Abstract
Following a demonstration that mouse-optimized cytosine-guanosine dinucleotide (CpG) oligodeoxynucleotides stimulated innate immune protection against intracellular pathogens, we tested the ability of CpG 7909, a primate-optimized Toll-like receptor 9 (TLR9) agonist, to stimulate rhesus macaques to produce interferon-inducible protein-10 (IP-10), a biomarker of immune activation. This study was performed prior to a similar trial with humans in order to facilitate the development of CpG 7909 as an immunomodulator for biodefense. A single subcutaneous dose of clinical-grade CpG 7909 was given to four groups of healthy adult rhesus macaques (0-mg dose [n = 5], 0.75-mg dose [n = 9], 1.5-mg dose [n = 9], and 3.0-mg dose [n = 9]). Directed physical examination findings, clinical laboratory values, and serum IP-10 concentrations were collected at scheduled intervals for 28 days. All three dose levels of CpG 7909 were safe and not associated with significant clinical or laboratory abnormality. The time to peak serum IP-10 concentration was 1.0 days at the 0.75-mg dose and 0.5 days at the 1.5- and 3.0-mg doses. A dose-dependent response was observed for the magnitude and duration of IP-10 concentrations, which remained significantly above baseline for 3 days for the 3.0-mg and 1.5-mg dose groups but above baseline for only 2 days for the 0.75-mg dose group. There were no nonresponders to CpG 7909. These rhesus macaque safety and IP-10 response data closely parallel a subsequent phase 1 human study of subcutaneously administered CpG 7909. A single dose of clinical-grade CpG 7909 induced a rapid, sustained IP-10 response, a biomarker for activation of the innate immune system. Given the similar susceptibilities of humans and rhesus macaques to infectious diseases, the rhesus macaque appears to be a suitable model to evaluate the potential of CpG 7909-mediated innate immune activation to protect humans against pathogens.
The activated innate immune system has the capacity to recognize, contain, and eliminate a wide array of pathogens long before the adaptive immune system has had a chance to respond. Activation of the innate immune system by pharmacological means offers the potential to rapidly protect vulnerable populations against diverse pathogens (2). The discovery of Toll-like receptors (TLRs) and their associated ligands has led to the development of pharmacological agents capable of rapidly activating protective innate immune defenses. Preexposure treatment of mice with a single dose of nonantisense oligodeoxynucleotides (ODNs) containing umethylated cytosine-guanosine dinucleotide (CpG) sequences significantly improves survival after exposure to lethal bacteria, mycobacteria, viruses, and parasites (7). However, there are significant species-specific differences in the optimal CpG sequences for potency, as well as species-specific differences in the cellular distributions of TLR9, the ligand for CpG ODNs. Thus, studies with primates in which optimal primate CpG ODNs are used are more relevant in modeling human responses than are optimal mouse CpG ODNs in mouse models.
As part of the Defense Advanced Research Projects Agency Pathogen Countermeasures Program (http://www.darpa.mil/dso/archives/pc/index.htm), we evaluated for the first time the ability of a single subcutaneous dose of clinical-grade CpG ODN 7909 to activate innate immunity in a rhesus macaque. CpG 7909 is an immunomodulating synthetic class B ODN that directly activates primate TLR9-bearing plasmacytoid dendritic cells and B cells in vitro. GMP CpG 7909 is in clinical development as an adjuvant for vaccines and as an immunotherapeutic agent for allergy, asthma, cancer, and infectious diseases (11). In an effort to determine a safe, well-tolerated systemic dose that would activate the innate immune system of primates, we selected three different dose levels of CpG 7909 and measured the subsequent immune activation by a determination of serum interferon (IFN)-inducible protein-10 (IP-10). Human IP-10, also known as CXCL 10, is a chemokine induced by gamma IFN (IFN-γ) and by IFN-α and is thought to indicate activation of the immune system (8). Although we were aware of no studies with humans describing potential biomarkers of the pharmacological activation of protective innate immunity, IP-10 was selected as a putative biomarker for the presently described primate studies based upon its anticipated induction by CpG 7909-stimulated expression of the short-lived cytokine IFN-α. This dose-ranging work was conducted as part of a comprehensive program to develop CpG ODNs for the immunoprophylaxis of infectious diseases in 2001 immediately following the nationally publicized anthrax bioterrorism attacks in the United States and before initial trials of subcutaneous CpG 7909 in humans (6).
MATERIALS AND METHODS
Ethical approval.
Animal work was performed under a protocol approved by the WRAIR institutional animal care and use committee. All animal experiments described herein were in compliance with the Animal Welfare Act, adhered to the principles of the Guide for the Care and Use of Laboratory Animals, and were in accordance with all applicable U.S. Department of Agriculture, Office of Laboratory Animal Welfare, and Department of Defense guidelines.
CpG 7909.
The lyophilized test article (CpG 7909, PF-3512676 [ProMune]; Coley Pharmaceutical Group, Wellesley, MA) was reconstituted using sterile saline 0.9% for injection, USP. The vial containing 100 mg CpG 7909, lot ACZ-01D-006-M, dated 29 November 2001, was initially reconstituted with 2.00 ml sterile saline to generate a stock solution of 50 mg/ml. Subsequent dilutions with sterile saline were made to yield three final concentrations of 6.0, 3.0, and 1.5 mg/ml. The injection volume of 0.5 ml therefore delivered 3.0, 1.5, and 0.75 mg/injection, respectively.
Enrollment and allocation.
This open-label study was conducted with healthy, colony-bred Macaca mulatta macaques of Indian origin. There were 13 females and 19 males, with ages ranging from 5 to 13 years and weights from 5.4 to 13.4 kg. The monkeys were allocated into one group of five monkeys and three groups of nine monkeys each to achieve balance in terms of sex, age, and body weight. The control group and the three experimental groups were assigned to receive a saline control and 0.75, 1.5, and 3.0 mg of CpG 7909, respectively.
Procedures.
Animals were sedated using one of three intramuscular combination regimens (ketamine-acepromazine, ketamine-diazepam, or tiletamine-zolazepam, chosen based on prior experience with the individual animal) before examination or blood draw. A patch of fur was shaved over the right deltoid and a 2-cm-diameter circle drawn with a skin-marking pen. CpG 7909 was diluted in phosphate-buffered saline and the 0.5-ml final volume administered by subcutaneous injection into the shaved right deltoid region by use of a 0.5-ml insulin syringe. The monkeys were examined and blood samples were taken just prior to the time of initial injection and 12, 24, 48, 72, and 96 h later as well as 7, 14, and 28 days later.
Injection site assessments.
The injection site area of erythema was measured for width and length and the mean diameter reported in mm. The area of local skin swelling (edema) was similarly measured, and in addition an estimate was made of the maximum increase in skin fold thickness. Swelling was then reported as the product of the mean diameter and the depth in mm.
Fever.
Fever was defined as an instance of rectal temperature 2 standard deviations greater than an individual animal's baseline temperature as measured in over 20 separate observations during the preceding 6-month period.
Spleen and lymph node.
Spleens were scored on a ordinal scale of 0, 1, 2, and 3, where 0 was normal or not palpable, 1 was slightly enlarged or slightly firmer than normal, 2 was moderately enlarged and/or firmer than normal, and 3 was markedly enlarged and firm. Axillary lymph nodes were scored on the following ordinal scale: 0 was a node that was small, soft, and flat; 1 was either larger than normal, firmer or rounder than normal, or more numerous than normal; 2 had two of the above-described conditions; and 3 had all three. Lymph node data were then transformed by comparison to the contralateral nodes, and the differences were reported. Individual monkey data were then reported by treatment group and by day of study.
Clinical pathology.
At 0, 1, 2, 3, 4, 7, 14, and 28 days, the following parameters were tested: red blood cell count, white blood cell count, platelets, blood urea nitrogen, serum creatinine, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyl transferase, and creatine kinase.
Two-milliliter blood samples for hematologic determinations were collected into Vacutainer K3 EDTA tubes (Becton Dickinson Inc., Franklin Lakes, NJ) and analyzed on a Coulter AcT 10 hemocytometer (Beckman Coulter Inc., Miami, FL) according to the manufacturers’ recommendations. Serum from whole blood collected in uncoated Vacutainer tubes was analyzed on a Vitros 250 chemistry system (Ortho-Clinical Diagnostics Inc., Rochester, NY) by the Clinical Pathology Laboratory, Division of Veterinary Medicine of the Walter Reed Army Institute of Research.
IP-10 determination.
IP-10 serum samples were collected at 0, 0.5, 1, 2, 3, 7, 14, and 28 days and frozen for batch processing. IP-10 was assayed in serum by use of a commercial enzyme-linked immunosorbent assay kit (Quantikine; R&D Systems Inc, Minneapolis, MN). Other studies have validated the cross-reactivity of this kit with Macaca mulatta IP-10 (1, 5, 9). The manufacturer's instructions were exactly followed. In every case, a standard curve with the supplied recombinant IP-10 was included as part of the day's experiment; curve-fitting software inherent in SoftMax Pro 3.1 plate-reading software was used to solve for the absorbance of each test sample and produce a value in pg/ml IP-10.
Data analysis.
Data were compiled, graphed, and analyzed using Excel 5.0 (Microsoft, Seattle, WA) and SigmaStat version 3.5 (Systat Software, Richmond, CA). IP-10 group responses were initially compared using Kruskal-Wallis one-way analysis of variance on ranks. Selected two-way comparisons between groups were made using Dunn's method without adjustments for multiple comparisons.
RESULTS
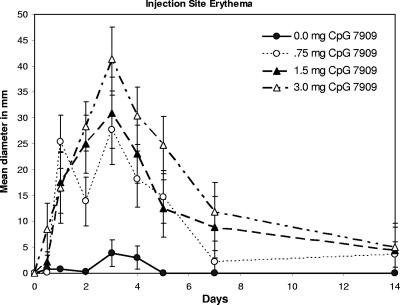
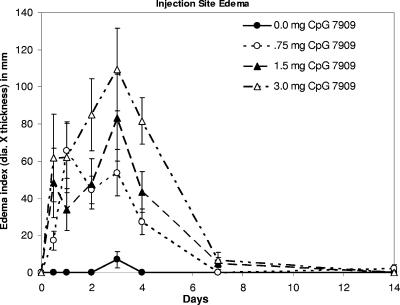
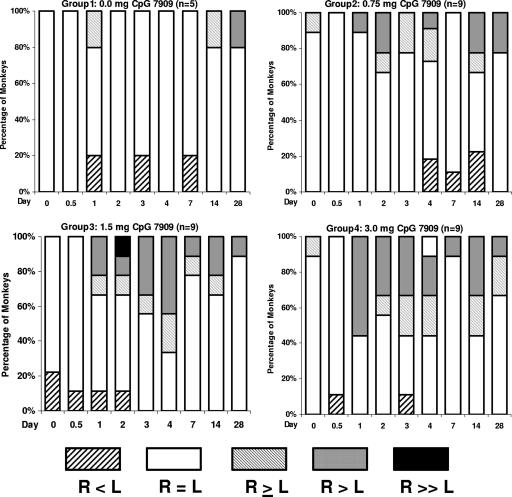
Groups were comparable in terms of weight, age, and sex, and all 32 monkeys completed the study (Table 1) . All injections were safe and very well tolerated. There was only one instance of fever as defined by the study protocol; on day 28 after injection, one animal had a temperature 0.1°C above the normal range defined for that animal. A trend toward mild local injection site erythema (Fig. 1) and swelling (Fig. 2) was noted for some individual monkeys in all three CpG 7909 groups; these trends peaked at day 3 and appeared somewhat dose dependent. However, none of the experimental group responses were proven to be statistically different from the control group responses at day 7. There were no ulcerations, granulomas, or other injection site abnormalities through day 28. Transient ipsilateral axillary lymph node enlargement occurred in the 1.5-mg and 3.0-mg dose groups, which was significantly different from what was seen for the control group only on day 1 (Fig. 3). No changes were noted in spleen size.
TABLE 1.
Baseline characteristics of groups
| Group (dosage) | Mean age (yr) (SD) | Mean wt (kg) (SD) | No. of males/total (% male) |
|---|---|---|---|
| Saline control | 8.8 (3.7) | 7.8 (0.7) | 3/5 (60) |
| CpG 7909 (0.75 mg) | 7.7 (3.6) | 8.1 (2.0) | 5/9 (55.6) |
| CpG 7909 (1.5 mg) | 9.0 (3.6) | 8.3 (2.3) | 5/9 (55.6) |
| CpG 7909 (3.0 mg) | 9.2 (3.3) | 8.6 (1.5) | 6/9 (66.7) |
FIG. 1.
Erythematous reaction to CpG administration by dose group over time. Each point depicts the group average diameter of injection site erythema in millimeters. Error bars depict the standard errors of the mean. All erythema had resolved by day 28.
FIG. 2.
Edematous reaction to CpG administration by dose group over time. Each point depicts the group average diameter (dia.) of edema multiplied by the estimated depth of edema in millimeters. Error bars depict the standard errors of the mean. All edema had resolved by day 28.
FIG. 3.
Relative size differences between right and left axillary lymph nodes after CpG 7909 administration by dose group over time.
Group average red blood cell count, white blood cell count, blood urea nitrogen, serum creatinine, alanine aminotransferase, and gamma-glutamyl transferase values remained within normal ranges. Transient elevations above the normal range for aspartate aminotransferase and creatine kinase peaked at day 1 and were observed for all animals, including the saline controls, but the CpG 7909 groups did not differ from the saline controls. Similar elevations have always been observed previously from the effects of intramuscularly delivered sedation. There were no deaths, serious illnesses, or unexpected medical events during the study.
IP-10 concentrations for all groups were equivalent at baseline, but experimental groups differed from the control group at 12 h (P < 0.001), 24 h (P = 0.004), 48 h (P = 0.001), and 72 h (P = 0.040) but not at 7, 14, or 28 days. A summary graph of the average serum IP-10 concentration over time is presented in Fig. 4 (data not shown for 14 and 28 days).
FIG. 4.
Serum IP-10 kinetics by dose group after subcutaneous injection of CpG 7909. Each point depicts the group average IP-10 concentration in pg/ml. Error bars depict the standard errors of the mean. All CpG 7909 group values were greater than the control group values at 12, 24, and 48 h. The 1.5-mg and 3.0-mg CpG 7909 group values were greater than the control group values at 72 h.
A dose-dependent effect was observed for the time to peak IP-10 concentration. The 3.0-mg dose group had a more rapid peak of serum IP-10 concentrations, with six of nine individual monkeys having their highest value at the 12-h time point. In the 1.5-mg dose group, four of nine monkeys had their highest individual value at 12 h, and in the 0.75-mg dose group, only two of nine monkeys peaked at that time point. All other monkey peak serum IP-10 values were noted at 24 h.
A dose-dependent effect was also observed for the duration of significant IP-10 elevation. The 3.0- and 1.5-mg dose groups were statistically different from control group at 12, 24, 48, and 72 h by Dunn's method (P < 0.05). In contrast, the 0.75-mg group IP-10 concentrations differed from those for controls only at 24 and 48 h and did not differ from controls at 72 h.
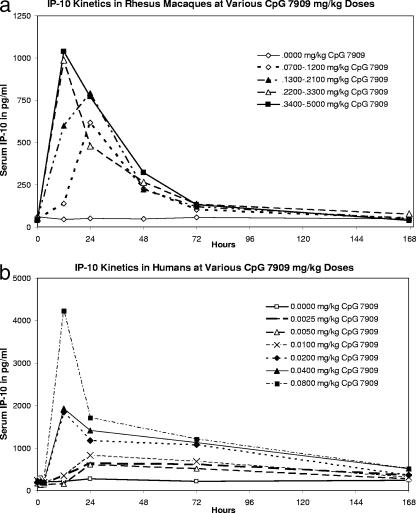
In order to compare these results with a recent clinical study of CpG 7909 administered subcutaneously to humans on a mg-per-kg of body weight basis (6), we replotted the present study's experimental groups according to the CpG dose in mg per kg of weight (Fig. 5a) and coplotted the human data (Fig. 5b). For both Homo sapiens and Macaca mulatta, higher CpG 7909 doses in mg/kg were associated with earlier, higher, and more sustained IP-10 concentrations than were lower doses. Of note was the fact that the highest human dose of 0.0800 mg/kg CpG 7909 was equivalent to the lowest rhesus dose group range of 0.0700 to 0.1200 mg/kg. In humans, the peak IP-10 responses were approximately fourfold greater than the peak IP-10 responses in rhesus macaques and remained significantly above baseline for up to 7 days rather than the 3-day extent of significant elevation above the control group observed for the two higher doses of CpG 7909 in rhesus macaques.
FIG. 5.
Comparison of CpG 7909 dose in mg/kg versus IP-10 in rhesus macaques (a) and in humans (b). Each point depicts IP-10 in pg/ml. In the rhesus study, there were five to eight animals per group. In the human study, there were six or seven subjects per group.
DISCUSSION
We have demonstrated the safety of a single subcutaneous 0.75-mg dose of CpG 7909 along with its ability to rapidly induce readily detectable concentrations of serum IP-10 for 2 days in rhesus macaques, as well as the ability of single doses of 1.5 mg or 3.0 mg of CpG 7909 to induce detectable concentrations of serum IP-10 for at least a 3-day period in rhesus macaques. The injections were well tolerated, as evidenced by the absence of fever, significant local reaction, or hematologic or biochemical effects at all three dosages. The absence of toxicity suggests that there is no safety or tolerability contraindication to extend dose-ranging or to repeat-dosing studies of CpG 7909 by the subcutaneous route in rhesus macaques. Similar safety and tolerability results for comparable doses of subcutaneously administered CpG 7909 in adult men and women support the use of the rhesus macaque as a model for human safety responses (6).
We have also demonstrated that the rhesus macaque models the human IP-10 response to a single subcutaneous dose of CpG 7909, albeit at levels apparently lower than those found in humans. The detection of serum IP-10 is strong presumptive evidence of immune system activation, and the known TLR9 ligand association, as well as the rapid timing, indicates an innate rather than an adaptive response. CpG 7909 acts by activating primate TLR9-expressing plasmacytoid dendritic cells and B cells. Nuclear factor-κΒ and other intracellular signaling pathways are rapidly upregulated and initiate a rapid innate immune response. The humoral effects of TLR9 agonists include the secretion of IFN-α, proinflammatory cytokines (interleukin-6 [IL-6], tumor necrosis factor alpha), anti-inflammatory cytokines (IL-10, IL-1RA), and IFN-inducible chemokines and cytokines (including IP-10) (7). We measured IP-10 concentrations, a more persistent biomarker of the other antecedent, more transiently expressed, and more rapidly consumed cytokines.
The protective effect of CpG ODNs in mice against diverse intracellular pathogens and the ability of CpG ODNs to stimulate IP-10 in mice have been described in numerous but separate reports. One study with mice has described CpG ODN induction of IP-10 in the context of protection against an extracellular pathogen (12), but the utility of IP-10 in mice, rhesus, or humans as a biomarker of innate immunity-mediated protection has not been formally examined.
The induction of IP-10 by CpG 7909 in rhesus macaques is extrapolated to humans more readily than are similar data from mice for two reasons. First, CpG ODN sequences optimized for primates stimulate human cells more potently than CpG ODN sequences optimized for murine cells (4, 9); second, the distribution of TLR9 in primate and humans is limited to plasmacytoid dendritic cells and B cells, unlike the universal distribution of TLR9 in mouse cells. CpG ODNs have successfully stimulated protective innate immune responses against Leishmania amazonensis and Leishmania major in both simian immunodeficiency virus-infected and healthy rhesus macaques (3, 10). CpG ODN induction of IP-10 in rhesus macaques has been demonstrated in vitro (1, 5, 9) and in one instance in vivo, when a hepatitis B vaccine was adjuvanted with CpG ODN 7909 (5).
We conclude that safety and immune responses to CpG 7909 in the rhesus macaque may prove to be a useful model of human responses to CpG 7909 and may eventually be used to validate IP-10 as a biomarker of innate immune system activation against pathogens.
Acknowledgments
This effort was funded by the Military Infectious Diseases Research Program, Fort Detrick, MD, and by the Unconventional Pathogens Countermeasures Program, Directorate of Science and Technology, Defense Advanced Projects Research Agency, Arlington, VA.
A. M. Krieg holds patents governing the described test article and is an employee of and shareholder in the manufacturer, Coley Pharmaceutical Group Inc., Wellesley, MA.
The opinions and assertions contained herein are our private views and do not represent official policy or positions of the Department of the Army or the Department of Defense.
All work was performed in accordance with a protocol scientifically and ethically approved by the WRAIR Institutional Animal Use and Care Committee.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Abel, K., Y. Wang, L. Fritts, E. Sanchez, E. Chung, P. Fitzgerald-Bocarsly, A. M. Krieg, and C. J. Miller. 2005. Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells. Clin. Diagn. Lab. Immunol. 12:606-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amlie-Lefond, C., D. A. Paz, M. P. Connelly, G. B. Huffnagle, N. T. Whelan, and H. T. Whelan. 2005. Innate immunity for biodefense: a strategy whose time has come. J. Allergy Clin. Immunol. 116:1334-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn, B., V. Wang, D. L. Sacks, R. A. Seder, and D. Verthelyi. 2005. Prevention and treatment of cutaneous leishmaniasis in primates by using synthetic type D/A oligodeoxynucleotides expressing CpG motifs. Infect. Immun. 73:4948-4954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann, G., A. Marschner, P. R. Viveros, C. Stahl-Hennig, M. Eisenblätter, Y. S. Suh, E. Stefan, K. Tenner-Racz, K. Überla, P. Racz, R. M. Steinman, and R. Ignatius. 2005. CpG oligonucleotides induce strong humoral but only weak CD4+ T cell responses to protein antigens in rhesus macaques in vivo. Vaccine 23:3310-3317 [DOI] [PubMed] [Google Scholar]
- 6.Krieg, A. M., S. M. Efler, M. Wittpoth, M. J. Al Adhami, and H. L. Davis. 2004. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 27:460-471. [DOI] [PubMed] [Google Scholar]
- 7.Krieg, A. M. 2007. Antiinfective applications of toll-like receptor 9 agonists. Proc. Am. Thorac. Soc. 4:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neville, L. F., G. Mathiak, and O. Bagasra. 1997. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): a novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 8:207-219. [DOI] [PubMed] [Google Scholar]
- 9.Puig, M., A. Grajkowski, M. Boczkowska, C. Ausín, S. L. Beaucage, and D. Verthelyi. 2006. Use of thermolytic protective groups to prevent G-tetrad formation in CpG ODN type D: structural studies and immunomodulatory activity in primates. Nucleic Acids Res. 34:6488-6495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verthelyi, D., M. Gursel, R. T. Kenney, J. D. Lifson, S. Liu, J. Mican, and D. M. Klinman. 2003. CpG oligodeoxynucleotides protect normal and SIV-infected macaques from Leishmania infection. J. Immunol. 170:4717-4723. [DOI] [PubMed] [Google Scholar]
- 11.Vollmer, J. 2005. Progress in drug development of immunostimulatory CpG oligodeoxynucleotide ligands for TLR9. Expert Opin. Biol. Ther. 5:673-682. [DOI] [PubMed] [Google Scholar]
- 12.Zeng, X., T. A. Moore, M. W. Newstead, J. C. Deng, S. L. Kunkel, A. D. Luster, and T. J. Standiford. 2005. Interferon-inducible protein 10, but not monokine induced by gamma interferon, promotes protective type 1 immunity in murine Klebsiella pneumoniae pneumonia. Infect. Immun. 73:8226-8236. [DOI] [PMC free article] [PubMed] [Google Scholar]