Fibrin-like vascular deposits | Glomerular congestion | Cortical congestion | Perivascular edema | |
| StxB1 | 0.78 (0.12)b | 22.1 (4.0) | 17.3 (2.4) | 5.34 (5.82)b | 0.06 (0.04)b |
| StxB1/LT | 0.78 (0.11)b | 10.0 (2.9) | 16.3 (1.7) | 4.80 (0.82)b | 0.03 (0.04)b |
| LT | 1.07 (0.11) | 23.3 (3.9) | 25.5 (1.8) | 10.9 (1.81) | 0.20 (0.08) |
| Naïve | 1.22 (0.24) | 11.8 (3.3) | 19.4 (2.2) | 12.2 (1.63) | 0.38 (0.11) |
Readings are based on 10 HPF per kidney section (see Materials and Methods).
Significant difference compared to LT and naïve groups.
FIG. 4.
Light micro graphs showing normal glomeruli (A) and cortex (B), congestion in glomeruli (C) and cortex (D), and heterophile (indicated by arrowheads) infiltration in glomerli (E). Hematoxylin and eosin staining was done. Magnifications, ×200 (A and C), ×100 (B and D), and ×400 (E).
FIG. 5.
Light micro graphs showing normal vessels (A), fibrin-like deposits in medium vessels (B) (indicated by an arrow), and vascular edema (C) (indicated by arrows) in an RDEC-H19-infected rabbit. Hematoxylin and eosin staining is shown. Magnification, ×200.
Immune responses.
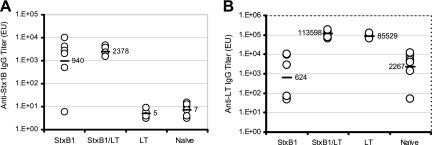
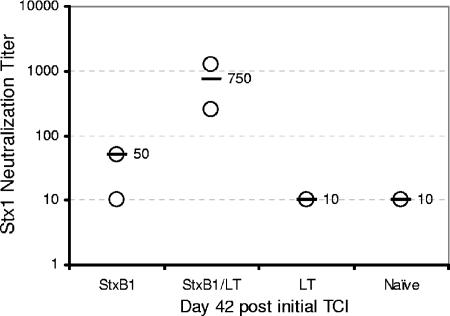
Serum Ig titers specific to StxB1 averaged 940 EU and 2,378 EU for rabbits receiving StxB1 and StxB1/LT, respectively. Five of six rabbits receiving StxB1 and all rabbits receiving StxB1/LT showed increased serum IgG titers specific to StxB1 compared to those prior to immunization (Fig. 6A). In contrast, rabbits receiving LT or naïve rabbits did not show serum IgG titers specific to StxB1. Statistical analysis indicated significantly higher (P < 0.04) serum IgG titers specific to StxB1 in rabbits receiving StxB1 than in the remaining two groups. A nonsignificant difference was seen in serum IgG specific to StxB1 between rabbits receiving StxB1 and those receiving StxB1 plus LT. Serum IgG titers specific to LT were seen among rabbits receiving LT, although low background levels were seen among the remaining two groups (Fig. 6B). No significant increase was observed for bile IgA in all experimental groups (data not shown). Toxin neutralization activities were seen in rabbits receiving StxB1 (1/50) or StxB1 plus LT (1/750) (Fig. 7).
FIG. 6.
Serum IgG titer specific to StxB1 (A) or LT (B) at 42 days after initial TCI. Bars represent the means. Numbers indicate actual EU.
FIG. 7.
Serum neutralization titers to Stx1 at 42 days after initial TCI. Bars represent the averages of the group. Numbers indicate actual titers.
DISCUSSION
TCI provides a novel alternative means for vaccination without the use of needles and has several advantages over parenteral routes of vaccination (9, 11, 38). The simplicity of TCI could reduce or eliminate the necessity for the administration of vaccines by trained personnel. Furthermore, the topical patch application could reduce the potential incidence of needle-borne infections and potentially increase the spectrum of availability by stabilizing vaccines for use outside the cold chain. Additionally, TCI offers an advantageous mode of vaccination due to the unique ability of cutaneous immune cells, especially Langerhans cells and other dendritic cells, to present antigens to the host immune system (24). These antigen-presenting dendritic cells migrate to draining lymph nodes through lymphatic vessels, where they encounter naïve T cells for the presentation of these antigens (31). This study demonstrates that the StxB1 protein delivered by TCI induces robust immune responses and protection of StxB1-immunized animals against Stx-induced vascular pathological changes. The observed increase in immune responses specific to StxB1 and LT in the current study suggests the efficient presentation of these antigens delivered by TCI. Compared to the parenteral immunization of StxB1 (3) or oral immunization with attenuated bacteria expressing StxB1 onto a cell surface-exposed loop of the LamB protein in Salmonella enterica serovar Typhimurium aroA (36) or with Vibrio cholerae carrying the StxB1 delivery plasmid (1), TCI provides an alternative route for antigen delivery.
Stx plays a key role in the molecular mechanism of HUS in EHEC infection. In a well-established rabbit model of infection, Stx-producing RDEC-H19A causes vascular changes in the rabbit intestine (34). In the current study, we demonstrated that the StxB1-immunized rabbits were protected from Stx-mediated intestinal pathological changes. These pathological changes are attributable to the interaction of Shiga toxin with the intestinal microvasculature, including the transmigration of heterophiles, endothelial swelling. The substantially less severe illness in these animals may contribute to the improved weight gain compared to that of rabbits receiving non-StxB1.
Although the precise sequence of events leading from ingestion of EHEC/STEC to the development of HUS is still unknown, it is well accepted that the intimate attachment of EHEC and Stx production play critical roles. A recent study suggested that Stx2 may contribute positively to adherence by influencing the surface of the gut epithelium, probably by enhancing the surface expression of nucleolin, which serves as a eukaryotic receptor for intimin (32, 33). In a recent bovine study, the Stx2-negative EHEC O157:H7 strain showed significantly lower levels of fecal shedding (14). We observed substantially reduced enterobacterial adherence in rabbits immunized StxB1 alone or in combination with LT. Whether this decreased bacterial adherence is directly linked to StxB1 immunity is not clear at this time.
Previous studies demonstrated that TCI in the presence of an ADP-ribosylating exotoxin, such as cholera toxin or LT or its mutant derivatives (LTK63 and LTR72), triggers protective immune responses against the toxin and the coadministered antigen (8, 10, 14). In the current study, we observed similar levels (P < 0.34) of serum antibody in both rabbit groups receiving StxB1 or StxB1/LT. However, the latter group showed more consistent IgG titers than the former group. Similarly, significantly higher (P < 0.05) serum neutralization titers were observed among rabbits immunized with StxB1/LT than among those receiving StxB1 alone. It appears that LT may play a role in enhancing immunity against StxB1.
The presence of Gb3 or galabiosyl ceramide receptors on cells has been found to determine the localization of tissue damage due to Stx in rabbits, mice, and humans (5, 29, 30). Natural and experimental infections with EHEC O153 develop renal histopathology characteristic of HUS (6). Our previous studies demonstrated similar findings using a well-characterized rabbit EHEC strain, RDEC-H19A (4). In the current study, we observed striking renal pathological changes following infection with RDEC-H19A, especially in the glomerular and cortical area. Notably, the reduction of glomerular heterophiles, congestion in the cortical area, and perivascular edema are suggestive of protection induced by immunization with StxB1. However, these parameters in the immunized rabbits are still comparable to those observed in naive animals challenged with the STEC strain. The observed intermediate degrees of congestion in the glomeruli and cortex, as well as FLD in vessels, are indicative of some degree of inflammation of the kidney, even in vaccinated animals. This may suggest that modified vaccination strategies are needed to achieve complete systemic functional protection. It may be necessary to provide mucosal as well as systemic immunity to Stx to achieve full protection from Stx-induced pathology.
Acknowledgments
We thank the Baltimore VA animal care section for technical assistance and Yevgeniy Elbert for assistance in the statistical analysis.
This work was supported by NIDDK 5R01DK059012 and a Veterans Administration merit review award (E.C.B.).
Footnotes
Published ahead of print on 14 November 2007.
REFERENCES
- 1.Acheson, D. W., S. B. Calderwood, S. A. Boyko, L. L. Lincicome, A. V. Kane, A. Donohue-Rolfe, and G. T. Keusch. 1993. Comparison of Shiga-like toxin I B-subunit expression and localization in Escherichia coli and Vibrio cholerae by using trc or iron-regulated promoter systems. Infect. Immun. 61:1098-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agin, T. S., C. Zhu, L. A. Jonson, T. E. Thate, Z. Yang, and E. C. Boedeker. 2005. Protection against hemorrhagic colitis in an animal model by oral immunization with isogenic rabbit enteropathogenic Escherichia coli attenuated by truncating intimin. Infect. Immun. 73:6608-6619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bielaszewska, M., I. Clarke, M. A. Karmali, and M. Petric. 1997. Localization of intravenously administered verocytotoxins (Shiga-like toxins) 1 and 2 in rabbits immunized with homologous and heterologous toxoids and toxin subunits. Infect. Immun. 65:2509-2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boedeker, E. C., C. Zhu, H. Rios, and K. Davis. 2006. Rabbit enteropathogenic strains expressing Shiga toxin 1 (Stx1) or 2 (Stx2) induce intestinal and renal lesions of hemolytic uremic syndrome (HUS) in Dutch Belted rabbits, abstract P10.2.10. Abstr. 6th Int. Symp. Shiga Toxin (Verotoxin)-Producing E. coli Infect., Melbourne, Australia.
- 5.Boyd, B., and C. Lingwood. 1989. Verotoxin receptor glycolipid in human renal tissue. Nephron 51:207-210. [DOI] [PubMed] [Google Scholar]
- 6.Garcia, A., and J. G. Fox. 2003. The rabbit as a new reservoir host of enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 9:1592-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia, A., C. J. Bosques, J. S. Wishnok, Y. Feng, B. J. Karalius, J. R. Butterton, D. B. Schauer, A. B. Rogers, and J. G. Fox. 2006. Renal injury is a consistent finding in Dutch Belted rabbits experimentally infected with enterohemorrhagic Escherichia coli. J. Infect. Dis. 193:1125-1134. [DOI] [PubMed] [Google Scholar]
- 8.Glenn, G. M., T. Scharton-Kersten, R. Vassell, C. P. Mallett, T. L. Hale, and C. R. Alving. 1998. Transcutaneous immunization with cholera toxin protects mice against lethal mucosal toxin challenge. J. Immunol. 161:3211-3214. [PubMed] [Google Scholar]
- 9.Glenn, G. M., M. Rao, G. R. Matyas, and C. R. Alving. 1998. Skin immunization made possible by cholera toxin. Nature 391:851. [DOI] [PubMed] [Google Scholar]
- 10.Glenn, G. M., T. Scharton-Kersten, R. Vassell, G. R. Matyas, and C. R. Alving. 1999. Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants. Infect. Immun. 67:1100-1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glenn, G. M., R. T. Kenney, S. A. Hammond, and L. R. Ellingsworth. 2003. Transcutaneous immunization and immunostimulant strategies. Immunol. Allergy Clin. North Am. 23:787-813. [DOI] [PubMed] [Google Scholar]
- 12.Grif, K., M. P. Dierich, H. Karch, and F. Allerberger. 1998. Strain-specific differences in the amount of Shiga toxin released from enterohemorrhagic Escherichia coli O157 following exposure to subinhibitory concentrations of antimicrobial agents. Eur. J. Clin. Microbiol. Infect. Dis. 17:761-766. [DOI] [PubMed] [Google Scholar]
- 13.Griffin, P. M., and R. V. Tauxe. 1991. The epidemiology of infections caused by Escherichia coli O157:H7, other enterohemorrhagic E. coli, and the associated hemolytic uremic syndrome. Epidemiol. Rev. 13:60-98. [DOI] [PubMed] [Google Scholar]
- 14.Hoffman, M., A. C. Menge, T. A. Casey, W. Laegreid, and B. T. Bosworth, and E. A. Dean-Nystrom. 2006. Bovine immune response to Shiga-toxigenic Escherichia coli O157:H7. Clin. Vaccine Immunol. 13:1322-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hovde, C. J., S. B. Calderwood, J. J. Mekalanos, and R. J. Collier. 1988. Evidence that glutamic acid 167 is an active-site residue of Shiga-like toxin I. Proc. Natl. Acad. Sci. USA 85:2568-2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson, M. P., J. W. Newland, R. K. Holmes, and A. D. O'Brien. 1987. Nucleotide sequence analysis of the structural genes for Shiga-like toxin I encoded by bacteriophage 933J from Escherichia coli. Microb. Pathog. 2:147-153. [DOI] [PubMed] [Google Scholar]
- 17.Jackson, M. P., R. L. Deresiewicz, and S. B. Calderwood. 1990. Mutational analysis of the Shiga toxin and Shiga-like toxin II enzymatic subunits. J. Bacteriol. 172:3346-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson, M. P. 1990. Structure-function analyses of Shiga toxin and the Shiga-like toxins. Microb. Pathog. 8:235-242. [DOI] [PubMed] [Google Scholar]
- 19.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2:123-140. [DOI] [PubMed] [Google Scholar]
- 20.Karmali, M. A. 2004. Infection by Shiga toxin-producing Escherichia coli: an overview. Mol. Biotechnol. 26:117-122. [DOI] [PubMed] [Google Scholar]
- 21.Karmali, M. A., B. T. Steele, M. Petric, and C. Lim. 1983. Sporadic cases of haemolytic-uraemic syndrome associated with faecal cytotoxin and cytotoxin-producing Escherichia coli in stools. Lancet i:619-620. [DOI] [PubMed] [Google Scholar]
- 22.Kenney, R. T., J. Yu, M. Guebre-Xabier, S. A. Frech, A. Lambert, B. A. Heller, L. R. Ellingsworth, J. E. Eyles, E. D. Williamson, and G. M. Glenn. 2004. Induction of protective immunity against lethal anthrax challenge with a patch. J. Infect. Dis. 190:774-782. [DOI] [PubMed] [Google Scholar]
- 23.Kohler, B., H. Karch, and H. Schmidt. 2000. Antibacterials that are used as growth promoters in animal husbandry can affect the release of Shiga-toxin-2-converting bacteriophages and Shiga toxin 2 from Escherichia coli strains. Microbiology 146:1085-1090. [DOI] [PubMed] [Google Scholar]
- 24.Loser, K., A. Mehling, S. Loeser, J. Apelt, A. Kuhn, S. Grabbe, et al. 2006. Epidermal RANKL controls regulatory T-cell numbers via activation of dendritic cells. Nat. Med. 12:1372-1379. [DOI] [PubMed] [Google Scholar]
- 25.Mead, P. S., L. Slutsker, V. Dietz, L. F. McCaig, J. S. Bresee, C. Shapiro, P. M. Griffin, and R. V. Tauxe. 1999. Food-related illness and death in the United States. Emerg. Infect. Dis. 5:607-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11:142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noel, J. M., and E. C. Boedeker. 1997. Enterohemorrhagic Escherichia coli: a family of emerging pathogens. Dig. Dis. 15:67-91. [DOI] [PubMed] [Google Scholar]
- 28.O'Brien, A. D., V. L. Tesh, A. Donohue-Rolfe, M. P. Jackson, S. Olsnes, K. Sandvig, A. A. Lindberg, and G. T. Keusch. 1992. Shiga toxin: biochemistry, genetics, mode of action, and role in pathogenesis. Curr. Top. Microbiol. Immunol. 180:65-94. [DOI] [PubMed] [Google Scholar]
- 29.Obrig, T. G., C. B. Louise, C. A. Lingwood, B. Boyd, L. Barley-Maloney, and T. O. Daniel. 1993. Endothelial heterogeneity in Shiga toxin receptors and responses. J. Biol. Chem. 268:15484-15488. [PubMed] [Google Scholar]
- 30.Oosterwijk, E., A. Kalisiak, J. C. Wakka, D. A. Scheinberg, and L. J. Old. 1991. Monoclonal antibodies against Gal alpha 1-4Gal beta 1-4Glc (Pk, CD77) produced with a synthetic glycoconjugate as immunogen: reactivity with carbohydrates, with fresh frozen human tissues and hematopoietic tumors. Int. J. Cancer 48:848-854. [DOI] [PubMed] [Google Scholar]
- 31.Randolph, G. J., V. Angeli, and M. A. Swartz. 2005. Dendritic-cell trafficking to lymph nodes through lymphatic vessels. Nat. Rev. Immunol. 5:617-628. [DOI] [PubMed] [Google Scholar]
- 32.Robinson, C. M., J. F. Sinclair, M. J. Smith, and A. D. O'Brien. 2006. Shiga toxin of enterohemorrhagic Escherichia coli type O157:H7 promotes intestinal colonization. Proc. Natl. Acad. Sci. USA 103:9667-9672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinclair, J. F., and A. D. O'Brien. 2001. Cell-surface localized nucleolin is a eucaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157: H7. J. Biol. Chem. 277:76-85. [DOI] [PubMed] [Google Scholar]
- 34.Sjogren, R., R. Neill, D. Rachmilewitz, D. Fritz, J. Newland, D. Sharpnack, C. Colleton, J. Fondacaro, P. Gemski, and E. C. Boedeker. 1994. Role of Shiga-like toxin I in bacterial enteritis: comparison between isogenic Escherichia coli strains induced in rabbits. Gastroenterology 106:306-317. [DOI] [PubMed] [Google Scholar]
- 35.Smith, M. J., L. D. Teel, H. M. Carvalho, A. R. Melton-Celsa, and A. D. O'Brien. 2006. Development of a hybrid Shiga holotoxoid vaccine to elicit heterologous protection against Shiga toxins types 1 and 2. Vaccine 24:4122-4129. [DOI] [PubMed] [Google Scholar]
- 36.Su, G. F., H. N. Brahmbhatt, J. Wehland, M. Rohde, and K. N. Timmis. 1992. Construction of stable LamB-Shiga toxin B subunit hybrids: analysis of expression in Salmonella typhimurium aroA strains and stimulation of B subunit-specific mucosal and serum antibody responses. Infect. Immun. 60:3345-3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tesh, V. L., and A. D. O'Brien. 1991. The pathogenic mechanisms of Shiga toxin and the Shiga-like toxins. Mol. Microbiol. 5:1817-1822. [DOI] [PubMed] [Google Scholar]
- 38.Yu, J., F. Cassels, T. Scharton-Kersten, S. A. Hammond, A. Hartman, E. Angov, B. Corthésy, C. Alving, and G. M. Glenn. 2002. Transcutaneous immunization using colonization factor and heat-labile enterotoxin induces correlates of protective immunity for enterotoxigenic Escherichia coli. Infect. Immun. 70:1056-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang, X., A. D. McDaniel, L. E. Wolf, G. T. Keusch, M. K. Waldor, and D. W. Acheson. 2000. Quinolone antibiotics induce Shiga toxin-encoding bacteriophages, toxin production, and death in mice. J. Infect. Dis. 181:664-670. [DOI] [PubMed] [Google Scholar]
- 40.Zhu, C., T. S. Agin, S. J. Elliott, L. A. Johnson, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2001. Complete nucleotide sequence and analysis of the locus of enterocyte effacement from rabbit diarrheagenic Escherichia coli RDEC-1. Infect. Immun. 69:2107-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu, C., S. Feng, T. E. Thate, J. B. Kaper, and E. C. Boedeker. 2006. Towards a vaccine for attaching/effacing Escherichia coli: a LEE encoded regulator (ler) mutant of rabbit enteropathogenic Escherichia coli is attenuated, immunogenic, and protects rabbits from lethal challenge with the wild-type virulent strain. Vaccine 24:3845-3855. [DOI] [PubMed] [Google Scholar]
- 42.Zhu, C., F. Ruiz-Perez, Z. Yang, Y. Mao, V. L. Hackethal, K. M. Greco, W. Choy, K. Davis, J. R. Butterton, and E. C. Boedeker. 2006. Delivery of heterologous protein antigens via hemolysin or autotransporter systems by an attenuated ler mutant of rabbit enteropathogenic Escherichia coli. Vaccine 24:3821-3831. [DOI] [PubMed] [Google Scholar]