Abstract
Treatments for human immunodeficiency virus type 1 (HIV-1)-positive individuals that augment HIV-1 suppression and have potential for achieving long-term control of HIV-1 viremia in the absence of antiretroviral therapy (ART) are urgently needed. We therefore conducted a phase I, clinical safety trial of a dendritic cell (DC)-based vaccination strategy as immunotherapy for HIV-1-positive individuals on ART. We studied 18 HIV-1-positive subjects on ART who underwent leukapheresis to obtain peripheral blood mononuclear cells for DC generation from monocytes cultured with cytokines. Mature DC were pulsed with three HIV-1 HLA*A0201 Gag, Env, and Pol peptides and one influenza A virus matrix protein peptide. The vaccine was administered to donors randomized to receive two vaccinations, either intravenously or subcutaneously. The primary end points were safety and tolerability of two doses of peptide-DC vaccine (3 million versus 10 million). Secondary end points included gamma interferon (IFN-γ) enzyme-linked immunospot assay responses and clinical correlates of an immune response to vaccination. Autologous DC-peptide vaccine was safe, well tolerated, and feasible for use in all participants. Adverse events were rare. Although the trial was not powered to assess an immunologic response, a significantly increased frequency of HIV-1 peptide-specific IFN-γ-positive cells was observed 2 weeks following the second vaccine, with three individuals responding to all four peptides. DC vaccination was safe, was feasible, and showed promise of immunogenicity in ART-treated, HIV-1-positive individuals. Additional studies of DC immunization strategies for HIV-1 infection are warranted.
Administration of potent antiretroviral therapy (ART) results in dramatic declines in the morbidity and mortality due to human immunodeficiency virus type 1 (HIV-1) infection in the majority of treated persons. Initiation of ART is usually accompanied by substantial decreases in HIV-1 viral load and increases in CD4+ T-lymphocyte counts (21, 22). Studies with long-term survivors and with subjects treated during acute infection show that vigorous HIV-1-specific cytotoxic T-lymphocyte (CTL) responses and strong HIV-1-specific TH1 responses correlate with long-term control of viral replication in the absence of therapy, resulting in prolonged, symptom-free survival (17, 19, 23, 24, 30). Unfortunately, limited restoration of HIV-1 specific T-helper cell and CTL reactivity in persons with chronic HIV-1 infection on ART (3, 19, 27) mandates lifelong therapy and generally persistent, albeit low-level, HIV-1 replication. While innate and acquired immune responses appear to be responsible for the initial control of viremia in acute HIV-1 infection, naturally occurring immune responses appear to fade over time in the majority of HIV-1-infected individuals, resulting in disease progression (4, 10, 15). HIV-1-infected individuals on ART with suppressed plasma viral loads progressively lose HIV-1-specific CTLs, suggesting that persistent viremia is required to maintain high frequencies of HIV-specific CTLs. In chronic HIV-1 infection, discontinuation of ART has been uniformly accompanied by a rapid rebound of plasma HIV-1 RNA to pretreatment, steady-state levels, indicating persistence of the HIV reservoir and absence of immune control (8, 20, 25). For these reasons, immunotherapeutic strategies with the potential to suppress viral replication and with the goal of eliciting broad, robust, and durable HIV-1-specific TH1 and CTL responses are needed.
Dendritic cells (DC) are known to play a critical role in the generation of highly specific immune responses against a variety of pathogens. DC acquire antigens in the periphery and, upon appropriate activation, migrate to lymph nodes, where they initiate generation of antigen-specific CD4+ and CD8+ T-cell responses (16). DC have been used extensively as adjuvants and antigen carriers in vaccination strategies for a variety of disease states, primarily malignancies, to induce antigen-specific T-cell responses with little or no toxicity (29, 32).
We and others have proposed that antigen-expressing DC could be an effective immunotherapy for HIV-1 infection (6). For this test-of-concept, phase I safety study, we used autologous DC loaded with highly conserved, immunodominant, HLA*A0201-restricted CTL peptides, including three different HIV-1 peptides and a single influenza A virus matrix peptide for immunization. Peptide-based vaccines are limited by the requirement for HLA specificity. We therefore chose for this study three HIV-1 CTL peptides that represent a selected subset of highly conserved HIV-1 synthetic peptides which were derived from the three major structural proteins of HIV-1 (Env, Gag, and Pol) and exhibit cross-reactive major histocompatibility complex binding to the HLA-A2 supertype family (1). Each of these HIV-1 CTL epitope peptides induces recall CTL responses from HIV-1-infected individuals, indicating that they are naturally processed and presented during the course of HIV-1 infection (1). Influenza A matrix peptide 58-66 represents a dominant epitope recognized by the majority of HLA*A0201-positive individuals. It has been safely and effectively used as an immunogen in numerous vaccination studies. Here we present the results of a phase I clinical trial designed to evaluate the safety and feasibility of autologous DC loaded with synthetic HIV-1 peptides.
MATERIALS AND METHODS
Study participants.
Eighteen HIV-1-infected adults on ART with plasma HIV-1 RNA levels of ≤400 copies/ml plasma for at least 6 months and <50 copies/ml at screening were enrolled at a single site between March 2003 and September 2005. All subjects expressed the HLA*A0201 allele and were on stable ART for at least 4 weeks prior to entry. Subjects were free from opportunistic infections or malignancies, had an expected survival of at least 12 months, had Karnofsky scores of greater than 70, and met additional laboratory-based safety criteria. Subjects had not received previous experimental HIV-1 vaccines, nor had they received other immunologic therapies, such as steroids or immunosuppressive agents, within 30 days of study entry. All subjects gave written informed consent, and the study was reviewed and approved by the Institutional Review Board of the University of Pittsburgh.
Study design.
Participants were randomized to receive autologous DC-HIV-1 peptide vaccines either intravenously (i.v.) or subcutaneously (s.c.). Each subject received two vaccine doses, 3 weeks apart, by the same route of administration. Two dose levels were studied: low dose, 1 million to 3 million cells (n = 6), and high dose, 5 million to 10 million cells (n = 12). The low-dose group was enrolled first. The enrollment of the high-dose group began after review of the safety data for the low-dose group by the Data Safety Monitoring Board. Subjects were observed for 30 min following each vaccination and called on the day following vaccination to evaluate safety. Study visits were conducted weekly for 8 weeks and then at weeks 12, 16, 20, 24, 36, and 48. Vaccinations were given at study weeks 1 and 4; peripheral blood specimens for immunologic studies were obtained at weeks 6, 8, and 12. The study was reviewed quarterly by an independent Data Safety Monitoring Board convened by the Office of Clinical Research at the University of Pittsburgh.
Vaccine production.
Subjects underwent a single leukapheresis at study entry for 2.5 times blood volume to obtain peripheral blood mononuclear cells (PBMC). Vaccine production has been thoroughly described previously (7). In brief, monocytes were isolated by plastic adherence and cultured in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4 (IL-4) for 6 days as previously described (13, 26). DC were then matured in the presence of IL-1β, IL-6, and tumor necrosis factor alpha for 24 h and incubated for 2 to 4 h with 10 μg/ml of each of the HIV-1 peptides (Gag 386 to 394, VLAEMSQV; Env 134 to 142, KLTPLCVTL; and Pol 498 to 506, ILKEPVHGV) and influenza A virus matrix protein peptide (58 to 66, GILGFVFTL). DC were pulsed with each peptide separately. The DC were harvested, combined, washed, and counted; assayed for viability, sterility, purity, and maturity by phenotype; and administered to study subjects. The vaccines were produced and evaluated by the Cellular Products Laboratory, which operates as a Current Good Manufacturing Practice facility at the University of Pittsburgh Cancer Institute.
Peptide synthesis.
The 9-amino-acid synthetic HIV-1 Gag, HIV-1 Pol, HIV-1 Env, and influenza A virus matrix peptides were synthesized, purified, and stored as described previously (5, 7). Prior to their use for DC pulsing, the peptides were tested for sterility, mycoplasma, and endotoxin.
Immunologic assays. (i) IFN-γ ELISPOT.
Direct enzyme-linked immunospot (ELISPOT) assays to enumerate gamma interferon (IFN-γ)-producing cells were performed using previously described methods (2, 28) at the Immunologic Monitoring Laboratory operated as a Good Laboratory Practice facility at the University of Pittsburgh Cancer Institute and as described previously (7). Briefly, unseparated PBMC were plated in 96-well antibody-coated plates. Stimulator cells, i.e., autologous DC pulsed with one of the peptides, were then added. Control wells contained unpulsed DC plus unseparated PBMC. The ratio of unseparated PBMC to stimulator cells was 10:1. When experimental values (unseparated PBMC + stimulator cells) were significantly different from the mean number of spots in control wells (background values) as determined by a permutation test, the background values were subtracted from the experimental values. The coefficient of variation for the assay was determined to be 15% (n = 50). For each subject, PBMC obtained before and after vaccination were batched and analyzed in the same assay to avoid interassay variability.
(ii) IL-12 production assay.
The IL-12 production assays were performed as described elsewhere (7). In brief, mature DC (mDC) and peptide-pulsed mDC were cocultured in the presence of the CD40L-expressing cell line J558 for 24 h at 37°C in wells of a 96-well U-bottom plate. Supernatants were harvested, cryopreserved, and tested for IL-12p70 levels by enzyme-linked immunosorbent assay (R&D Systems) performed on thawed and batched supernatants in the same assay. These assays were performed at the Immunologic Monitoring Laboratory, University of Pittsburgh Cancer Institute.
(iii) Flow cytometry.
The phenotype and maturity of DC were assessed by flow cytometry following DC staining with monoclonal antibodies specific for DC surface markers and purchased from Coulter Beckman (Miami, FL). Conditions used for staining were previously described (12, 31), and isotype controls were used in all experiments. Three- or four-color flow cytometry analysis was performed as previously described using a Coulter Epics XL cytometer with a single 488-nm argon ion laser (12, 31). The amplification and compensation were set according to the standard procedure, using negative controls (isotype control immunoglobulin G1-fluorescein isothiocyanate or immunoglobulin G1-phycoerythrin antibodies). At least 10,000 cells were acquired for analysis, and the data were analyzed using the Expo-32 software.
(iv) Statistical analysis.
The primary end point of this prospective phase I study was to define the safety and tolerability of two doses of peptide-pulsed, autologous, cultured DC administered either i.v. or s.c. to HIV-1-infected subjects. Secondarily, we planned to (i) determine whether immunization with peptide-pulsed DC increased CD8+ T-cell responses to HIV-1 and influenza virus peptides in ELISPOT assay, (ii) compare the immune responses to HIV-1 peptide-pulsed, autologous DC given i.v. or s.c. and at a low versus high dose, and (iii) evaluate the relationship between immunologic responses and clinical parameters.
For analysis of ELISPOT assay results, the nonparametric Wilcoxon signed rank test was used. Mean spot counts from triplicate wells at baseline (prior to vaccination) were compared to mean spot counts from triplicate wells at study weeks 4, 6, 8, and 12. While several baseline results were taken, the difference between these was not statistically significant (data not shown), and the decision was made to use a single baseline for comparison. The nonparametric Wilcoxon rank sum test was used to compare mean spot counts of test wells between high and low doses, between the i.v. versus s.c. route, and between subjects with low nadir (≤150 cells/mm3) and high nadir (>150 cells/mm3) CD4+ T cell counts. Individually, a positive response to antigen at baseline as measured in IFN-γ ELISPOT assays was defined as the number of spots (mean from triplicate wells) being greater by at least 2 standard deviations than the negative control (unseparated PBMC plus DC) for that assay. A positive response to vaccination was defined as the number of spots being greater, by at least 2 standard deviations, than the baseline count and exceeding the baseline count by at least 20 spots/106 PBMC at any later time point.
RESULTS
Study participants.
Baseline characteristics of the study population are listed in Table 1. The study subjects were mostly male (15/18) and white (16/18), with a median age of 44 years. The median baseline CD4+ T-cell count was 645 cells/mm3, and the median CD4+ T-cell nadir was 259 cells/mm3 (n = 17); six subjects had nadir CD4+ T-cell count of ≤150 cells/mm3, and one subject had no nadir CD4 value available. All subjects completed at least 24 weeks of follow-up; one subject withdrew consent (after week 24) and a second died due to causes unrelated to the study vaccine (week 40).
TABLE 1.
Clinical characteristics of the study participants
| Subject | Age (yr) | Gendera | CD4+ T-cell count (cells/mm3)b
|
Duration of disease (yr) | Pretherapy viral load (copies/ml) | Vaccine dose | Route of administration | |
|---|---|---|---|---|---|---|---|---|
| Baseline | Nadir | |||||||
| 1 | 25 | M | 1,195 | 442 | 5 | 67,273 | Low | s.c. |
| 2 | 37 | M | 819 | 564 | 11 | 503 | Low | i.v. |
| 3 | 45 | M | 642 | 311 | 4 | 68,100 | Low | s.c. |
| 4 | 48 | M | 699 | 350 | 7 | NAc | Low | i.v. |
| 5 | 45 | F | 809 | 241 | 15 | 41,552 | Low | s.c. |
| 6 | 42 | M | 739 | 49 | 15 | NA | Low | i.v. |
| 7 | 32 | M | 974 | 340 | 4 | 38,058 | High | i.v. |
| 8 | 65 | F | 645 | 64 | 10 | 1,067,110 | High | s.c. |
| 9 | 48 | F | 558 | 59 | 11 | 55,031 | High | i.v. |
| 10 | 47 | M | 471 | 468 | 4 | 56,759 | High | s.c. |
| 11 | 43 | M | 744 | 310 | 11 | 79,000 | High | s.c. |
| 12 | 59 | M | 567 | 150 | 13 | 10,899 | High | i.v. |
| 13 | 34 | M | 464 | 236 | 10 | 69,171 | High | i.v. |
| 14 | 41 | M | 1,606 | 723 | 6 | NA | High | s.c. |
| 15 | 44 | M | 349 | 83 | 9 | 2,161,402 | High | i.v. |
| 16 | 47 | M | 646 | NA | 19 | NA | High | s.c. |
| 17 | 38 | M | 457 | 13 | 8 | 200,000 | High | i.v. |
| 18 | 47 | M | 475 | 259 | 5 | 67,273 | High | s.c. |
M, male; F, female.
The baseline count was obtained at the time of screening for the protocol. The nadir count is the lowest recorded value for each patient.
NA, not available.
Safety.
The autologous DC-HIV peptide immunizations were generally safe and well tolerated. Seventeen subjects received both doses of vaccine. One subject (high dose, s.c.) experienced an undocumented grade 3 (104°F) fever on the day following the first vaccination, which was considered possibly related to the vaccine, and the second vaccine was withheld. No other grade 3 or 4 laboratory or clinical events related to study vaccinations were observed. CD4+ T-cell counts remained stable and viral loads remained suppressed to less than 50 copies/ml for the duration of follow-up.
Vaccine production.
One subject (low dose, i.v.) was unable to undergo leukapheresis because of anatomical irregularities. For this subject, ample DC were obtained from a 100-ml peripheral blood draw performed 1 week prior to each vaccine dose. For the remaining subjects, substantial DC were generated from a single leukapheresis product to allow preparation of multiple vaccine doses. DC generated from study participants were similar to DC obtained from a cohort of uninfected volunteers with respect to the yield, viability, purity, and phenotype (Table 2) (described in more detail elsewhere [5]). The mean yield was 9.1 × 107 (range, 1.3 × 107 to 2.7 × 108) mature DC, compared with 2.5 × 108 (range, 2.3 × 108 to 3.5 × 108) for an uninfected control cohort. The mean viability and purity were 93% (range, 69 to 99%) and 100% (range, 47% to 100%), respectively, compared with 90% (range, 85% to 95%) and 77% (range, 75% to 89%) for uninfected controls. IL-12p70 production, a measure of DC function (11), was variable but not statistically different from that of normal donors (Table 2). There were no statistically significant differences between the phenotypic characteristics of immature DC or mDC generated from monocytes of the 18 study participants with chronic HIV-1 infection and those of 15 normal donors, as shown previously (7).
TABLE 2.
Comparison of properties of mDC derived from study subjects with those of mDC derived from a cohort of normal donors
| Subjects (n) | Mean (range)
|
|||
|---|---|---|---|---|
| Yield | Viability (%) | Purity (%) | IL-12 (pg/ml)b | |
| Study participants (18) | 9.1 × 107 (1.3 × 107-2.7 × 108) | 93 (69-99) | 100 (47a-100) | 347.7 (11-1,605)c |
| Uninfected volunteers (5) | 2.5 × 108 (2.3 × 108-3.5 × 108) | 90 (85-95) | 77 (75-89) | 270.0 (8-999)d |
In one subject, a second plastic adherence was required to increase purity as defined by positivity for CD86.
IL-12 production levels in DC supernatants were measured as described in Materials and Methods.
n = 15: three subjects had insufficient mature DC at the time of scheduled vaccination to permit IL-12 testing.
n = 1.
Immune responses.
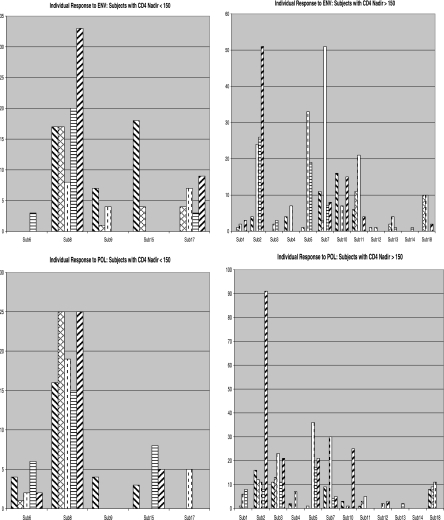
At baseline, 9/18 individuals had responses to one or more HIV-1 peptides, and 12/18 had a response to the influenza A virus matrix protein peptide. Three individuals had significant responses to all four peptides (Fig. 1). Following vaccination, in aggregate, statistically significant increases in the frequency of CD8+ T cells responding to HIV-1 Gag (P = 0.02) and HIV-1 Pol (P = 0.04) at week 6 (Fig. 2) were seen. No significant differences were observed between the i.v. and s.c. routes of administration. A significant difference was observed between the high and low doses at week 4 for HIV-1 Env (P = 0.04), and a trend toward a significant response was observed at week 4 for Gag (P = 0.07) and at weeks 6 (P = 0.1) and 8 (P = 0.05) for HIV-1 Pol (Fig. 3). Considerable interindividual variability was noted in the overall increase in CD8+ T-cell responses, and the resulting significance was attributable to strong responses seen with cells of a few individuals (Fig. 4). Finally, we investigated the effect of pre-ART CD4 nadir by grouping patients based their nadir CD4 (≤150 versus >150 cells/mm3). A slight trend toward more immunogenicity in the cohort with higher nadir CD4+ cell counts was observed, particularly at week 6 for HIV-1 Env (P = 0.1), but no significant differences were seen (Fig. 5).
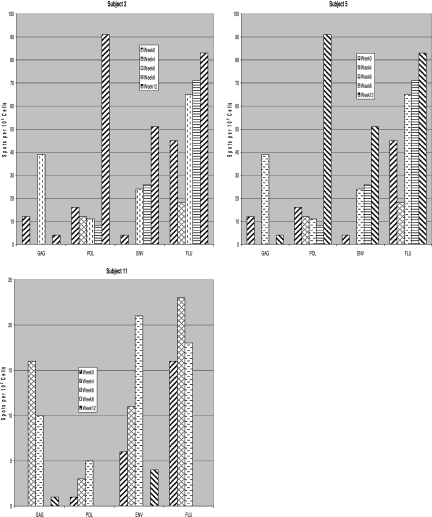
FIG. 1.
ELISPOT responses to vaccine peptides over time for three individuals (subjects 2, 5, and 11) who had significant responses to all vaccine peptides. Although the responses were variable, the best responses were observed at week 6, 2 weeks following the second vaccination. FLU, influenza A virus matrix protein.
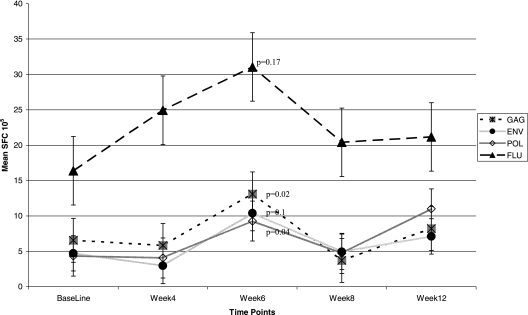
FIG. 2.
Actual mean ELISPOT responses per 105 PBMC of all 18 participants. From baseline to week 6, on average, an increase in the frequency of the HIV-1-specific lymphocytes was observed. Responses were highest for the FLU, influenza A virus matrix protein (FLU) peptide, probably as a result of multiple, previous influenza virus vaccinations, but were statistically significant for the Pol and Gag peptides, and there was a trend toward significance for the Env peptide. P values represent a statistically significant increase in spot-forming cells (SFC)/105 PBMC above the prevaccination values. Error bars indicate standard deviations.
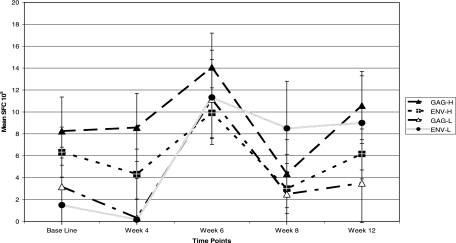
FIG. 3.
CD8+ T-cell responses to Gag and Env peptides observed from week 0 through week 12 after low- and high-dose vaccination (P = 0.07 for Gag and 0.04 for Env by Wilcoxon rank sum test). SFC, spot-forming cells. Error bars indicate standard deviations.
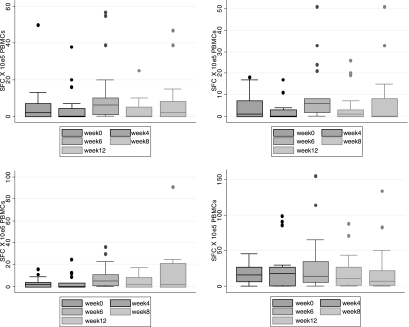
FIG. 4.
Box plots for each peptide, showing the medians of the groups as a whole. The results demonstrate that within each group there was considerable variability of response as indicated by the frequency of peptide-specific T cells in the peripheral circulation. FLU, influenza A virus matrix protein. SFC, spot-forming cells. Error bars indicate 95% confidence intervals.
FIG. 5.
Actual spot counts (spots/105 cells on the y axis) for responses to Pol (bottom panels) and Env (top panels) by individual subjects (x axis). Hatch patterns represent weeks 0, 4, 6, 8, and 12, respectively. Overall, subjects with higher nadir CD4 cell counts (>150 cells/mm3) (right panels) appeared to respond better to vaccination than subjects with lower nadir CD4 cell counts (≤150 cells/mm3) (left panels).
DISCUSSION
In this study, we have demonstrated that vaccination with autologous DC pulsed with HLA-A2-restricted HIV-1 peptides was safe and feasible in chronically HIV-1-infected, ART-treated subjects. Further, although only two vaccinations were given, generation of HIV-1-specific immunologic responses was observed in the setting of chronic HIV-1 infection. DC-based vaccination represents a potentially promising tool to augment host immune response against HIV-1 infection. This study represents the largest and most completely described trial of autologous DC vaccination in ART-treated individuals and sets a foundation for future trials of increasingly used immunogenic, immune-based therapies. Immune-based therapies in this population have the potential to be a crucial addition to currently available ART as we strive to cure, rather than merely treat, HIV-1 infection. Despite years of research, it remains unknown whether immune manipulation is safe and can decrease viremia and improve immunologic control in chronically HIV-1-infected persons. Our trial examined the safety and efficacy of a therapeutic vaccine in HIV-1-infected subjects on ART.
The results of this study as well as additional characterization of DC properties from the study participants at baseline indicated that monocyte-derived DC obtained from leukapheresis of chronically HIV-1-infected subjects with suppressed viral loads were, with few exceptions, phenotypically and functionally comparable to DC from normal donors (7). The choices of antigen and adjuvant are of critical importance in all vaccination strategies. Using DC as an adjuvant for vaccination was largely developed in cancer trials, where the use of synthetic peptides as antigens has several advantages: they are relatively easy to produce, they enable monitoring of the peptide-specific cellular responses, they exclude the possibility of infection from the vaccine product, and they minimize the risk of generating immunity to self antigens. For these reasons, HIV-1-specific synthetic peptides were chosen for this phase I safety and feasibility study of DC-based immunization in this ART-treated cohort. It remains unknown and is a subject of much study as to what will emerge as the optimal antigen for HIV-1 vaccination, whether therapeutic or prophylactic. Here we have shown that administration of only two doses of synthetic peptide-pulsed DC, albeit with relatively narrow specificity, represents a feasible and potentially immunogenic strategy.
The goal of immunotherapeutic strategies for HIV-1 infection, i.e., to decrease viral loads and increase CD4+ cell counts, is known from years of clinical trials and observations to correlate with decreased morbidity and mortality resulting from HIV-1 infection. This study was neither designed nor powered to detect a clinical or immunologic response. Despite this, we have shown not only that viral loads and CD4 counts remained unchanged but that certain individuals appeared to increase their HIV-1 peptide-specific CD8+ T-cell response.
The results of three other phase I autologous DC-based therapeutic immunization trials have recently been published. Lu et al. (18) used autologous DC pulsed with inactivated, autologous HIV-1 in 18 ART-naïve Brazilian subjects. These DC immunizations appeared to be safe and resulted in an increase in HIV-1-specific CD4+ and CD8+ T-cell immunity. The observed increase in immune response was associated with a prolonged reduction in plasma HIV-1 RNA in eight of the subjects. Garcia et al. (9) reported that immunotherapy in 12 HIV-1-infected, ART-treated individuals with autologous DC pulsed with inactivated, autologous HIV-1 was safe and resulted in an increase in T-cell lymphoproliferative and HIV-1-specific CTL responses after an analytic treatment interruption. This immunologic effect was associated with a decrease in plasma HIV-1 RNA levels in 4 of the 12 subjects. Finally, Ide et al. (14) administered HIV-1 peptide-pulsed DC to four HIV-1-infected individuals on ART who subsequently underwent analytic treatment interruption. DC vaccination was safe, with two of four subjects demonstrating significant CD8+ T-cell IFN-γ responses to some of the HIV-1 peptides (14).
While the methods of DC maturation, antigens, and patient populations of these three studies varied compared to those in our clinical trial, there are enough similarities to conclude, based on the safety and limited clinical success of this therapy, that administration of autologous DC vaccines shows substantial promise as an immunotherapeutic strategy for treatment of chronic HIV-1 infection. Our phase I trial demonstrated, for the first time, that vaccination with autologous DC matured and pulsed with HLA-A2-restricted HIV-1 peptides was feasible and safe in a cohort of ART-treated, HIV-1-infected individuals. We have also shown that a significant increase in the frequency of CD8+ T cells responsive to HIV-1 peptides was achieved following peptide-pulsed DC vaccine. We have demonstrated that a higher dose (5 million to 10 million DC) appeared to be more effective than a lower dose (1 million to 3 million DC) in increasing the frequencies of HIV-1 peptide-reactive T cells. Significant interindividual variability in the immunologic response to the vaccine peptides was observed, with a trend toward a better response among those with the higher CD4+ T-cell nadir. We observed no differences in immunogenicity between the s.c. and i.v. routes of administration. This study was limited by a relatively small sample size which consisted of predominantly male subjects with a restricted age range. In summary, our study represents a significant advance in showing that under very rigorously controlled conditions, very small doses of a limited antigen can be immunogenic in a subset of individuals, and therefore this shows substantial promise as an immunotherapeutic strategy for chronic HIV-1 infection. Additional studies with virologic end points are urgently needed to evaluate alternative antigen-DC combinations and to further our comprehension of the mechanisms responsible for the interindividual immunologic response variability.
Acknowledgments
We thank Carol Oriss, Chris Tripoli, and Pat Paulson for their invaluable work coordinating this study. Thanks also go to Joanna Stanson, Toni Temples, and the entire IMCPL staff for product preparation and outcome analysis. We express our sincere appreciation for the generosity and dedication of our study volunteers.
This work was supported by NIH grants PO1-AI55794 and PO1-DE12321 and NIH/NCRR/GCRC grant M01-RR000056.
Footnotes
Published ahead of print on 17 October 2007.
REFERENCES
- 1.Altfeld, M. A., B. Livingston, N. Reshamwala, P. T. Nguyen, M. M. Addo, A. Shea, M. Newman, J. Fikes, J. Sidney, P. Wentworth, R. Chesnut, R. L. Eldridge, E. S. Rosenberg, G. K. Robbins, C. Brander, P. E. Sax, S. Boswell, T. Flynn, S. Buchbinder, P. J. Goulder, B. D. Walker, A. Sette, and S. A. Kalams. 2001. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 75:1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai, T., W. J. Storkus, and T. L. Whiteside. 2000. Evaluation of the modified ELISPOT assay for gamma interferon production in cancer patients receiving antitumor vaccines. Clin. Diagn. Lab. Immunol. 7:145-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 4.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Connolly, N., S. A. Riddler, C. R. Rinaldo, C. C. Wilson, S. Ferrone, J. Stanson, and T. L. Whiteside. 2004. Dendritic cell vaccination: a novel therapy for HIV-1-infected subjects. Pre-clinical characterization of peptide-loaded, autologous dendritic cells for use as a therapeutic vaccine. Abstr. XV Int. AIDS Conf., Bangkok, Thailand, abstr. no. WePeB5683.
- 6.Connolly, N., B. Colleton, and C. R. Rinaldo. 2007. Treating HIV-1 infection with dendritic cells. Curr. Opin. Mol. Ther. 9:353-363. [PubMed] [Google Scholar]
- 7.Connolly, N., S. Riddler, J. Stanson, W. Gooding, C. r. Rinaldo, S. Ferrone, and T. W. Whiteside. 2007. Evaluating DC function in chronically HIV-1 infected subjects: levels of antigen processing machinery components in monocyte-derived dendritic cells generated for therapeutic vaccines. AIDS 21:1683-1692. [DOI] [PubMed] [Google Scholar]
- 8.Davey, R. T., Jr., N. Bhat, C. Yoder, T. W. Chun, J. A. Metcalf, R. Dewar, V. Natarajan, R. A. Lempicki, J. W. Adelsberger, K. D. Miller, J. A. Kovacs, M. A. Polis, R. E. Walker, J. Falloon, H. Masur, D. Gee, M. Baseler, D. S. Dimitrov, A. S. Fauci, and H. C. Lane. 1999. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc. Natl. Acad. Sci. USA 96:15109-15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garcia, F., M. Lejeune, N. Climent, C. Gil, J. Alcami, V. Morente, L. Alos, A. Ruiz, J. Setoain, E. Fumero, P. Castro, A. Lopez, A. Cruceta, C. Piera, E. Florence, A. Pereira, A. Libois, N. Gonzalez, M. Guila, M. Caballero, F. Lomena, J. Joseph, J. M. Miro, T. Pumarola, M. Plana, J. M. Gatell, and T. Gallart. 2005. Therapeutic immunization with dendritic cells loaded with heat-inactivated autologous HIV-1 in patients with chronic HIV-1 infection. J. Infect. Dis. 191:1680-1685. [DOI] [PubMed] [Google Scholar]
- 10.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 11.Hilkens, C. M., P. Kalinski, M. de Boer, and M. L. Kapsenberg. 1997. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood 90:1920-1926. [PubMed] [Google Scholar]
- 12.Hoffmann, T. K., J. Muller-Berqhaus, R. L. Ferris, J. T. Johnson, W. J. Storkus, and T. L. Whiteside. 2002. Alterations in the frequency of dendritic cell subsets in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clin. Cancer Res. 8:1787-1793. [PubMed] [Google Scholar]
- 13.Hoffmann, T. K., N. Meidenbauer, J. Muller-Berghaus, W. J. Storkus, and T. L. Whiteside. 2001. Proinflammatory cytokines and CD40 ligand enhance cross-presentation and cross-priming capability of human dendritic cells internalizing apoptotic cancer cells. J. Immunother. 24:162-171. [PubMed] [Google Scholar]
- 14.Ide, F., T. Nakamura, M. Tomizawa, A. Kawana-Tachikawa, T. Odawara, N. Hosoya, and A. Iwamoto. 2006. Peptide-loaded dendritic-cell vaccination followed by treatment interruption for chronic HIV-1 infection: a phase 1 trial. J. Med. Virol. 78:711-718. [DOI] [PubMed] [Google Scholar]
- 15.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lichtner, M., C. Maranon, P. O. Vidalain, O. Azocar, D. Hanau, P. Lebon, M. Burgard, C. Rouzioux, V. Vullo, H. Yagita, C. Rabourdin-Combe, C. Servet, and A. Hosmalin. 2004. HIV type 1-infected dendritic cells induce apoptotic death in infected and uninfected primary CD4 T lymphocytes. AIDS Res. Hum. Retroviruses 20:175-182. [DOI] [PubMed] [Google Scholar]
- 17.Lifson, J. D., J. L. Rossio, M. Piatak, Jr., T. Parks, L. Li, R. Kiser, V. Coalter, B. Fisher, B. M. Flynn, S. Czajak, V. M. Hirsch, K. A. Reimann, J. E. Schmitz, J. Ghrayeb, N. Bischofberger, M. A. Nowak, R. C. Desrosiers, and D. Wodarz. 2001. Role of CD8+ lymphocytes in control of simian immunodeficiency virus infection and resistance to rechallenge after transient early antiretroviral treatment. J. Virol. 75:10187-10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, W., L. C. Arraes, W. T. Ferreira, and J. M. Andrieu. 2004. Therapeutic dendritic-cell vaccine for chronic HIV-1 infection. Nat. Med. 10:1359-1365. [DOI] [PubMed] [Google Scholar]
- 19.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 20.Oxenius, A., D. A. Price, H. F. Gunthard, S. J. Dawson, C. Fagard, L. Perrin, M. Fischer, R. Weber, M. Plana, F. Garcia, B. Hirschel, A. McLean, and R. E. Phillips. 2002. Stimulation of HIV-specific cellular immunity by structured treatment interruption fails to enhance viral control in chronic HIV infection. Proc. Natl. Acad. Sci. USA 99:13747-13752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Palella, F. J., Jr., K. M. Delaney, A. C. Moorman, M. O. Loveless, J. Fuhrer, G. A. Satten, D. J. Aschman, S. D. Holmberg, et al. 1998. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. N. Engl. J. Med. 338:853-860. [DOI] [PubMed] [Google Scholar]
- 22.Powderly, W. G., A. Landay, and M. M. Lederman. 1998. Recovery of the immune system with antiretroviral therapy: the end of opportunism? JAMA 280:72-77. [DOI] [PubMed] [Google Scholar]
- 23.Rinaldo, C., X. L. Huang, Z. F. Fan, M. Ding, L. Beltz, A. Logar, D. Panicali, G. Mazzara, J. Liebmann, M. Cottrill, et al. 1995. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J. Virol. 69:5838-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 25.Ruiz, L., J. Martinez-Picado, J. Romeu, R. Paredes, M. K. Zayat, S. Marfil, E. Negredo, G. Sirera, C. Tural, and B. Clotet. 2000. Structured treatment interruption in chronically HIV-1 infected patients after long-term viral suppression. AIDS 14:397-403. [DOI] [PubMed] [Google Scholar]
- 26.Sallusto, F., and A. Lanzavecchia. 1994. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J. Exp. Med. 179:1109-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 28.Schultes, B. C., and T. L. Whiteside. 2003. Monitoring of immune responses to CA125 with an IFN-gamma ELISPOT assay. J. Immunol. Methods 279:1-15. [DOI] [PubMed] [Google Scholar]
- 29.Sinkovics, J. G., and J. C. Horvath. 2000. Vaccination against human cancer. Int. J. Oncol. 16:81-96. [DOI] [PubMed] [Google Scholar]
- 30.Wang, B., W. B. Dyer, J. J. Zaunders, M. Mikhail, J. S. Sullivan, L. Williams, D. N. Haddad, G. Harris, J. A. Holt, D. A. Cooper, M. Miranda-Saksena, R. Boadle, A. D. Kelleher, and N. K. Saksena. 2002. Comprehensive analyses of a unique HIV-1-infected nonprogressor reveal a complex association of immunobiological mechanisms in the context of replication-incompetent infection. Virology 304:246-264. [DOI] [PubMed] [Google Scholar]
- 31.Whiteside, T. L., Y. Zhao, T. Tsukishiro, E. M. Elder, W. Gooding, and J. Baar. 2003. Enzyme-linked immunospot, cytokine flow cytometry, and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin. Cancer Res. 9:641-649. [PubMed] [Google Scholar]
- 32.Wierecky, J., M. Mueller, and P. Brossart. 2006. Dendritic cell-based cancer immunotherapy targeting MUC-1. Cancer Immunol. Immunother. 55:63-67. [DOI] [PMC free article] [PubMed] [Google Scholar]