Abstract
Serological tests are the main laboratory procedures used for diagnosis during the indeterminate and chronic stages of Chagas' disease. A serological regression to negativity is the main criterion used to define parasitological cure in treated patients. The aim of this work was to monitor the individual specificities of antibody levels for 3 years posttreatment in 18 adult patients. Conventional serological techniques (hemagglutination assays and enzyme-linked immunosorbent assay [ELISA]) were modified by using recombinant antigens to detect early markers of treatment effectiveness. For this purpose, serum samples were taken before and during treatment and every 6 months after treatment for at least 3 years. When hemagglutination assays were used, a decrease in antibody levels was observed in only one patient. When ELISA with serum dilutions was used, antibody clearance became much more apparent: in 77.7% (14/18) of the patients, antibody titers became negative with time. This was observed at serum dilutions of 1/320 and occurred between the 6th and the 30th months posttreatment. The immune response and the interval for a serological regression to negativity were different for each patient. For some of the recombinant antigens, only 50% (9/18) of the patients reached the serological regression to negativity. Recombinant antigen 13 might be a good marker of treatment effectiveness, since 66.6% (six of nine) of the patients presented with an early regression to negativity for specific antibodies to this antigen (P = 0.002).
Chagas' disease, or South American trypanosomiasis, a zoonosis caused by the flagellate protozoan parasite, Trypanosoma cruzi, is the main cause of heart disease and affects about 12 million people in Central and South America (27). The disease has different clinical stages: acute, indeterminate, and chronic. The indeterminate stage is asymptomatic, while in the chronic stage there are three types of manifestations: cardiac, gastrointestinal, and neurologic (14). Because infection with T. cruzi causes the polyclonal activation of B and T lymphocytes and high levels of anti-T. cruzi antibodies, serological tests are the main laboratory procedures used for diagnosis. Currently, the most widely used serological tests are the indirect hemagglutination assay (IHA), the indirect immunofluorescence assay, and the enzyme-linked immunosorbent assay (ELISA) (4). The antibodies reacting in these tests are defined as the antibodies of conventional serology.
Since the 1930s, several attempts have been made to discover an appropriate drug for treatment of the infection. Only nifurtimox and benznidazole have been relatively successful in Argentina and most other countries where the disease is endemic. The first attempts to regulate the treatment of the infection were made in 1983, in which treatment only for children was recommended (13). In 1997, the original regulations were revised and new procedures were approved. Currently, the “norms for the care of chagasic patients” recommend treatment for adult patients in the indeterminate phase of the disease or with an incipient cardiac form of Chagas' disease (22).
Since the pathology of Chagas' disease and its diagnosis are related to the immune response, the measure of such a response is of potential interest as a marker of the evolution of the disease (6).
Circulating parasites are scarce in the chronic phase, and antibodies become the hallmark of this phase because they are often present at high titers. In an infected individual successfully treated during the acute or the chronic phase, the parasites disappear, as do those antibodies that were formerly present. Since the parasites are extremely antigenic, producing a strong antibody response, even after cure, the progressive decrease in titers usually takes years or decades until serology becomes negative. This occurs more slowly in adults than in children. The parasitological tests (xenodiagnosis, hemoculture, and PCR) are meaningful only when they are positive, indicating a therapeutic failure. This means that negative parasitological test results are not a reliable criteria for cure. The only accepted criterion of parasitological cure is the absence of anti-T. cruzi antibodies measured by conventional assays (ELISA, indirect immunofluorescence assay, and IHA) with crude parasite antigens, which are available on the market as kits in every country where the disease is endemic (2, 3, 5, 16, 17, 18, 26). ELISA is the most precise, quantitative, and widely used technique. In Argentina, the usual serological methods used are IHA and ELISA. Both can be quantitated by either limiting dilution (IHA) or optical density (OD) measurements.
In this work we screened the different T. cruzi antigens used in commercially available diagnostic kits against a panel of sera from treated patients. The aim of this work was to search for an antigen which provides specificity, good diagnostic sensitivity, and the ability to cause the specific antibody response to regress or become negative in successfully treated patients during a short time of follow-up. For this purpose we used different combinations of antigen and/or serum dilutions and IHAs or ELISAs.
MATERIALS AND METHODS
Subjects.
The study included 18 adult patients infected with T. cruzi. The patients either were in the indeterminate stage or had incipient cardiopathy (Kuschnir groups 0 and 1) (12). The patients were identified as bearing T. cruzi infection by two initial serological commercial test kits based on different principles (IHA and ELISA): the Wiener Chagatest-HAI and the Wiener Chagatest-ELISA.
The age of the patients ranged from 19 to 41 years (mean age, 25 years; median age, 23 years) at the beginning of treatment. The population studied included 11 women and 7 men. All patients were treated with benznidazole (5 mg/kg of body weight/day) for 60 days.
Clinical examination.
Clinical analyses were carried out, including hemograms with platelet counts and tests for urea, creatine, transaminases, cholesterol, alkaline phosphatase, and bilirubin. These analyses were performed during the pretreatment period and on days 30 and 60 posttreatment (11).
Serum samples were taken once before treatment and every 6 months until the 32nd month (minimum) posttreatment. These were processed shortly (2 weeks) after admission, but aliquots of all serum samples were preserved frozen at −20°C and were tested simultaneously for the comparative evaluation at the end of the study.
Serological reactions. (i) IHA.
The specifications of the commercial IHA test kit (Wiener Chagatest-HAI) were followed. Briefly, the patient's serum was mixed with red blood cells sensitized with T. cruzi cytoplasmic and membrane antigens. If the patient's serum contains anti-T. cruzi antibodies, these will produce a specific agglutination. The samples were serially diluted, and agglutination at dilutions greater than or equal to 1/16 was indicative of infection. The titer was defined as the highest serum dilution presenting agglutination.
(ii) ELISA.
The ELISA reaction is based on the formation of immunocomplexes between fixed antigens and antibodies in the serum. The sera were serially diluted from 1/20 to 1/320 in phosphate-buffered saline-Tween 20 (0.05%)-1% bovine serum albumin and were allowed to react with the antigen in plastic multiwell microplates, according to the Wiener Chagatest-ELISA kit specifications. ELISAs with three different combinations of antigens were used. First, whole cytoplasmic and membrane antigens of T. cruzi were used in an ELISA (CMA-ELISA) for initial diagnosis and also to carry out assays with serial serum dilutions at different times posttreatment to check for time-related antibody variations. Second, an ELISA with a recombinant antigen mixture (RAM-ELISA) comprising six recombinant proteins of the T. cruzi epimastigote and trypomastigote stages, largely tested in Latin America, was performed. These antigens consisted of shed acute-phase antigen (SAPA), which is reactive during the acute phase of infection; antigens 1, 2, and 30, which detect antibodies mainly in individuals in the chronic stage; and antigens 13 and 36, which are reactive during both the acute and the chronic stages (7, 10, 25). Third, an ELISA with individual recombinant antigens (IRA-ELISA) was performed. ELISAs were carried out with each recombinant antigen individually with the pre- and posttreatment sera. The antigens in the second and third assays were used to monitor the antibody variations at different times posttreatment, with the sera diluted 1:20 according to the specifications of the recombinant (version 3) Wiener Chagatest-ELISA (9).
ELISA procedure.
The antigens were coated with carbonate buffer (100 mM, pH 9.6) for 18 h at 4°C and the optimal concentrations in 96-well microwell plates. Following dilution, the serum samples were added to the microwell plates and the plates were incubated at 37°C for 30 min. After five washes (NaCl [1.4 M] in phosphate buffer [concentrated to 100 mM]) to remove the unbound immunoglobulin (Ig), the samples were incubated at 37°C for 30 min with a 1:40,000-diluted anti-human IgG conjugate labeled with peroxidase. Unbound conjugate was removed by another wash step. The microwell plates were then revealed with hydrogen peroxide (60 mM in citrate buffer [50 mM], pH 3.2) and tetramethybenzidine (0.01 mM in chlorhydric acid [0.1 N]). The solution develops a blue color with an intensity proportional to the concentration and affinity of the anti-T. cruzi antibodies in the sample. The reaction was stopped by the addition of sulfuric acid (2 N), and the color of the solution turned to yellow. Within 20 min the strips were read at a wavelength of 450 nm. A sample is considered nonreactive if the absorbance is lower than the cutoff value. On the other hand, a sample is considered reactive if the absorbance is equal to or greater than the cutoff value (19).
Ethical considerations.
The patients signed an informed consent, which informed them about the advantages and the drawbacks of the treatment, as well as the time and frequency of the posttreatment control evaluations. This study was approved by the Ethics Committee of the Faculty of Health Sciences at the National University of Salta and by the Ministry of Public Health, Salta, Argentina.
Statistical analysis.
The chi-square and Fisher's exact tests were used to perform statistical analyses with the EPI-Info statistical program package (version 6.0).
RESULTS
Treatment follow-up.
Benznidazole treatment caused some adverse effects, which were relatively well managed and tolerated with simple additional medication. It was not necessary to interrupt or delay treatment in any case. The side effects detected in the 18 benznidazole-treated patients were allergic dermatitis (39%), which disappeared after antihistamine administration; headache (33%), which ceased with analgesics; gastrointestinal intolerance (22%), which responded well to diet and antacids; and arthralgia or distal peripheral neuritis (11%), which disappeared with nonsteroidal anti-inflammatory drugs and B-complex vitamin supplements. Other side effects were slight increases in hepatic enzyme levels (11%) and asthenia (6%). No collateral effects were observed in 33% of the treated patients.
Serologic follow-up included data from 18 patients. Five patients were evaluated for 36 months or less (24 to 36 months) posttreatment, three patients were evaluated for 42 months posttreatment, three patients were evaluated for 48 months posttreatment, three patients were evaluated for 54 months posttreatment, three patients were evaluated for 60 months posttreatment, and one patient was evaluated for 66 months posttreatment.
IHA.
The IHA result showed a decrease of more than two titers in 8/18 patients between the pretreatment and the last control evaluation. One patient (patient 9) reached titers lower than 1/16. This decrease could be observed before the 18th month, and the negative titer (less than 1/16) remained constant until the last control evaluation (Table 1).
TABLE 1.
Antibody titers obtained at different times of follow-up by IHA in 9/18 patients who showed a regression
| Patient no. | Antibody titer at the following mo of follow-up
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 6 | 12 | 18 | 24 | 30 | 36 | 42 | 48 | 54 | 60 | 66 | |
| 1 | 1,024 | 1,024 | 1,024 | 512 | 128 | 64 | NDa | ND | ND | ND | ND | ND |
| 2 | 128 | 32 | 32 | 32 | 32 | 32 | ND | ND | ND | ND | ND | ND |
| 3 | 1,024 | 512 | 512 | 128 | 128 | 128 | 128 | 128 | 128 | 128 | ND | ND |
| 6 | 1,024 | 1,024 | 512 | ND | 512 | 64 | 64 | 128 | 128 | 128 | ND | ND |
| 7 | 1,024 | 512 | 1,024 | ND | 256 | 128 | 128 | 256 | ND | ND | ND | ND |
| 9 | 64 | 64 | 64 | 32 | 64 | 16 | 16 | 32 | 16 | 8 | ND | 8 |
| 13 | 1,024 | ND | 2,048 | 512 | 1,024 | 512 | 64 | 64 | 512 | 256 | 256 | ND |
| 15 | 1,024 | 128 | 512 | ND | 32 | 256 | 256 | ND | ND | ND | ND | ND |
| 16 | 2,048 | 512 | 512 | 512 | 128 | 64 | 64 | 64 | 64 | 64 | ND | 64 |
ND, not determined.
Semiquantitative ELISA (CMA-ELISA).
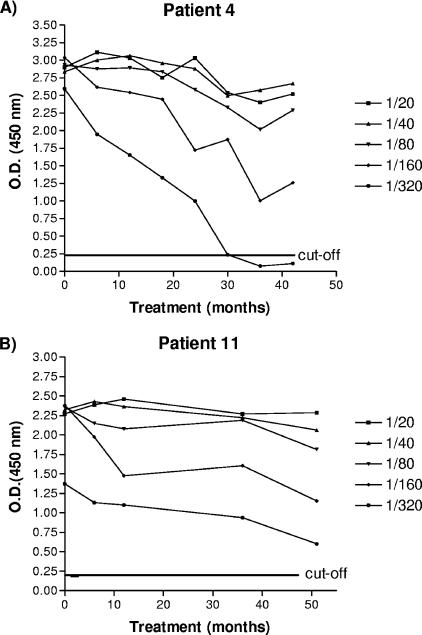
Five dilutions of the serum samples obtained from each patient at the beginning of the study and at different times posttreatment were tested. This consisted of >540 combinations (18 patients × five dilutions × more than six tests). All 18 patients presented a decrease in antibody levels at different times during the posttreatment period (Fig. 1; see the supplemental material). However, four of them (patients 1, 7, 11, and 17) did not reach values under the cutoff (0.22) at any time posttreatment or at any dilution. The 77.7% (14/18) of the remaining patients (patients 2, 3, 4, 5, 6, 8, 9, 10, 12, 13, 14, 15, 16, and 18) presented negative titers at different dilutions and times. For 78.5% (11/14) of the patients, negative values were observed at a 1/320 dilution at times ranging from 6 to 30 months posttreatment. Then, 4/11 patients (36.36%) displayed a negative seroconversion and did so at a 1/320 dilution and at periods of as short as 6 months posttreatment. One patient (patient 9) reached OD values under the cutoff (0.22) at a 1/160 dilution at month 12 posttreatment. Patients 12 and 1 presented values lower than the cutoff at a 1/80 dilution at 18 and 24 months, respectively (Table 2).
FIG. 1.
Variations in ODs by the CMA-ELISA reaction as a function of time and serum dilution. The results for patient 4 are representative of those for 14 patients. The results for patient 11 are representative of those for four patients. Note that the 1/320 dilution of serum provided the best rate of regression to antibody negativity for 11/18 (61%) of patients.
TABLE 2.
Cumulative numbers of patients who achieved negative antibody titers at different dilutions and times, as determined by CMA-ELISA
| Time of follow-up (mo) | Cumulative no. of patients with negative antibody titers at the following serum dilution
|
||
|---|---|---|---|
| 1/80 | 1/160 | 1/320 | |
| 6 | 0 | 0 | 4 (3, 6, 14, 15)a |
| 12 | 0 | 1 (9) | 6 (16, 8) |
| 18 | 1 (12) | 1 | 8 (10, 2) |
| 24 | 2 (1) | 1 | 9 (5) |
| 30 | 2 | 1 | 11 (4, 13) |
Numbers in parentheses identify the patient numbers of the new patients who became seronegative.
RAM-ELISA.
Sera from all patients were tested with the recombinant antigen mixture at the 1/20 dilution recommended by the manufacturer of the kit. No decrease in the absorbance values was observed at any time posttreatment.
IRA-ELISA.
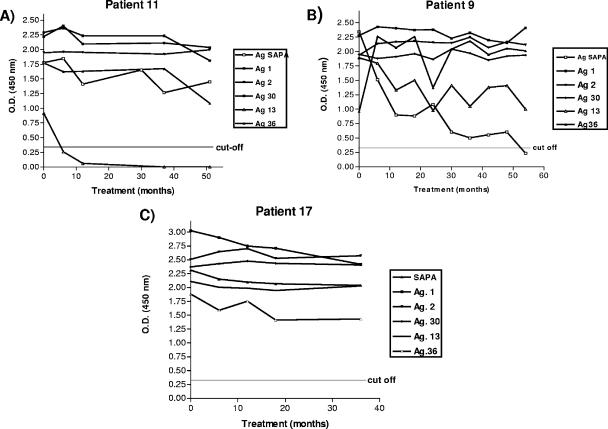
Before treatment all 18 patients responded to antigen 30. For SAPA and antigens 1 and 2, the response rates were 94.4% (17/18 patients). For antigen 36, the response rate was 88.9% (16/18), and for antigen 13 the response rate was 72.2% (13/18). For some antigens, only 50% (9/18) of the patients presented absorbance levels after treatment less than those for the pretreatment samples. Antigen 13 displayed the best ability to reveal this effect, since of nine patients originally positive for this antigen, six (patients 1, 3, 4, 5, 11, and 15 [67%]) became negative after treatment. Next, SAPA displayed a good ability to detect regressions, since in three of nine patients (patients 9, 10, and 15 [33.33%]) the titers clearly decreased and all patients tested displayed some degree of regression. Antigen 36 showed a regression in patients 15 and 18 (Fig. 2; see the supplemental material). Finally, antigens 1, 2, and 30 had lesser abilities to reveal regressions, although a general trend toward a regression to negativity was observed, even with the last three antigens (Table 3).
FIG. 2.
Patients becoming negative for antibody to antigen 13, SAPA, and antigen 36 by IRA-ELISA. The results for patient 11 are representative of those for six others patients, who became negative for antigen 13 early after treatment. The results for patient 9 are representative of those for three patients, who became negative for SAPA. The results for patient 17 are representative of those for nine patients, who did not become negative for antibody to any recombinant antigen.
TABLE 3.
Comparative results obtained by IHA with red blood cells sensitized with cytoplasmic and membrane antigens, CMA-ELISA, and IRA-ELISA
| Patient no. | Age (yr) | Antibody reduction
|
||||
|---|---|---|---|---|---|---|
| IHAa | IRA-ELISA
|
CMA-ELISA
|
||||
| Best indicator antigen | Mo of neg. resultb | Best dilution | Mo of neg. result | |||
| 1 | 25 | Yes | 13 | 6 | None | Anyc |
| 3 | 27 | Yes | 13 | 12 | 1/320 | 6 |
| 4 | 20 | No | 13 | 30 | 1/320 | 30 |
| 5 | 41 | No | 13 | 30 | 1/320 | 24 |
| 9 | 23 | Yes | SAPA | 54 | 1/160 | 12 |
| 10 | 23 | No | SAPA | 30 | 1/320 | 18 |
| 11 | 26 | No | 13 | 6 | None | None |
| 15 | 20 | Yes | SAPA, 13, 36 | 36 | 1/320 | 6 |
| 18 | 21 | No | 36 | 42 | 1/80 | 24 |
| 2 | 19 | Yes | Response only to 2 and 30 | 1/320 | 18 | |
| 6 | 22 | Yes | 13 | Began to descend | 1/320 | 6 |
| 7 | 35 | Yes | Any | Any | None | None |
| 8 | 22 | No | Any | Any | 1/320 | 12 |
| 12 | 23 | No | 36 | Began to descend | 1/80 | 18 |
| 13 | 21 | Yes | Any | Any | 1/320 | 30 |
| 14 | 29 | No | Any | Any | 1/320 | 6 |
| 16 | 21 | Yes | Any | Any | 1/320 | 12 |
| 17 | 38 | No | Any | Any | None | None |
The results indicate whether a regression of more than two titers between the pretreatment and the posttreatment evaluations occurred.
Mo of neg. result, month in which a patient became negative by any given test.
Any, positivity for all of the recombinant antigens.
DISCUSSION
The specific antiparasitic treatment for chronic Chagas' disease has been the subject of much controversy. The age and the disease phase at which patients receive treatment are important factors in treatment success. For children or adults treated during the early chronic phase, a decrease in titers is observed during the first 5 years of follow-up and a decline to negative titers is obtained after 5 to 10 years. When treatment is given during the late chronic phase, follow-up control evaluations should extend up to 20 years (17, 18, 26). Until now, there has been a lack of markers to show the decrease in antibody titers during a short follow-up in successfully treated patients. In this study, we tested the conventional diagnostic tests (IHA and ELISA) with different combinations of antigens and/or serum dilutions and looked for an early marker of serological regression to negativity posttreatment, which would allow a shorter period of follow-up.
The serological responses were different according to the antigen mixture or serological test used. A decrease in antibody titers was very difficult to observe by IHA. This is probably because this technique measures IgG, IgA, IgE, and even IgM. This nonspecific Ig detection could be the reason for the detection of only 3 of 9 patients (patients 1, 3, and 15) who became negative for antibodies by IRA-ELISA and 8 of 14 patients (patients 2, 3, 5, 6, 9, 13, 15, and 16) who presented with negative values at different dilutions by CMA-ELISA but merely showed lower titers by IHA. On the other hand, the results of the ELISA techniques (CMA-ELISA and IRA-ELISA) were more comparable because they measure IgG exclusively. With these techniques we were able to find differences depending on each patient; in particular, of the nine patients (patients 1, 3, 4, 5, 9, 10, 11, 15, and 18) who became negative for some recombinant antigen, seven of them (patients 3, 4, 5, 9, 10, 15, and 18) also showed values below the cutoff by the use of any dilution (Table 3).
To answer the question of whether the evaluation of antibody production may indicate patient cure, the functions of antibodies are briefly addressed. Humoral responses usually induce the persistent production of antibodies and the generation of long-lived memory B cells, apparently without antigenic stimulation (1). Thus, the antibody concentrations measured could be caused by different memory B-cell clones that continue replicating for several years. For example, because of their lower half-lives in dendritic and B cells, the regression to negativity occurs earlier for antigen 13 and SAPA than for antigens 2 and 30. Moreover, absorbance is an additive property (21): if a patient does not produce specific antibodies to one antigen, the total of the absorbance values generated by other antibodies may surpass the cutoff, resulting in positive values. This was clearly demonstrated with the patients who became negative for antigen 13 and SAPA but who were still seropositive with antigen mixtures.
Host-related factors are responsible for the variability in antigen recognition (24). One of our patients responded to only two recombinant antigens and not to the rest of them. This observation is relevant: if our tests did not include multiple antigens, some specific responses might have been missed, explaining the often observed case in which patients have a negative serology result and a positive PCR result (20). Great variability can be observed in populations from different countries. Frasch and colleagues tested 30 serum samples from Chilean chagasic patients for antigens 1, 2, 13, and 36 and SAPA (25). Their results were different from ours. In the population studied, they observed that the highest response was to antigens 2 and 36. The proportions of patients reacting with the remaining antigens were 92% for peptide 2, 65% for peptide 36, 44% for peptides 1 and 13, and 32% for SAPA (25).
According to the “gold standard” definition of cure (17), only patient 9 was “cured” because he showed negative antibody titer values by three different assays. Several research groups have used serological tests to monitor patients with Chagas' disease for treatment success. After the treatment of children younger than 14 years old, four groups (Britto et al. [5], da Silveira et al. [7], Sosa Estani and Segura [22], and Streiger et al. [23]) detected significant titer reductions or regression to negative results for >50% of the patients, particularly when treatment was given during the acute phase. Other authors (Fabbro de Suasnábar et al. [8], Pereira-Chioccola et al. [16], and Viotti et al. [26]) attempted to treat adults during the unapparent or chronic phase of infection. Although the observation periods, the sites of the trials, and the serological tests used varied among the studies, a trend toward a titer reduction and regression to antibody negativity by serology was also apparent, particularly in studies with long follow-up periods (8, 26). These results were similar to the ones that we obtained with the CMA-ELISA. However, when we compared the results of the CMA-ELISA with those of tests that use recombinant antigens, particularly SAPA and antigen 13, using the same serum samples, the latter antigens revealed faster regression trends in response to treatment.
The standard criterion for cure is still regression to negativity for anti-T. cruzi antibodies by conventional serology, since it is appropriate for the purposes of diagnosis in public health policies (15). On the basis of our results, we recommend the use of 1:320 serum dilutions in the CMA-ELISA. The titers should be evaluated at months 12, 24, and 36 posttreatment; and the samples should be tested in parallel by the conventional IHA. Further studies will be required to test the use of antigen 13 and SAPA with a larger cohort or in follow-up studies after the treatment of children.
Supplementary Material
Acknowledgments
This work was supported by Project 1003 of the Research Council of the National University of Salta. M. Á. Basombrío received an international research scholar grant from the Howard Hughes Medical Institute. The Wiener laboratory donated antigens and serological kits.
We thank the personnel of the Health Department of the National University of Salta and Public Health Center 15 for the recruitment and follow-up of patients.
Footnotes
Published ahead of print on 5 December 2007.
Supplemental material for this article may be found at http://cvi.asm.org/.
REFERENCES
- 1.Abbas, A. K., A. H. Lichtman, and J. S. Pober. 2002. Activación de las células B y producción de anticuerpos, p. 189-215. In Inmunología celular y molecular. McGraw-Hill Interamericana, Madrid, Spain.
- 2.Andrade, S. G., S. A. Freitas, S. Peyrol, A. R. Pimentel, and M. Sadigurski. 1991. Experimental chemotherapy of Trypanosoma cruzi infection: persistence of parasite antigens and positive serology in parasitologically cured mice. Bull. W. H. O. 69:191-197. [PMC free article] [PubMed] [Google Scholar]
- 3.Barclay, C. A., J. A. Cerisola, and H. Lugones. 1978. Aspectos farmacológicos y resultados terapéuticos del benznidazol en el tratamiento de la infección chagásica. Prensa Med. Argentina 65:239-244. [Google Scholar]
- 4.Brener, Z., and R. T. Gazzinelli. 1997. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int. Arch. Allergy Immunol. 114:103-110. [DOI] [PubMed] [Google Scholar]
- 5.Britto, C., C. Silveira, M. A. Cardozo, P. Marques, A. Luquetti, V. Macedo, and O. Fernández. 2001. Parasite persistence in treated chagasic patients revealed by xenodiagnosis and polymerase chain reaction. Mem. Inst. Oswaldo Cruz 96:823-826. [DOI] [PubMed] [Google Scholar]
- 6.Cervetta, L., B. Basso, I. Castro, N. Santamarina, and E. Moretti. 1997. Reactividad serológica hacia antígenos crudos y semipurificados de T. cruzi en distintos grupos clínicos de pacientes. Medicina (Buenos Aires). 57:161-168. [PubMed] [Google Scholar]
- 7.da Silveira, J. F., E. S. Umezawa, and A. O. Luquetti. 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol. 17:286-291. [DOI] [PubMed] [Google Scholar]
- 8.Fabbro de Suasnábar, D., E. Arias, M. Streiger, M. Piacenza, M. Infaramo, M. del Barco, and N. Amicone. 2000. Evolutive behavior towards cardiomyopathy of treated (nifurtimox or benznidazole) and untreated chronic chagasic patients. Rev. Inst. Med. Trop. Sao Paulo 42:99-109. [DOI] [PubMed] [Google Scholar]
- 9.Gariglio, R. C., M. V. Felcaro, E. M. Toplikar, and G. A. Capriotti. 2000. Boletín del servicio bibiográfico de Wiener Laboratorios SAIC 111. Wiener Laboratorios, Santa Fé, Argentina.
- 10.Ibáñez, F. G., J. I. Affrachino, R. A. Macina, M. B. Reyes, S. Leguizamon, M. E. Camargo, L. Aslund, S. Pettersson, and A. C. Frasch. 1988. Multiple Trypanosoma cruzi antigens containing tandemly repeated amino acid sequence motifs. Mol. Biochem. Parasitol. 30:27-34. [DOI] [PubMed] [Google Scholar]
- 11.Ióvine, E., and A. A. Selva. 1985. El Laboratorio en la clínica. Metodología analítica, fisiopatología e interpretación semiológica. Editorial Médica Panamericana, Buenos Aires, Argentina.
- 12.Lacunza, C. D., O. Sánchez Negrette, M. C. Mora, M. A. Segura, A. Uncos, F. Ramos, M. Garaizabal, N. Del Castillo, and M. A. Basombrío. 2004. Tratamiento de infectados por T. cruzi adultos jóvenes con benznidazol, seguidos durante 3 años. Rev. Med de Salta 7:14-19. [Google Scholar]
- 13.Ministerio de Salud y Acción Social. 1983. Normas para atención médica del infectado chagásico. COFESA, Buenos Aires, Argentina.
- 14.Morales, J. R. 1996. Aspectos clínicos de la enfermedad de Chagas, p. 71-88. In Boletín de la Academia Nacional de Medicina de Buenos Aires, Buenos Aires, Argentina.
- 15.Ostermayer A., and A. Rassi. 2000. Diagnosis and treatment of the infection by T. cruzi. Mem. Inst. Oswaldo Cruz 95:37. [Google Scholar]
- 16.Pereira-Chioccola, V. L., A. A. Fragata-Filho, A. M. Aparecida Levy, M. M. Rodrigues, and S. Schenkman. 2003. Enzyme-linked immunoassay using recombinant trans-sialidase of Trypanosoma cruzi can be employed for monitoring of patients with Chagas' disease after drug treatment. Clin. Diagn. Lab. Immunol. 10:826-830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rassi, A., and A. O. Luquetti. 2003. Specific treatment for Trypanosoma cruzi infection (Chagas disease), p. >117-125. In American trypanosomiasis. Kluwer Academic Publishers, Boston, MA.
- 18.Romeau Cançado, J. 1999. Criteria of Chagas disease cure. Mem. Inst. Oswaldo Cruz 94:331-335. [DOI] [PubMed] [Google Scholar]
- 19.Sáenz-Alquézar, A., W. Marques, and M. B. Botini. 2004. Evaluación de un kit ELISA para la detección de enfermedad de Chagas. Boletín del Servicio bibliográfico de Wiener Laboratorios SAIC. 125. Wiener Laboratorios, Santa Fé, Argentina.
- 20.Salomone, O. A., A. L. Basqueira, A. Sembaj, A. M. Aguerri, M. E. Reyes, M. Omelianik, R. A. Fernandez, J. Enders, A. Palma, J. Moreno Barral, and R. J. Madoery. 2003. Trypanosoma cruzi in persons without serologic evidence of disease, Argentina. Emerg. Infect. Dis. 9:1558-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skoog, D., and D. M. West. 1988. Fundamentos de Química analítica, p. 661. In Aplicaciones de la absorbancia molecular. Editorial Reverté S.A., Barcelona, Spain.
- 22.Sosa Estani, S., and E. Segura. 1999. Tratamiento de la infección por T. cruzi en fase indeterminada. Experiencia y normatización actual en la Argentina. Medicina (Buenos Aires) 59:166-170. [PubMed] [Google Scholar]
- 23.Streiger, M. L., M. L. del Barco, D. L. Fabbro, E. D. Arias, and N. A. Amicone. 2004. Estudo longitudinal e quimioterapia específica em crianças, com doença de Chagas cronica, residentes em área de baixa endemicidade da República Argentina. Rev. Soc. Bras. Med. Trop. 37:365-375. [DOI] [PubMed] [Google Scholar]
- 24.Umezawa, E. S., S. F. Bastos, M. E. Camargo, L. M. Yamauchi, M. R. Santos, A. González, B. Zingales, M. J. Levin, O. Sousa, R. Rangel-Aldao, and J. F. Da Silveira. 1999. Evaluation of recombinant antigens for serodiagnosis of Chagas' disease in South and Central America. J. Clin. Microbiol. 37:1554-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vergara, U., M. Lorca, C. Veloso, A. Gonzalez, A. Engstrom, L. Aslund, U. Pettersson, and A. C. Frasch. 1991. Assay for detection of T. cruzi antibodies in human sera based on reaction with synthetic peptides. J. Clin. Microbiol. 29:2034-2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viotti, R., C. Vigliano, H. Armenti, and E. Segura. 1994. Treatment of chronic Chagas' disease with benznidazole. Clinical and serologic evolution of patients with long-term follow up. Am. Heart J. 127:151-162. [DOI] [PubMed] [Google Scholar]
- 27.World Health Organization. 2002. Control of Chagas disease. Report of WHO Expert Committee. Technical report series 905. World Health Organization, Geneva, Switzerland. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.