Abstract
Using 42 isolates contributed by laboratories in Canada, The Netherlands, the United Kingdom, and the United States, we compared the results of analyses done with seven Clostridium difficile typing techniques: multilocus variable-number tandem-repeat analysis (MLVA), amplified fragment length polymorphism (AFLP), surface layer protein A gene sequence typing (slpAST), PCR-ribotyping, restriction endonuclease analysis (REA), multilocus sequence typing (MLST), and pulsed-field gel electrophoresis (PFGE). We assessed the discriminating ability and typeability of each technique as well as the agreement among techniques in grouping isolates by allele profile A (AP-A) through AP-F, which are defined by toxinotype, the presence of the binary toxin gene, and deletion in the tcdC gene. We found that all isolates were typeable by all techniques and that discrimination index scores for the techniques tested ranged from 0.964 to 0.631 in the following order: MLVA, REA, PFGE, slpAST, PCR-ribotyping, MLST, and AFLP. All the techniques were able to distinguish the current epidemic strain of C. difficile (BI/027/NAP1) from other strains. All of the techniques showed multiple types for AP-A (toxinotype 0, binary toxin negative, and no tcdC gene deletion). REA, slpAST, MLST, and PCR-ribotyping all included AP-B (toxinotype III, binary toxin positive, and an 18-bp deletion in tcdC) in a single group that excluded other APs. PFGE, AFLP, and MLVA grouped two, one, and two different non-AP-B isolates, respectively, with their AP-B isolates. All techniques appear to be capable of detecting outbreak strains, but only REA and MLVA showed sufficient discrimination to distinguish strains from different outbreaks.
Since Bartlett et al. (2) implicated Clostridium difficile as an agent of antimicrobial-associated colitis nearly 30 years ago, C. difficile strain typing methods have been explored as aids in epidemiologic investigations. Early typing techniques reflected the technology of the time and were based on phenotypic characteristics such as antibiotic resistance patterns, soluble protein patterns (11, 34), bacteriophage and bacteriocin patterns (29), slide agglutination schemes (8), and Western immunoblotting (13). In the 1980s, typing methods began to evolve from phenotype-based methods toward genotype-based methods such as restriction endonuclease analysis (REA) of the total bacterial genome (13, 17), pulsed-field gel electrophoresis (PFGE), and arbitrarily primed PCR (AP-PCR) (9, 15, 26). Brazier et al. compared the results of different typing methods using international isolates, but their study included results from only one genotypic typing method and was conducted before the emergence of the current international epidemic strain of C. difficile (5).
Currently, a strain known as type BI by REA, North American (NA) pulsed-field type 1 (NAP1) by PFGE, and 027 by PCR-ribotyping (BI/NAP1/027) is the single most important epidemic strain causing C. difficile-associated disease (CDAD) in North America and Europe (16, 21, 24). The emergence of this hypervirulent strain has increased interest in C. difficile typing and stimulated the application of newer genotype-based methods such as PCR-ribotyping, amplified fragment length polymorphism (AFLP), multilocus sequence typing (MLST), multilocus variable-number tandem-repeat analysis (MLVA), and surface layer protein A gene sequence typing (slpAST). In view of the emergence of BI/NAP1/027 and the proliferation of typing techniques, we conducted an international multilaboratory comparison of seven typing methods to determine their utility in epidemiologic investigations of C. difficile outbreaks.
MATERIALS AND METHODS
Isolates.
The pool of C. difficile isolates for testing consisted of 42 isolates submitted by four countries: 10 from Canada, 10 from the United Kingdom, 10 from The Netherlands, and 12 from the United States. Each country was instructed to submit five epidemic strains (including three of a single, identical subtype from different geographic locales and two additional strain subtypes, if available; otherwise, these were also to be from different locales) and five nonepidemic strains (three of the most common strains and two of the next most common strains). Two laboratories did not examine all 42 isolates because some isolates were nonviable upon receipt.
All isolates were further characterized by toxinotyping (27), PCR for the presence of the cdtB binary toxin gene using primers described previously by Stubbs et al. (32), and PCR for a tcdC DNA deletion using primers tcdc1 (GCACCTCATCACCATCTTC) and tcdc2 (TGGTTCAAAATGAAAGACGAC), prepared by the CDC core facility. The tcdC PCR was performed on a 50-μl sample containing 5 μl Amplitaq Gold 10× buffer (Applied Biosystems, Foster City, CA), 1.5 mM MgCl2, 25 pmol each primer, and 2.5 U Amplitaq Gold PCR polymerase. Amplification was performed on an Applied Biosystems model 9700 thermocycler with 1 cycle at 95°C for 5 min; 35 cycles at 95 C° for 30 s, 53 C° for 30 s, and 72 C° for 30 s; and 1 cycle at 72 C° for 2 min. PCR products were detected by gel electrophoresis in 2% agarose-1× Tris-borate-EDTA (TBE) buffer, with an applied voltage of 200 V for 4 h. Gels were stained with ethidium bromide and photographed.
Typing techniques.
All seven of the typing methods that we assessed were DNA based. Two methods (REA and PFGE) used enzyme restriction of total genomic DNA, two methods (MLVA and PCR-ribotyping) relied on PCR products alone, two methods (MLST and slpAST) relied on sequencing of selected gene segments, and one method (AFLP) used a combination of enzyme restriction and PCR.
The seven typing methods (AFLP, slpAST, PCR ribotyping, MLST, MLVA, REA, and PFGE) were evaluated in six laboratories, and PCR ribotyping was evaluated twice because the U.S. and United Kingdom laboratories used different primer sets (Table 1). MLVA was performed as described previously by van den Berg et al. (33). AFLP was performed as described previously by Mohammadi et al. (22): isolates clustering with ≥86% similarity were considered to be the same AFLP type.
TABLE 1.
C. difficile typing techniques evaluated
| Laboratory | Technique used | Target |
|---|---|---|
| Leiden University Medical Center, Leiden, The Netherlands | MLVA | DNA repeat units |
| VU University Medical Center, Amsterdam, The Netherlands | AFLP | PstI and MseI restriction sites |
| National Institute of Infectious Diseases, Tokyo, Japan | slpAST | Surface layer protein A gene |
| Anaerobe Reference Unit, PHL, Cardiff, United Kingdom | PCR ribotyping | 16S-23S spacer region |
| Loyola University Medical Center and Hines VA Hospital | REA | HindIII restriction sites |
| Duke University | MLST | Housekeeping loci |
| Duke University | PCR-ribotyping | 16S-23S spacer region |
| CDC | PFGE | SmaI restriction sites |
United Kingdom PCR-ribotyping was performed as described previously by Stubbs et al. (31): isolates were considered to be of a new PCR ribotype if their pattern was at least one band different from previously named patterns. U.S. PCR-ribotyping was performed according to the method described previously by Bidet et al. (3), with a slight modification, in that the thermocycling protocol denaturation, annealing, and extension times were 30 s instead of 1 min, and the PCR products were run on 3% SeaKem LE agarose gels in 1× TBE at 85 V for 4 h. Again, isolates were considered to be of a new PCR ribotype if their patterns were at least one band different from previously named patterns.
MLST was performed according to a method described previously by Lemee et al. (18). REA was done by HindIII digestion of total genomic DNA as described previously by Clabots et al. (6). slpAST was done by sequencing a PCR product from the variable region of the surface layer protein A gene (slpA) and comparing the amino acid sequences deduced from the DNA sequences: a new type was established if the deduced amino acid sequences differed from existing types by more than 20 amino acid residues. Subtypes consisted of groups differing by ≤20 amino acid residues, as described previously by Kato et al. (14).
For PFGE, broth cultures of the isolates were harvested at 7 h, and agar plugs were prepared. Plugs were incubated overnight at 37°C with lysozyme (5 mg/ml) and RNase (0.4 mg/ml) and treated with proteinase K (1 mg/ml). DNA plugs were digested with SmaI for 4 h at room temperature. DNA was separated on 1% SeaKem Gold agar in 0.5% TBE buffer for 18 h with the use of a 5- to 40-s switch time. Thiourea (200 μM) was added to the running buffer and gel for any DNA samples showing degradation. PFGE patterns were analyzed with BioNumerics 4.01 (Applied Maths, Austin, TX) software. To show the similarity between PFGE patterns, we created dendrograms by the unweighted-pair group method with arithmetic mean using Dice coefficients with position tolerance and optimization of 1.10%. We considered clusters with ≥80% similarity to be distinct NAP types and clusters with ≥95% similarity within a type to be distinct NAP subtypes.
Discrimination index.
To compare the discriminating capacity of the seven methods, we used an index of discrimination (D) based on Simpson's index of diversity according to the following formula described previously by Hunter and Gaston (12):
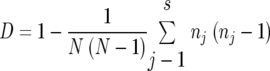 |
where N is the total number of strains in the sample population for which data were obtained, s is the total number of types/subtypes described, and n is the number of strains of the jth type. Confidence intervals for D were determined by a method described previously by Grundmann et al. (10).
RESULTS
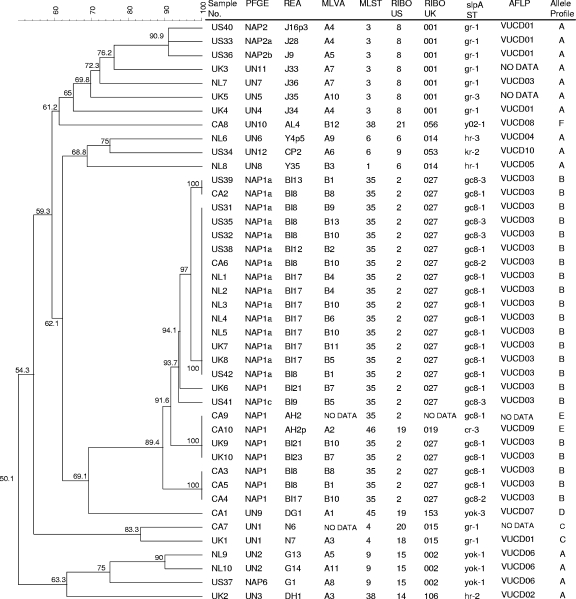
As shown in Fig. 1, a dendrogram produced from PFGE data with the results of the other typing methods distributed along it, we found a high concordance of groupings by all methods.
FIG. 1.
PFGE dendrogram with results of all methods aligned. RIBO US, PCR-ribotyping performed in the United States; RIBO UK, PCR-ribotyping performed in the United Kingdom.
All isolates belonged to one of six allele profiles (AP-A through AP-F) defined by toxinotype, binary toxin PCR results, and the presence or absence of a deletion in the tcdC gene. AP-A (toxinotype 0, binary toxin negative, and no tcdC deletion) was considered to be the archetype profile. Isolates with variant APs (i.e., those differing from the archetypal allele profile) tended to group together by all methods, whereas isolates with profile A were distributed among several groups (Table 2).
TABLE 2.
Grouping of isolates by AP
| AP and samplea | Isolate type byb:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| REA | PFGE | MLVA | MLST | PCR-ribotyping
|
slpAST | AFLP | ||
| United States | United Kingdom | |||||||
| AP-A | ||||||||
| US34 | CP2 | UN12 | A6 | 6 | 9 | 053 | kr-2 | VUCD10 |
| UK2 | DH1 | UN3 | A3 | 38 | 14 | 106 | hr-2 | VUCD02 |
| US37 | G1 | NAP6 | A8 | 9 | 15 | 002 | yok-1 | VUCD06 |
| NL9 | G13 | UN2 | A5 | 9 | 15 | 002 | yok-1 | VUCD06 |
| NL10 | G14 | UN2 | A11 | 9 | 15 | 002 | yok-1 | VUCD06 |
| US33 | J28 | NAP2a | A4 | 3 | 8 | 001 | gr-1 | VUCD01 |
| US36 | J9 | NAP2b | A5 | 3 | 8 | 001 | gr-1 | VUCD01 |
| US40 | J16p3 | NAP2 | A4 | 3 | 8 | 001 | gr-1 | VUCD01 |
| UK3 | J33 | UN11 | A7 | 3 | 8 | 001 | gr-1 | No data |
| UK4 | J34 | UN4 | A4 | 3 | 8 | 001 | gr-1 | VUCD01 |
| UK5 | J35 | UN5 | A10 | 3 | 8 | 001 | gr-3 | No data |
| NL7 | J36 | UN7 | A7 | 3 | 8 | 001 | gr-1 | VUCD03 |
| NL8 | Y35 | UN8 | B3 | 1 | 6 | 014 | hr-1 | VUCD05 |
| NL6 | Y4p5 | UN6 | A9 | 6 | 6 | 014 | hr-3 | VUCD04 |
| AP-B | ||||||||
| US38 | BI 12 | NAP1a | B2 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| US39 | BI 13 | NAP1a | B1 | 35 | 2 | 027 | gc8-3 | VUCD03 |
| UK7 | BI 17 | NAP1a | B11 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| UK8 | BI 17 | NAP1a | B5 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| NL1 | BI 17 | NAP1a | B4 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| NL2 | BI 17 | NAP1a | B4 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| NL3 | BI 17 | NAP1a | B10 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| NL4 | BI 17 | NAP1a | B6 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| NL5 | BI 17 | NAP1a | B10 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| CA4 | BI 17 | NAP1 | B10 | 35 | 2 | 027 | gc8-2 | VUCD03 |
| UK6 | BI 21 | NAP1 | B7 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| UK9 | BI 21 | NAP1 | B10 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| UK10 | BI 23 | NAP1 | B7 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| CA2 | BI 8 | NAP1a | B8 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| CA3 | BI 8 | NAP1 | B8 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| CA5 | BI 8 | NAP1 | B1 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| CA6 | BI 8 | NAP1a | B10 | 35 | 2 | 027 | gc8-2 | VUCD03 |
| US31 | BI 8 | NAP1a | B9 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| US32 | BI 8 | NAP1a | B10* | 35 | 2 | 027 | gc8-3 | VUCD03 |
| US35 | BI 8 | NAP1a | B13* | 35 | 2 | 027 | gc8-3 | VUCD03 |
| US42 | BI 8 | NAP1a | B1 | 35 | 2 | 027 | gc8-1 | VUCD03 |
| US41 | BI 9 | NAP1c | B5 | 35 | 2 | 027 | gc8-3 | VUCD03 |
| AP-C | ||||||||
| CA7 | N6 | UN1 | No data | 4 | 20 | 015 | gr-1 | No data |
| UK1 | N7 | UN1 | A3 | 4 | 18 | 015 | gr-1 | VUCD01 |
| AP-D | ||||||||
| CA1 | DG1 | UN9 | A1 | 45 | 19 | 153 | yok-3 | VUCD07 |
| AP-E | ||||||||
| CA9 | AH2 | NAP1 | No data | 46 | 18 | No data | cr-3 | No data |
| CA10 | AH2p | NAP1 | A2 | 46 | 19 | 019 | cr-3 | VUCD09 |
| AP-F | ||||||||
| CA8 | AL4 | UN10 | B12 | 38 | 21 | 056 | y02-1 | VUCDO8 |
| D Index | 0.933 | 0.843 | 0.964 | 0.699 | 0.700 | 0.688 | 0.815 | 0.631 |
AP-A, toxinotype 0, binary toxin negative, and no tcdC gene deletion; AP-B, toxinotype III, binary toxin positive, and an 18-bp tcdC gene deletion; AP-C, toxinotype 0, binary toxin negative, and an 18-bp tcdC gene deletion; AP-D, toxinotype III, binary toxin positive, and no tcdC gene deletion; AP-E, toxinotype IX, binary toxin positive, and no tcdC gene deletion; AP-F, toxinotype XII, binary toxin positive, and no tcdC gene deletion.
*, identical upon repeat testing. “No data” indicates that isolates were not tested because they were nonviable for the test laboratory.
All typing methods were able to type 100% of the isolates examined. With the use of thiourea in the gel, PFGE was able to type the previously described REA type “J” strains (AP-A) that were reported to have degraded with the use of earlier PFGE procedures (7, 28). Samples US32 and US35 were the same isolate submitted to participating laboratories in duplicate as a limited indicator of reproducibility, and all methods except MLVA showed identical patterns for the duplicate sample on initial testing. The initial MLVA results for the duplicate sample showed a low level of target amplification, but upon repeat testing, the duplicate samples showed identical patterns, and the results with other samples remained unchanged.
Four methods (MLVA, REA, slpAST, and PFGE) recognized subtypes under primary types, and the other three (PCR-ribotyping, MLST, and AFLP) recognized only primary types. The D values were considerably higher for methods that recognized subtypes (>0.900 for MLVA and REA, 0.843 for PFGE, and 0.819 for slpAST) than for those that did not (0.631 for AFLP and 0.688 to 0.700 for United Kingdom and U.S. PCR-ribotyping and MLST) (Table 3).
TABLE 3.
Discrimination indices of compared methodsa
| Method | No. of types | No. of subtypes | D | 95% CI |
|---|---|---|---|---|
| MLVA | 2 | 24 | 0.964 | 0.94-0.99 |
| REA | 10 | 27 | 0.933 | 0.88-0.98 |
| PFGE | 15 | 19 | 0.843 | 0.75-0.93 |
| slpAST | 7 | 13 | 0.815 | 0.72-0.91 |
| PCR-ribotyping (United States | 10 | NA | 0.700 | 0.56-0.83 |
| PCR-ribotyping (United Kingdom) | 10 | NA | 0.688 | 0.55-0.83 |
| MLST | 9 | NA | 0.699 | 0.56-0.83 |
| AFLP | 10 | NA | 0.631 | 0.46-0.80 |
CI, confidence interval; NA, not applicable.
The current international epidemic strain (BI/NAP1/027) is defined as being AP-B in this study. REA, PFGE, and PCR-ribotyping grouped each of these 22 AP-B isolates together with or without subtypes. The type names assigned to this group by various methods did not occur in other AP groups except for the two AP-E isolates typed as NAP1 by PFGE, one AP-A isolate typed as VUCD03 by AFLP, and one AP-A isolate and one AP-F isolate typed as B by MLVA (Table 2). Among the other variant APs, profiles C and E contained two isolates each, and profiles D and F contained one isolate each. AP-C and AP-E isolates were found to have the same type by REA, PFGE, MLST, and slpAST but different types by PCR-ribotyping (U.S. laboratories only).
DISCUSSION
Because of the sporadic occurrence of geographically widespread CDAD outbreaks caused by a common strain, it is important to have tools to detect and track these strains. Since the discovery of the “J” strain in the 1990s, typing methods have relied increasingly on a strain's genotypic characteristics; however, only a few studies have compared the typing performances of these newer techniques. Early in this decade, BI/NAP1/027 emerged as the single most important epidemic strain causing CDAD in North America and Europe (16, 21, 24). Our study provides the most comprehensive comparison of typing techniques since the emergence of the BI/NAP1/027 strain and is the first to compare the discrimination and typing capabilities of some of the newer genotypic techniques.
To be useful, a strain typing method should cluster isolates that appear to be related (e.g., similar antibiotic resistance profiles, toxin production levels, and epidemiologic links) and exclude those that do not. Most of the techniques in this study were able to do this. Although those methods that distinguish subtypes of C. difficile had higher D values than those that did not, the groupings of isolates by primary type were very similar for all methods tested, and all methods appeared to be adequate for detecting an outbreak strain in a particular institution. However, only REA and MLVA were able to distinguish NA isolates of AP-B organisms from European (EU) isolates. REA separated the 10 EU AP-B isolates into three types, including seven classified as being type BI 17, whereas only 1 of 12 NA AP-B isolates was identified as being BI 17. REA separated the NA isolates into five types, with type BI 8 accounting for 8 of 12 of the NA AP-B isolates and none of 10 EU AP-B isolates. MLVA separated the NA and EU AP-B isolates into six and seven types, respectively, with only two types (type B5 [one isolate] and type B10 [three isolates]) occurring in both NA and EU isolates. Using slpAST, all EU AP-B isolates tested here were typed into gc8-1, while three subtypes (gc8-1, gc8-2, and gc8-3) were found in NA AP-B isolates. All of the other techniques showed little to no type variation between NA and EU AP-B isolates. Although several typing methods grouped the isolates into subtypes, the subtypes were not necessarily congruent (Table 2).
While MLVA showed a high level of discrimination and separated the AP-B isolates from the non-AP-B isolates, the dendrogram based on MLVA results (Fig. 2) is so discriminating that it obscures strain similarities that are evident in dendrograms based on PFGE (Fig. 1) and AFLP results (not shown). Using different MLVA targets and different analytic methods, others have found MLVA to be a useful tool in tracking health care-related C. difficile infections (20).
FIG. 2.
MLVA dendrogram and assigned types. The dark vertical line is the cutoff value separating MLVA types A and B; the dark horizontal line separates MLVA types A and B.
PFGE typing of C. difficile has proven to be problematic in the past because of the DNA degradation observed with some isolates, particularly those of the REA “J” type (28) or serogroup G (7). Bidet et al. (4) previously found good concordance between results of PFGE and PCR-ribotyping. We found that no sample was untypeable by PFGE, including samples of the epidemic “J” strain (28). The use of thiourea in the gel has proven effective in alleviating typing problems attributable to DNA degradation (7).
Non-sequence-based methods rely on some form of band size comparison in agarose or acrylamide gels. Some of these techniques, such as REA, are very labor-intensive and may require analysts to compare isolates on the same gel, making interlaboratory data comparisons very difficult. The standardization of PFGE gel runs and the use of computer-assisted gel analyses have made interlaboratory data comparisons somewhat easier, but PFGE is still labor-intensive.
Once the reactions are optimized, AFLP and PCR-ribotyping both require relatively little labor. In this study, we found that PCR-ribotyping results had very good interlaboratory agreement; even though the laboratories involved used two different primer sets, they disagreed on only one isolate. The sequence-based methods MLST and slpAST also performed well, although MLST is much more labor-intensive because it sequences multiple loci, whereas slpAST sequences only one locus. One advantage of sequenced-based techniques is that their data are highly transportable from laboratory to laboratory.
The limitations of the study include a small sample size and minimal data with which to evaluate intralaboratory and interlaboratory reproducibility. Only one isolate was included in duplicate to check intralaboratory reproducibility. A larger sample size may have better defined the concordance of test methods among the APs other than AP-A and AP-B. An evaluation of interlaboratory reproducibility was not possible because no two laboratories used exactly the same method. A nonrandomized sample selection was used to determine the predominant C. difficile types in different geographic regions and to increase strain variety for testing the discrimination of each method. The inclusion of more isolates of variant APs other than AP-B might have better defined the concordance of the methods with variant APs.
The epidemiology of C. difficile is changing, due in part to the emergence of more virulent CDAD in North America and Europe (16, 21, 24) and the emergence of CDAD in the community (1). Additionally, C. difficile appears to be a growing problem in food production animals (25, 30) and has even been isolated from food products (26). Thus, CDAD is no longer strictly a health care-associated phenomenon, and risk factors other than antimicrobial exposure might be important as we begin to understand the broader scope of CDAD. MacCannell et al. (19) previously reported the frequent occurrence of BI/NAP1/027 in community-associated isolates, emphasizing the need for good clinical histories and typing methods to define the epidemiology of CDAD in community and health care populations. Sorting out the answers to these questions will require the ability to discriminate among strains from all of these sources even if they share AP characteristics and the same type designations.
Our results showed that the methods evaluated can identify the current BI/NAP1/027 epidemic strain, the “J” strain, and would likely recognize a new outbreak strain if one occurred in an institution. However, because some typing techniques do not distinguish all APs, in the event of an outbreak, it may be beneficial to do a complete allele profile characterization. If one wishes to track outbreak strains geographically and do intratypic discrimination, one should consider the use of one of the more discriminating techniques such as REA or MLVA. Recently, Pasqualotto et al. (23) found that there might be size differences of up to six bases between sequencing data and sizing data produced by capillary electrophoresis and size differences of up to three bases among data produced by different capillary electrophoresis instruments. This would make the use of MLVA in strain tracking problematic and interlaboratory comparisons less reliable unless, as suggested by Pasqualotto et al., correction factors are used (23). Because REA is not widely used, its interlaboratory reproducibility is unknown. In short, although many methods can detect an outbreak strain within an institution, there is currently no method for interinstitutional strain tracking and intratypic discrimination that has proven laboratory-to-laboratory reproducibility.
Acknowledgments
This work was supported by research grants from the U.S. Department of Veterans Affairs Research Service to D.N.G. and S.J.
The findings and conclusions in this report have not been formally disseminated by the CDC and should not be construed to represent any agency determination or policy.
Footnotes
Published ahead of print on 26 November 2007.
REFERENCES
- 1.Barbut, F., B. Gariazzo, L. Bonné, V. Lalande, B. Burghoffer, R. Luiuz, and J.-C. Petit. 2007. Clinical features of Clostridium difficile-associated infections and molecular characterization of strains: results of a retrospective study, 2000-2004. Infect. Control Hosp. Epidemiol. 28131-139. [DOI] [PubMed] [Google Scholar]
- 2.Bartlett, J. G., T. W. Chang, M. Gurwith, S. L. Gorbach, and A. B. Onderdonk. 1978. Antibiotic-associated pseudomembranous colitis due to toxin-producing clostridia. N. Engl. J. Med. 298531-534. [DOI] [PubMed] [Google Scholar]
- 3.Bidet, P., F. Barbut, V. Lalande, B. Burghoffer, and J.-C. Petit. 1999. Development of a new PCR-ribotyping method for Clostridium difficile based on ribosomal RNA gene sequencing. FEMS Microbiol. Lett. 175261-266. [DOI] [PubMed] [Google Scholar]
- 4.Bidet, P., V. Lalande, B. Saluaze, B. Burghoffer, V. Avesani, M. Delmee, A. Rossier, F. Barbut, and J.-C. Petit. 2000. Comparison of PCR-ribotyping, arbitrarily primed PCR, and pulsed-field gel electrophoresis for typing Clostridium difficile. J. Clin. Microbiol. 382484-2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brazier, J. S., M. E Mulligan, M. Delmee, S. Tabaqchali, and the International Clostridium difficile Study Group. 1997. Preliminary findings of the International Typing Study on Clostridium difficile. Clin. Infect. Dis. 25(Suppl. 2)S199-S201. [DOI] [PubMed] [Google Scholar]
- 6.Clabots, C. R., S. Johnson, K. M. Bettin, P. A. Mathie, M. E. Mulligan, D. R. Schaberg, L. R. Peterson, and D. N. Gerding. 1993. Development of a rapid and efficient restriction endonuclease analysis typing system for Clostridium difficile and correlation with other typing systems. J. Clin. Microbiol. 311870-1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corkill, J. E., R. Graham, C. A. Hart, and S. Stubbs. 2000. Pulsed-field gel electrophoresis of degradation-sensitive DNAs from Clostridium difficile PCR ribotype 1 strains. J. Clin. Microbiol. 382791-2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmee, M., M. Homel, and G. Wauters. 1985. Serogrouping of Clostridium difficile strains by slide agglutination. J. Clin. Microbiol. 21323-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fawley, W. N., and M. H. Wilcox. 2002. Pulsed-field gel electrophoresis can yield DNA fingerprints of degradation-susceptible Clostridium difficile strains. J. Clin. Microbiol. 403546-3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundmann, H., S. Hori, and G. Tanner. 2001. Determining confidence intervals when measuring genetic diversity and the discriminatory abilities of typing methods for microorganisms. J. Clin. Microbiol. 394190-4192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heard, S. R., S. O'Farrell, D. Holland, S. Crook, M. J. Barnett, and S. Tabaqchali. 1986. The epidemiology of Clostridium difficile with use of a typing scheme: nosocomial acquisition and cross-infection among immunocompromised patients. J. Infect. Dis. 153159-162. [DOI] [PubMed] [Google Scholar]
- 12.Hunter, P. R., and M. A. Gaston. 1988. Numerical index of the discriminatory ability of typing systems: an application of Simpson's index of diversity. J. Clin. Microbiol. 262465-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato, H., J. Cavallaro, N. Kato, S. L. Bartley, G. Killgore, K. Watanabe, and K. Ueno. 1993. Typing of Clostridium difficile by Western immunoblotting with 10 different antisera. J. Clin. Microbiol. 31413-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kato, H., T. Yokoyama, and Y. Arakawa. 2005. Typing by sequencing the slpA gene of Clostridium difficile strains causing multiple outbreaks in Japan. J. Med. Microbiol. 54167-171. [DOI] [PubMed] [Google Scholar]
- 15.Killgore, G. E., and H. Kato. 1994. Use of arbitrary primer PCR to type Clostridium difficile and comparison of results with those by immunoblot typing. J. Clin. Microbiol. 321591-1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuijper, E. J., B. Coignard, P. Tüll, and the ESCMID Study Group. 2006. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin. Microbiol. Infect. 12(Suppl. 6)2-18. [DOI] [PubMed] [Google Scholar]
- 17.Kuijper, E. J., J. H. Oudbier, W. N. H. M. Stuifbergen, A. Jansz, and H. C. Zanen. 1987. Application of whole-cell DNA restriction endonuclease profiles to the epidemiology of Clostridium difficile-induced diarrhea. J. Clin. Microbiol. 25751-75313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lemee, L., A. Dhalluin, M. Pestrel-Carson, J.-F. Lemeland, and J.-L. Pons. 2004. Multilocus sequence typing analysis of human and animal Clostridium difficile isolates of various toxigenic types. J. Clin. Microbiol. 422609-2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.MacCannell, D. R., T. J. Louie, D. B. Gregson, M. Laverdiere, A.-C. Labbe, F. Laing, and S. Henwick. 2006. Molecular analysis of Clostridium difficile PCR ribotype 027 isolates from Eastern and Western Canada. J. Clin. Microbiol. 442147-2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marsh, J. W., M. M. O'Leary, K. Shutt, A. W. Pasculle, S. Johnson, D. N. Gerding, C. A. Muto, and L. H. Harrison. 2006. Multilocus variable-number tandem-repeat analysis for investigation of Clostridium difficile transmission in hospitals. J. Clin. Microbiol. 442558-2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald, L. C., G. E. Killgore, A. Thompson, R. C. Owens, Jr., S. V. Kazakova, S. P. Sambol, S. Johnson, and D. N. Gerding. 2005. An epidemic, toxin gene-variant strain of Clostridium difficile. N. Engl. J. Med. 3532433-2441. [DOI] [PubMed] [Google Scholar]
- 22.Mohammadi, T., H. W. Reesink, R. N. Pietersz, C. M. Vandenbroucke-Grauls, and P. H. Savelkoul. 2005. Amplified-fragment length polymorphism analysis of Propionibacterium isolates implicated in contamination of blood products. Br. J. Haematol. 131403-409. [DOI] [PubMed] [Google Scholar]
- 23.Pasqualotto, A. C., D. W. Denning, and M. J. Anderson. 2007. A cautionary tale: lack of consistency in allele sizes between two laboratories for a published multilocus microsatellite typing system. J. Clin. Microbiol. 45522-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pépin, J., L. Valiquette, M.-E. Alary, P. Villemure, A. Pelletier, K. Forget, K. Pépin, and D. Chouinard. 2004. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. Can. Med. Assoc. J. 171466-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Palacios, A., H. R. Stämpfil, T. Duddield, A. S. Peregrine, L. A. Trotz-Williams, L. G. Arroyo, J. S. Brazier, and J. S. Weese. 2006. Clostridium difficile PCR ribotypes in calves, Canada. Emerg. Infect. Dis. 121730-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Palacios, A., H. R. Staempfli, T. Duffield, and J. S. Weese. 2007. Clostridium difficile in retail ground meat, Canada. Emerg. Infect. Dis. 13485-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rupnik, M., V. Avesani, M. Janc, C. von Eichel-Streiber, and M. Delmee. 1998. A novel toxinotyping scheme and correlation of toxinotypes with serogroups of Clostridium difficile isolates. J. Clin. Microbiol. 362240-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samore, M., G. Killgore, S. Johnson, R. Goodman, J. Shim, L. Venkataraman, S. Sambol, P. DeGirolami, F. Tenover, R. Arbeit, and D. Gerding. 1997. Multicenter typing comparison of sporadic and outbreak Clostridium difficile isolates from geographically diverse hospitals. J. Infect. Dis. 1761233-1238. [DOI] [PubMed] [Google Scholar]
- 29.Sell, T. L., D. R. Schaberg, and F. R. Fekety. 1983. Bacteriophage and bacteriocin typing scheme for Clostridium difficile. J. Clin. Microbiol. 171148-1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Songer, J. G. 2004. The emergence of Clostridium difficile as a pathogen of food animals. Anim. Health Res. Rev. 5321-326. [DOI] [PubMed] [Google Scholar]
- 31.Stubbs, S. L. J., J. S. Brazier, G. L. O'Neill, and B. I. Duerden. 1999. PCR targeted to the 16S-23S rRNA gene intergenic spacer region of Clostridium difficile and construction of a library consisting of 116 different PCR ribotypes. J. Clin. Microbiol. 37461-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stubbs, S., M. Rupnik, M. Gibert, J. Brazier, B. Duerden, and M. Popoff. 2000. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol. Lett. 186307-312. [DOI] [PubMed] [Google Scholar]
- 33.van den Berg, R. J., I. Schapp, K. E. Templeton, C. H. W. Klaassen, and E. J. Kuijper. 2007. Typing and subtyping of Clostridium difficile isolates using multiple-locus variable-number tandem-repeat analysis. J. Clin. Microbiol. 451024-1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wust, J., N. M. Sullivan, U. Hardegger, and T. D. Wilkins. 1982. Investigation of an outbreak of antibiotic-associated colitis by various typing methods. J. Clin. Microbiol. 161096-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]