Abstract
During the last few years, the use of type III secretion system-based bacterial vectors for immunotherapy purposes has been assessed in various applications. We showed that a type III secretion-based Pseudomonas aeruginosa vector delivering the ovalbumin (OVA) antigen induced an efficient specific CD8+ T-lymphocyte immune response against OVA-expressing cells. Because of the intrinsic toxicity of the vector, further virulence attenuation was needed. Therefore, we explored the effects of the deletion of quorum-sensing genes and the aroA gene toward toxicity and efficiency of the vector strain. The aroA mutation in our strain (making the strain auxotrophic for aromatic amino acids) conferred a strikingly reduced toxicity, with the bacterial lethal dose being more than 100 times higher than that of the parental strain. The quorum-sensing gene mutation alone was associated with a slightly reduced toxicity. In a prophylactic OVA-expressing melanoma mouse model, an OVA-delivering aroA-deficient mutant was the most efficient at a low dose (105), but dose enhancement was not associated with a greater immune response. The quorum-sensing-deficient strain was the most efficient at a mild dose (106), but this dose was close to the toxic dose. Combination of both mutations conferred the highest efficiency at an elevated dose (107), in agreement with the known negative effects of quorum-sensing molecules upon T-cell activation. In conclusion, we have obtained a promising immunotherapy vector regarding toxicity and efficiency for further developments in both antitumor and anti-infectious strategies.
The use of live bacteria and bacterial virulence factors as therapeutic tools in human medicine has been considered for more than a century. The observation that the onset of a bacterial infection could modify the course of a malignant disease (6) was a hallmark in this history, but in the end very few procedures (such as the intravesical administration of an attenuated Mycobacterium bovis strain for the cure of noninvasive urothelial carcinoma) have been routinely used. In the last 10 years, better characterization of bacterial mechanisms (mainly toxins and secretion systems) and extensive progress in genomic studies have allowed engineering of bacteria (mainly Escherichia coli and Salmonella spp.). These domesticated agents can be delivered to mammals for different purposes. Notably, the design of antigen-delivering bacteria that trigger antigen-specific cytotoxic CD8+ T-lymphocyte responses is an emerging field of investigation in vaccine development (5). Antigen delivery can be performed by using intrinsic properties of bacterial toxins (as with a Listeria monocytogenes-derived vector [21]) or secretion pathways normally used by bacteria to release toxins, such as the type III secretion system (TTSS). This system has been considered promising because it allows gram-negative rods to inject toxins into eukaryotic cell cytoplasm; therefore, epitopes delivered by this system are likely to be presented by antigen-presenting cell major histocompatibility complex I molecules and to activate cytotoxic T lymphocytes. Moreover, the bacterium-associated, Toll-like receptor-mediated danger signals would ensure the correct activation of antigen-presenting cells. Previous work showed that antigen-delivering TTSS-based Yersinia (24) and Salmonella (16) vectors can be used in antimicrobial and antitumor immunotherapies and induce simultaneous CD4 and CD8 antigen-specific lymphocytes (17). We recently showed that a TTSS-based Pseudomonas aeruginosa vector efficiently induced an antigen-specific CD8+ T-cell response and could be exploited in antitumor immunotherapy (8). The field of applications of these antigen-delivering vectors may be very large, and diverse disorders such as cancer, human immunodeficiency virus infection, or malaria are likely to be prevented or treated by microbe-based immunotherapy (12).
One limitation to the use of such vectors is their intrinsic toxicity. We had previously used a P. aeruginosa strain carrying deletions in the genes of the TTSS toxin exoenzymes ExoS and ExoT and a spontaneously ExoU-negative strain (strain CHA-OST), but toxicity reduction was not optimized. Here, we present the results concerning the toxicity and the efficiency obtained with much more attenuated P. aeruginosa TTSS-based vectors in an antitumor model.
MATERIALS AND METHODS
Bacterial strains.
CHA-OST, a ΔexoS Δorf1 ΔexoT Pseudomonas aeruginosa strain, has been previously described (20). Additional deletions of aroA, lasI, and rhlI were performed in CHA-OST using the Cre-lox system that we previously adapted to P. aeruginosa. This system allows performing multiple successive allelic exchanges in the same strain (20). The genes lasI and rhlI, encoding synthases of the quorum-sensing (QS) homoserine lactones (HSLs; 3-oxo-C12-HSL and C4-HSL, respectively), and the gene aroA were deleted by allelic exchange (see the primer sequences in Table 1). We generated three attenuated mutants from CHA-OST: CHA-OA (ΔexoS Δorf1 ΔexoT ΔaroA), CHA-OAL (ΔexoS Δorf1 ΔexoT ΔaroA ΔlasI), and CHA-ORL (ΔexoS Δorf1 ΔexoT ΔrhlI ΔlasI). Growth kinetic assays were performed in Luria-Bertani (LB) broth or Vogel-Bonner (VB) minimal broth by measuring the optical density at 600 nm (OD600).
TABLE 1.
Primer sequences for PCR fragments used in allelic exchange for deletion of genes aroA, lasI, and rhlI
| Gene | Primer direction | Primer sequence
|
|
|---|---|---|---|
| 5′-flanking region of gene | 3′-flanking region of gene | ||
| aroA | Forward | GCCGATTGTGCTAACCGCG | CGCTCATGTTCATACCTGTAG |
| Reverse | AGCCCTCCACTTCGGTGGT | TACGACATGCCGATGGCCAG | |
| lasI | Forward | AAGTGGCTATGTCGCCG | GGCCTGGACGTATCGCG |
| Reverse | AGTTTTTTATCGAACTCTTCGCGC | CTTAAGGAGTCGGACGGG | |
| rhlI | Forward | GCTCGGCGATCATGGCG | TGTCCGGAAATCCTCATGC |
| Reverse | CGCGGTGCGCCGCAAGG | GCGTCATCGGGCGTTCC | |
Plasmids and TTSS assay.
Production and delivery of nonbacterial proteins by the TTSS were obtained by transforming P. aeruginosa strains with plasmids pS54-Ova_ExsAi (to obtain ovalbumin [OVA] delivery) or pS54-GFP_ExsAi (to obtain green fluorescent protein [GFP] delivery). Briefly, these previously described plasmids (8) encode a transductional fusion between an optimized ExoS fragment (the first 54 amino acids of ExoS [ExoS54]) and ovalbumin (C-terminal fragment) or GFP under the control of an ExoS native promoter and an exsA gene encoding the ExoS transcriptional activator cloned under the control of an isopropyl-β-d-thiogalactopyranoside (IPTG)-inducible promoter. Strains transformed with these plasmids produce a fusion protein (S54-GFP or S54-Ova) when cultivated with IPTG; TTSS-mediated secretion of the fusion protein is obtained by calcium depletion or eukaryotic cell contact. Plasmid propagation is ensured by cultivating bacteria with 300 mg/liter carbenicillin.
Strains transformed by either pS54-Ova_ExsAi or pS54-GFP_ExsAi were cultivated from an OD600 of 0.2 in LB broth supplemented with 0.8 mM IPTG and/or 5 mM EGTA and 20 mM MgCl2 until they reached an OD600 of 1 to 2. ExoS54-fused GFP production was assessed in pellets after centrifugation and was expressed as the fluorescence intensity/OD600 ratio. ExsoS54-fused ovalbumin secretion was assessed in supernatants after centrifugation by using 10% trichloroacetic acid protein precipitation and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis.
For the immunization control, the CHA-OST strain was transformed with plasmid pExsAind. This previously described plasmid (9) contains the already-mentioned ExsA-inducible system without any fusion protein.
Mice.
Female C57BL/6 mice were purchased from Janvier SA (Le Genest-Saint-Isle, France) and used at 6 to 8 weeks of age. Experiments were approved by the Université J. Fourier committee for animal experimentation.
Vector injection.
Bacteria were grown in LB broth supplemented with 300 mg/liter carbenicillin and 0.8 mM IPTG from an OD600 of 0.2 to an OD600 of 1.5 and then resuspended in phosphate-buffered saline before a 100-μl subcutaneous injection in the right flank.
Mammalian cell lines.
B16-OVA is a melanoma cell line from C57BL/6 mice that constitutively expresses ovalbumin (3) and was cultivated in medium supplemented with 500 mg/liter Geneticin.
Tumor challenge.
A total of 2 × 105 B16-OVA cells were injected subcutaneously in the mouse left thigh. Tumor size was assessed every 2 days. Mice were sacrificed when the tumor diameter reached 1 cm.
RESULTS
Determination of time schedule.
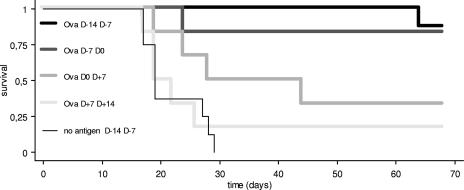
Using a partially attenuated CHA-OST strain, we determined which injection dates would be more appropriate in a two-injection schedule. Mice received subcutaneously 2 × 105 B16-OVA cells at day zero and were injected with 106 ovalbumin-delivering CHA-OST vectors either at days −14 and −7, at days −7 and 0, at days 0 and +7, or at days +7 and +14. As a negative control, another group received 106 CHA-OST vectors not delivering any antigen at days −14 and −7. Mice injected with the days −14 and −7 schedule demonstrated the best protection, with more than 80% of animals remaining tumor-free (Fig. 1); therefore, subsequent experiments with more attenuated strains were performed identically (unless indicated otherwise).
FIG. 1.
Determination of the best time schedule for vaccination using strain CHA-OST. Mice received the same dose of ovalbumin-delivering CHA-OST with different delays from tumor implantation (on day 0): at days −14 and −7, at days −7 and 0, at days 0 and day +7, or at days +7 and +14. A day −14 and day −7 schedule using a CHA-OST strain delivering no antigen was used as a negative control. Mice were sacrificed when the tumor diameter reached 1 cm.
Generation of attenuated vectors.
Target genes for further virulence attenuation were aroA and two genes participating in the QS system, lasI and rhlI. aroA-encoded 3-phosphoshikimate 1-carboxyvinyltransferase is a key enzyme in aromatic amino acid synthesis; the aroA deletion confers auxotrophy for aromatic amino acids and has been successfully used to elaborate attenuated P. aeruginosa strains for the purpose of anti-Pseudomonas vaccination (19). lasI and rhlI encode the two enzymes producing QS homoserine lactones 3-oxo-C12-HSL and C4-HSL, respectively; P. aeruginosa QS inactivation has been associated with virulence attenuation in various animal models for injury or illness, including those for pneumonia (14, 18), burns (23), and pyelonephritis (15).
We used the Cre-lox system that we previously adapted to Pseudomonas aeruginosa (20). By successive mutations, we generated three attenuated mutants from CHA-OST: CHA-OA (ΔexoS ΔexoT ΔaroA), CHA-OAL (ΔexoS ΔexoT ΔaroA ΔlasI), and CHA-ORL (ΔexoS ΔexoT ΔrhlI ΔlasI). All mutants were verified genetically by PCR (data not shown) and then phenotypically for growth rates and TTSS function.
In vitro characterization of attenuated vectors.
As the aroA deletion was previously reported to confer reduction of growth rates, we measured the growth of CHA-OST, CHA-OA, CHA-OAL, and CHA-ORL mutant vectors in LB rich medium and VB minimal medium and observed that CHA-OA and CHA-OAL grew slower than CHA-OST and CHA-ORL in both media (Table 2). This phenotype that results from aroA deletion is likely associated with reduced in vivo toxicity but could also result in a TTSS deficiency.
TABLE 2.
Growth kinetics (doubling times) of mutants CHA-OST, CHA-ORL, CHA-OA, and CHA-OAL in LB and VB broths
| Mutant | Mean doubling time (min) during exponential growth
|
|
|---|---|---|
| LB broth | VB broth | |
| CHA-OST | 44 | 68 |
| CHA-ORL | 45 | 55 |
| CHA-OA | 81 | 104 |
| CHA-OAL | 88 | 127 |
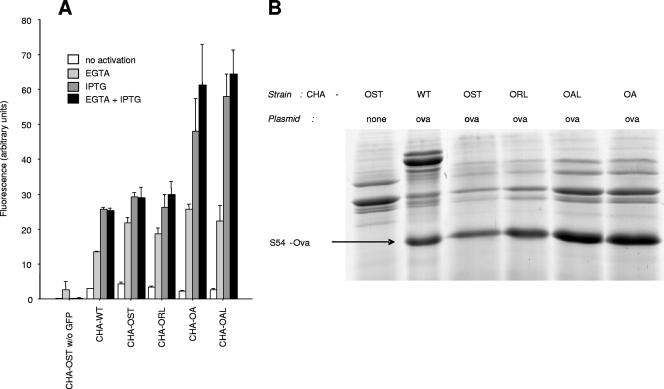
We therefore compared the in vitro TTSS efficiencies of the four strains. We obtained transformants with plasmid pS54-GFP_ExsAind or pS54-Ova_ExsAind and assessed the production and secretion by TTSS of ExoS54-fused proteins by the different strains. We used four growth conditions in LB medium: no TTSS stimulation, TTSS stimulation by calcium depletion induced by 5 mM EGTA (triggering production and secretion of the ExoS54-fused protein), exsA transcription induced by 0.8 mM IPTG (triggering only production of the ExoS54-fused protein), and supplementation with both EGTA and IPTG.
ExoS54-GFP production was assessed by measuring fluorescence in the culture pellet of pS54-Ova_ExsAi transformants. We observed that mutants CHA-ORL, -OA, and -OAL demonstrated identical (ORL) or even higher (OA and OAL) GFP production levels compared to CHA-OST or the wild-type CHA strain (Fig. 2A). This feature was promising for TTSS-based immunotherapy. The same results were obtained when assessing TTSS-mediated secretion of ExoS54-Ova by SDS-PAGE (we did not assess secretion of ExoS54-GFP secretion by fluorimetry because of the Luria-Bertani broth high background fluorescence). Mutants CHA-ORL, -OA, and -OAL secrete identical or higher amounts of the fusion protein compared with CHA-OST and wild-type CHA (Fig. 2B). This indicates that although the growth of aroA-deleted strains is affected, TTSS function is maintained.
FIG. 2.
In vitro TTSS efficiencies of mutants. (A) Fluorescence intensity in the culture pellet of the wild-type (WT) strain and different mutants transformed by plasmid pS54-GFP_ExsAi under different TTSS-activating conditions. The negative control was CHA-OST without plasmid. Error bars represent 1 standard error. (B) SDS-PAGE analysis of secretion of S54-Ova by the WT strain and different mutants transformed by plasmid pS54-Ova_Exsai. Culture medium was supplemented with both IPTG and EGTA. The negative control was CHA-OST without plasmid.
In vivo toxicity of attenuated mutants.
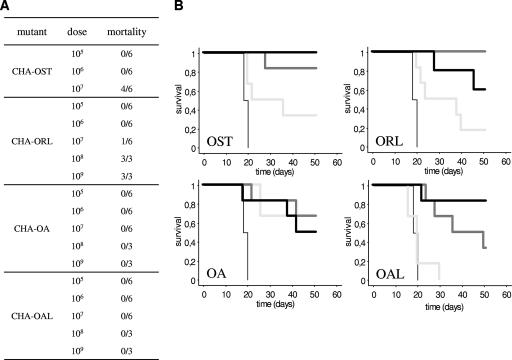
We then assessed the in vivo toxicity of these different strains by observing mortality after one subcutaneous injection of 105, 106, or 107 ovalbumin-delivering bacteria to 6-week-old female C57BL/6 mice (Fig. 3A). The pS54-Ova_ExsAi-transformed bacteria were grown in medium containing 300 mg/liter carbenicillin and 0.8 mM IPTG from an OD600 of 0.2 to an OD600 between 1 and 2. When injecting 107 bacteria, four of six mice injected with CHA-OST and one of six mice injected with CHA-ORL died in the first 40 h. No death was observed in the groups vaccinated with 107 CHA-OA or CHA-OAL or in other groups vaccinated with 105 or 106 bacteria. We then assessed the lethality of doses of 108 and 109 for CHA-ORL, CHA-OA, and CHA-OAL; all the mice injected with strain CHA-ORL died at both doses, while all the mice injected with CHA-OA or CHA-OAL remained alive. Therefore, these different mutants demonstrate a partially (CHA-ORL) or greatly (CHA-OA and CHA-OAL) reduced in vivo toxicity.
FIG. 3.
In vivo toxicity (A) and efficiency (B) of TTSS-based mutant vectors. (A) Mouse mortality after subcutaneous injection of 105, 106, 107, 108, or 109 mutants. (B) Survival after tumor implantation in mice vaccinated beforehand twice with 105 (thick pale gray line), 106 (thick dark gray line), or 107 (thick black line) mutant vectors delivering S54-Ova: CHA-OST, CHA-ORL, CHA-OA, and CHA-OAL. The negative control was 106 CHA-OST not delivering S54-Ova (thin line).
In vivo efficiency of attenuated mutants.
We then assessed the antigen delivery efficiency of these vectors in a mouse model of prophylactic immunotherapy using syngeneic B16 melanoma cells expressing ovalbumin as a model antigen (B16-OVA cell line). Surviving mice from the previous experiment (105, 106, and 107 groups) were subcutaneously injected with an identical dose of the same ovalbumin-delivering P. aeruginosa strain at day 8 (to complete the two-injection schedule), and a subcutaneous injection (in a different site) of 2 × 105 B16-OVA cells was performed at day 15. One more group received two injections of CHA-OST without ovalbumin as a negative control before tumor challenge. Mice were sacrificed when tumor diameters reached 1 cm. As we demonstrated previously, the ability of the vector to induce an efficient antiovalbumin cellular immune response is associated with inhibition of the onset of tumor growth.
We observed contrasting dose-dependent efficiencies (Fig. 3B). For the lowest vector dose (105), CHA-OST and CHA-ORL demonstrated mild tumor growth inhibition and CHA-OA and CHA-OAL had higher and lower efficiencies, respectively. At a mild dose (106), protection was almost complete or complete for CHA-OST and CHA-ORL, respectively; CHA-OAL showed an improved efficiency, and CHA-OA efficiency was comparable with the previous lower dose. At the highest dose (107), CHA-OAL showed an almost complete protection and CHA-ORL and CHA-OA showed comparable or lower efficiency, respectively; the two surviving mice injected with 107 CHA-OST did not develop tumors.
Influence of modification of the injection schedule.
We explored the influence of the immunization schedule (dose and frequency) upon the efficiency of the most attenuated vector, CHA-OAL, in the prophylactic anti-B16-OVA assay. The following conditions were assessed: one injection of either 105 or 106 bacteria at days 1 and 8, either one or two injections of 105 bacteria at days 1, 4, 7, 10, and 13, or either one or two injections of 106 bacteria at days 1, 4, 7, 10, and 13. Tumor challenge was performed on day 15.
Table 3 shows the proportion of tumor-free mice at day 45. We observed the same relation between dose and efficiency, and the vaccination schedule (two or five injections) of the same total dose (106 or 107) had no influence upon protection. Indeed, mice injected with a total dose of 106 bacteria were not protected, and mice vaccinated with a total dose of 5 × 106 or 107 were almost all protected; splitting the total dose in two or five injections did not influence the antitumor protection.
TABLE 3.
Antitumor protection after prophylactic vaccination using S54-Ova delivering CHA-OAL at different schedules and doses: proportion of tumor-free mice at day 45
| Exptl group | Dose (no. of mutant vectors/injection) | No. of injection rounds (no. of injections per round) | Total dose (no. of mutant vectors delivered) | No. of tumor-free mice at day 45/no. injected |
|---|---|---|---|---|
| NCa | 5 × 106 | 2 (1) | 107 | 0/5 |
| Vaccinated | 5 × 106 | 2 (1) | 107 | 4/6 |
| 1 × 105 | 2 (1) | 2 × 105 | 0/6 | |
| 1 × 105 | 5 (1) | 5 × 105 | 0/6 | |
| 5 × 105 | 2 (1) | 106 | 0/6 | |
| 1 × 105 | 2 (5) | 106 | 0/6 | |
| 1 × 106 | 2 (1) | 2 × 106 | 3/5 | |
| 1 × 106 | 5 (1) | 5 × 106 | 5/6 | |
| 1 × 106 | 2 (5) | 107 | 5/6 | |
| 1 × 107 | 2 (1) | 2 × 107 | 5/6 |
NC, negative control (CHA-OST with no antigen delivered).
DISCUSSION
The aim of this study was to generate mutants of our P. aeruginosa strain with reduced toxicity and conserved or enhanced efficiency for immunotherapy purposes. We chose two different mutagenesis targets: aromatic amino acid metabolism and the QS system.
Auxotrophic strains (notably auxotrophic for aromatic amino acids) have been generated to obtain attenuated vaccine strains in mammals (1, 4, 10) and fish (26) to trigger a protective antibacterial response and in immunotherapy studies to obtain safer vectors (16). Our ΔaroA mutants (CHA-OA and CHA-OAL) have reduced growth rates in LB and VB media, and the dramatic mortality reduction observed when injecting high amounts of these strains into C57BL/6 mice is likely to be related to a low multiplication in the host, allowing elimination of the bacteria. However, several works have demonstrated the link between metabolic processes and virulence in prokaryotes, notably for TTSS (7). Therefore, the low toxicities of our mutants could have been also related to a low virulence because of a decrease in TTSS function. In contrast, an in vitro study of strains transformed with plasmids pS54-Ova_ExsAi and pS54-GFP_ExsAi showed for both ExoS54-fused proteins an enhanced type III production or secretion level, demonstrating that toxicity reduction was not due to TTSS inactivation but mainly to a low replication capacity in the mice. The good results observed when assessing the performance of immunotherapy vectors of the two ΔaroA strains are due to this interesting association of poor replication and enhanced TTSS levels.
Considering QS system inactivation, previous experiments showed that it conferred a reduced virulence in various models, in accordance with the role of QS signaling of many virulence factors of P. aeruginosa. Several studies (2, 11) have determined that production of 3-oxo-C12-HSL and C4-HSL induces a down-regulation of the TTSS level, as observed when the bacterial density is high. Therefore, rhlI and/or lasI mutations were likely to be associated with greater, or at least unchanged, TTTS levels compared with the parental strain; this was confirmed when measuring S54-GFP production or S54-Ova secretion by CHA-ORL and CHA-OAL. Moreover, it has been demonstrated that HSLs display pleiotropic activity upon the immune system, particularly macrophages and T lymphocytes. Indeed, gamma interferon secretion by T lymphocytes after antigen-specific stimulation is decreased when cells are incubated with 3-oxo-C12-HSL (22), and the same HSLs display a negative influence upon T-lymphocyte proliferation after concanavalin A activation (25). This may explain why the ΔlasI vector CHA-OAL displayed better results at high doses (107) than CHA-OA. The use of this mutant may be associated with no down-regulation of the CD8+ T-lymphocyte response and therefore a better destruction of targeted cells.
In addition, it is also noteworthy that an aroA mutation, which alters the production of aromatic amino acids, may influence the synthesis of signal molecules important for virulence of P. aeruginosa (13).
To summarize, aroA mutation is likely to confer a higher TTSS functional level and a greatly reduced toxicity, along with a reduced intrahost multiplication, and a QS mutation is likely to confer a slightly reduced toxicity and a reduced negative effect of QS molecules upon the immune system.
Taken together, these results may lead investigators to choose CHA-OAL for further development. Indeed, CHA-ORL demonstrated the highest efficiency for a nonlethal dose (complete antitumor protection at 106), but toxicity reduction was mild. CHA-OA has a strikingly reduced lethality and demonstrated efficiency at a low dose (105); this great therapeutic index is a key property for further development. However, its efficiency is lower than those of CHA-ORL and CHA-OAL at higher doses. CHA-OAL shares the same toxicity reduction as CHA-OA but demonstrates a better efficiency at high doses. This maximally attenuated strain may represent the best compromise between virulence attenuation and efficiency.
Moreover, the exploration of various injection schedules (modification of dose and frequency) using CHA-OAL showed that two injections were enough to obtain an important protection and that it was not necessary to split the total dose among more injections. This simplicity is also an argument to consider this strain for immunotherapy purposes.
Acknowledgments
This work was supported by grants from the Association pour la Recherche contre le Cancer and from the Agence Nationale de la Recherche (“Emergence et Maturation de Projets de biotechnologie” BacVac 2007).
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Abd El Ghany, M., A. Jansen, S. Clare, L. Hall, D. Pickard, R. A. Kingsley, and G. Dougan. 2007. Candidate live, attenuated Salmonella enterica serovar Typhimurium vaccines with reduced fecal shedding are immunogenic and effective oral vaccines. Infect. Immun. 75:1835-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bleves, S., C. Soscia, P. Nogueira-Orlandi, A. Lazdunski, and A. Filloux. 2005. Quorum sensing negatively controls type III secretion regulon expression in Pseudomonas aeruginosa PAO1. J. Bacteriol. 187:3898-3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown D. M., T. L. Fisher, C. Wei, J. G. Frelinger, and E. M. Lord. 2001. Tumours can act as adjuvants for humoral immunity. Immunology 102:486-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buzzola, F. R., M. S. Barbagelata, R. L. Caccuri, and D. O. Sordelli. 2006. Attenuation and persistence of and ability to induce protective immunity to a Staphylococcus aureus aroA mutant in mice. Infect. Immun. 74:3498-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chabalgoity, J. A., G. Dougan, P. Mastroeni, and R. J. Aspinall. 2002. Live bacteria as the basis for immunotherapies against cancer. Expert Rev. Vaccines 1:495-505. [DOI] [PubMed] [Google Scholar]
- 6.Coley, W. B. 1893. The treatment of malignant tumours by repeated inoculations of erysipelas, with a report of ten original cases. Am. J. Med. Sci. 105:487-511. [PubMed] [Google Scholar]
- 7.Dacheux, D., O. Epaulard, A. de Groot, B. Guery, R. Le Berre, I. Attree, B. Polack, and B. Toussaint. 2002. Activation of the Pseudomonas aeruginosa type III secretion system requires an intact pyruvate dehydrogenase aceAB operon. Infect. Immun. 70:3973-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epaulard, O., B. Toussaint, L. Quénée, M. Derouazi, N. Bosco, C. Villiers, R. Le Berre, B. Guery, D. Filopon, L. Crombez, P. Marche, and B. Polack. 2006. Anti-tumour immunotherapy using a live Pseudomonas aeruginosa type III secretion system-based vector in a mouse model. Mol. Ther. 14:656-661. [DOI] [PubMed] [Google Scholar]
- 9.Filopon, D., A. Merieau, G. Bernot, J. P. Comet, R. Le Berre, B. Guery, B. Polack, and J. Guespin-Michel. 2006. Epigenetic acquisition of inducibility of type III cytotoxicity in P. aeruginosa. BMC Bioinformatics 7:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fittipaldi, N., J. Harel, B. D'Amours, S. Lacouture, M. Kobisch, and M. Gottschalk. 2007. Potential use of an unencapsulated and aromatic amino acid-auxotrophic Streptococcus suis mutant as a live attenuated vaccine in swine. Vaccine 25:3524-3535. [DOI] [PubMed] [Google Scholar]
- 11.Hogardt, M., M. Roeder, A. M. Schreff, L. Eberl, and J. Heesemann. 2004. Expression of Pseudomonas aeruginosa ExoS in controlled by quorum sensing and RpoS. Microbiology 150:843-851. [DOI] [PubMed] [Google Scholar]
- 12.Kotton, C. N., A. J. Lankowski, N. Scott, D. Sisul, L. M Chen, K. Raschke, G. Borders, M. Boaz, A. Spentzou, J. E. Galan, and E. L. Hohmann. 2006. Safety and immunogenicity of attenuated Salmonella enterica serovar Typhimurium delivering an HIV-1 Gag antigen via the Salmonella type III secretion system. Vaccine 24:6216-6224. [DOI] [PubMed] [Google Scholar]
- 13.Lesic, B., F. Lépine, E. Déziel, J. Zhang, Q. Zhang, K. Padfield, M. H. Castonguay, S. Milot, S. Stachel, A. A. Tzika, R. G. Tompkins, and L. G. Rahme. 2007. Inhibitors of pathogen intercellular signals as selective anti-infective compounds. PLoS Pathog. 3:1229-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lesprit, P., F. Faurisson, O. Join-Lambert, F. Roudot-Thoraval, M. Foglino, C. Vissuzaine, and C. Carbon. 2003. Role of the quorum-sensing system in experimental pneumonia due to Pseudomonas aeruginosa in rats. Am. J. Respir. Crit. Care Med. 167:1478-1482. [DOI] [PubMed] [Google Scholar]
- 15.Mittal, R., S. Sharma, S. Shhibber, and K. Harjai. 2006. Contribution of quorum-sensing systems to virulence of Pseudomonas aeruginosa in an experimental pyelonephritis model. J. Microbiol. Immunol. Infect. 39:302-309. [PubMed] [Google Scholar]
- 16.Nishikawa, H., E. Sato, G. Briones, L. M. Chen, M. Matsuo, Y. Nagata, G. Ritter, E. Jager, H. Nomura, S. Kondo, I. Tawara, T. Kato, H. Shiku, J. Old, J. E. Galan, and S. Gnatic. 2006. In vivo antigen delivery by a Salmonella typhimurium type III secretion system for therapeutic cancer vaccines. J. Clin. Investig. 116:1946-1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Panthel, K., K. M. Meinel, V. E. Domenech, H. Retzbach, E. I. Igwe, W. D. Hardt, and H. Russmann. 2005. Salmonella pathogenicity island 2-mediated overexpression of chimeric SspH2 proteins for simultaneous induction of antigen-specific CD4 and CD8 T cells. Infect. Immun. 73:334-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pearson J. P., M. Feldman, B. H. Iglewski, and A. Prince. 2000. Pseudomonas aeruginosa cell-to-cell signalling is required for virulence in a model of acute pulmonary infection. Infect. Immun. 68:4331-4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Priebe, G. P., G. J. Meluleni, F. T. Coleman, J. B. Goldberg, and G. B. Pier. 2003. Protection against fatal Pseudomonas aeruginosa pneumonia in mice after nasal immunization with a live, attenuated aroA deletion mutant. Infect. Immun. 71:1453-1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Quénée, L., D. Lamotte, and B. Polack. 2005. Combined sacB-based negative selection and Cre-lox antibiotic marker recycling for efficient gene deletion in Pseudomonas aeruginosa. BioTechniques 38:63-67. [DOI] [PubMed] [Google Scholar]
- 21.Radford, K. J., D. E. Higgins, S. Pasquini, E. J. Cheadle, L. Carta, A. M. Jackson, N. R. Lemoine, and G. Vassaux. 2002. A recombinant E. coli vaccine to promote MHC class I-dependent antigen presentation: application to cancer immunotherapy. Gene Ther. 9:1455-1463. [DOI] [PubMed] [Google Scholar]
- 22.Ritchie, A. J., A. Jansson, J. Stallberg, P. Nilsson, P. Lysaght, and M. A. Cooley. 2005. The Pseudomonas aeruginosa quorum-sensing molecule N-3-(oxododecanoyl)-l-homoserine lactone inhibits T-cell differentiation and cytokine production by a mechanism involving an early step in T-cell activation. Infect. Immun. 71:1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rumbaugh, K. P., J. A. Griswold, B. H. Iglewski, and A. N. Hamood. 1999. Contribution of QS to the virulence of Pseudomonas aeruginosa in burn wound infections. Infect. Immun. 67:5854-5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Russmann, H., U. Gerdemann, E. I. Igwe, K. Panthel, J. Heesemann, S. Garbom, H. Wolf-Watz, and G. Geginat. 2003. Attenuated Yersinia pseudotuberculosis carrier vaccine for simultaneous antigen-specific CD4 and CD8 T-cell induction. Infect. Immun. 71:3463-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telford, G., D. Wheeler, P. Williams, P. T. Tomkins, P. Appleby, H. Sewell, G. S. A. B. Stewart, B. W. Bycroft, and D. I. Pritchard. 1998. The Pseudomonas aeruginosa quorum-sensing signal molecule N-(3-oxododecanoyl)-l-homoserine lactone has immunomodulatory activity. Infect. Immun. 66:36-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vivas, J., J. Riano, B. Carracedo, B. E. Razquin, P. Lopez-Fierro, G. Naharro, and A. J. Villena. 2004. The auxotrophic aroA mutant of Aeromonas hydrophila as a live attenuated vaccine against A. salmonicida infections in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 16:193-206. [DOI] [PubMed] [Google Scholar]
- 27.Reference deleted.