Abstract
Burkholderia is an important bacterial genus with a complex taxonomy that contains species of both ecological and pathogenic importance, including nine closely related species collectively termed the Burkholderia cepacia complex (BCC). Unfortunately, 16S rRNA gene analysis has proven to be not sensitive enough to discriminate between species of the BCC. Alternative species identification strategies such as recA-based PCR followed by restriction fragment length polymorphism analysis, although initially useful, have proven to be inaccurate with the increasing species diversity of the BCC. recA gene sequence analysis is more discriminatory and corroborates other biochemical and polyphasic means of taxonomic differentiation. However, it is limited by the fact that certain BCC species are subdivided into discrete recA sequence subgroups that may confuse clinical diagnoses. In this study, an effective approach is described for the rapid differentiation of BCC species from both environmental and clinical sources by means of a single-locus sequencing and PCR assay using fur as a target gene that provides sequence phylogenies that are species specific and, with few exceptions, not divided into subspecies clusters. This assay is specific and can be used to correctly determine the species status of BCC strains tested following sequencing and amplification of the fur gene by both general and species-specific primers. Based on our results, this typing strategy is simpler than and as sensitive as established tests currently in use clinically. This assay is useful for the rapid, definitive identification of all nine current BCC species and potentially novel species groups.
The Burkholderia cepacia complex (BCC) is a closely related family of gram-negative betaproteobacteria that inhabit both environmental and clinical locations. First described by Burkholder (6) as the causative agent of “sour skin” disease of onion bulbs, the BCC is now considered to be an important group of bacterial pathogens of plants, animals, and humans (4, 11, 13). This is especially true for people with cystic fibrosis (CF), where BCC infection can significantly contribute to the deterioration of pulmonary function. Due to the high levels of antibiotic resistance displayed by members of the BCC, infections are difficult to treat and, in some cases, result in death (1). Certain BCC species, especially B. cenocepacia and B. multivorans, have been shown to be highly transmissible, epidemically spread pathogens exchanged between patients attending CF clinics. BCC strains are also capable of causing difficult-to-treat nosocomial infections in patients with other immunodeficient or debilitating conditions (16, 19). Importantly, all nine species of the BCC include strains capable of colonizing CF patients, even though the majority of clinically prevalent strains belong to the species B. cenocepacia and B. multivorans (30). The genetic similarity of the BCC has, in the past, required that multiphasic diagnostic tests be employed for accurate identification (8, 15). Misidentification of these species has occurred, leading to problems with patient care. McMenamin et al. (23) previously reported average false-positive and false-negative rates of 11% and 36%, respectively.
The accurate identification of BCC members is currently carried out by means of a polyphasic approach, employing biochemical metabolic testing, DNA-DNA genome hybridization, whole-cell protein electrophoresis, and recA gene sequence analysis. Various molecular typing methods are currently being evaluated to accurately identify and categorize strains belonging to the BCC, although a single test that does this has yet to be found. Although 16S rRNA gene sequence analysis is an acceptable means of differentiating many bacterial species, it is of limited use in accurately separating species of the BCC due to high (98 to 99%) sequence identity (5, 20, 21). Techniques using the recA gene locus have proven to be the most successful. Originally designed to be used primarily as an assay to separate strains at the species level, recent modifications to this procedure permit discrimination between all Burkholderia genus members (21, 25, 33). recA-based typing has been used to identify BCC strains from both environmental samples and sputa as well as to identify other Burkholderia species (7, 12, 24, 27). However, misidentification of BCC species has occurred using this approach as a restriction fragment length polymorphism-based strategy (23, 24). Furthermore, the medically important BCC member B. cenocepacia is divided into four different recA phylotypes, complicating the identification of this species (32).
Recently, a multilocus sequence typing (MLST) scheme has been developed for the precise differentiation of the species and strains of the BCC (3). PCR amplification of seven conserved housekeeping genes was used to first obtain sequencing targets, followed by DNA sequencing using nested oligonucleotide primers. Extensive nucleotide sequence diversity was found within all seven genetic loci, ranging from 13.1% for atpD to 37.4% for gyrB. This MLST scheme differentiated all nine BCC species and 114 of 119 BCC strains, but as expected, no differentiation was observed between strains obtained from environmental or clinical sources (3). Unfortunately, DNA sequencing and comparison of seven gene targets for each BCC isolate are not within the capabilities of many clinical laboratories, and therefore, simpler, effective strategies for BCC classification are still needed.
In spite of the numerous methods available for separating BCC species, a single well-conserved gene locus that would provide a simple method for unambiguous species-specific identification is highly desirable. While studying the potential of using virulence factor genes for the purpose of distinguishing virulent BCC strains from environmental strains, we discovered that the BCC fur gene (encoding the ferric uptake regulator protein) was useful for differentiating between members of the BCC. Subsequent to DNA sequencing and alignment, it was discovered that this single conserved gene contained sufficient polymorphisms to not only allow species level differentiation of the nine BCC species but also permit the creation of a PCR strategy that could be employed to rapidly identify specific BCC species.
MATERIALS AND METHODS
Bacterial strains.
The 73 bacterial strains and isolates used in this study are shown in Table 1. These were primarily members of the BCC experimental strain panel (22) and the updated BCC strain panel (10), obtained from the Belgium Coordinated Collection of Microorganisms LMG Bacteria Collection (Ghent, Belgium), the American Type Culture Collection (Manassas, VA), and the Canadian Burkholderia cepacia Complex Research and Referral Repository (Vancouver, British Columbia, Canada). Two BCC recA group K strains were also provided by the Canadian Burkholderia cepacia Complex Research and Referral Repository. Clinical isolates putatively identified as being BCC species by diagnostic metabolism tests were provided by the University of Alberta Hospitals (Pediatric/Adult) CF clinic. Growth of these isolates on Burkholderia cepacia selective agar (BCSA) (14) was assessed following overnight aerobic incubation at 30°C. For characterized strains, species assignment was based on results from previously published polyphasic analyses (10, 22, 32). Strains and isolates were maintained at −80°C by freezing in LB medium containing 20% glycerol. Before use, bacteria were streaked for aerobic growth on half LB plates and grown overnight at 30°C.
TABLE 1.
Strains and clinical isolates used in this studya
| Species | Strain/isolate | Source | Location | recA RFLP result | Growth on BCSA | Amplification with JD490/JD491 | Amplification with species-specific primers |
|---|---|---|---|---|---|---|---|
| Burkholderia cepacia | CEP509*/LMG 18821* | CF | Australia | E | NT | + | + |
| ATCC 25416T*/LMG 1222T* | ENV | United States | D | NT | + | + | |
| ATCC 17759*/LMG 2161* | ENV | Trinidad | E | NT | + | + | |
| BCC group K | CEP0964 | CF | Canada | K | NT | + | + |
| CEP1056 | CF | Canada | K | NT | + | + | |
| R445 | CF | Canada | + | + | + | ||
| Burkholderia multivorans | LMG 13010T* | CF | Belgium | NT | + | + | |
| ATCC 17616*/LMG 17588* | ENV | United States | F | NT | + | + | |
| C3430 | CF | Canada | NT | + | + | ||
| C5274 | CF | Canada | NT | + | + | ||
| C5393*/LMG 18822* | CF | Canada | F | NT | + | + | |
| C5568 | CF | Canada | NT | + | + | ||
| M1512 | CF | Canada | + | + | + | ||
| M1865 | CF | Canada | + | + | + | ||
| R810 | CF | Canada | + | + | + | ||
| R1159 | CF | Canada | + | + | + | ||
| Burkholderia cenocepacia | K56-2*/LMG 18863* | CF-e | Canada | G | NT | + | + |
| J2315*/LMG 16656* | CF-e | United Kingdom | G | NT | + | + | |
| C1257 | CF-e | Canada | NT | + | + | ||
| C4455 | CF-e | Canada | NT | + | + | ||
| C5424*/LMG 18827* | CF-e | Canada | G | NT | + | + | |
| C6433*/LMG 18828* | CF-e | Canada | NT | + | + | ||
| CEP511*/LMG 18830* | CF-e | Australia | I | NT | + | + | |
| D1 | ENV | United States | NT | + | + | ||
| PC184*/LMG 18829* | CF-e | United States | J′ | NT | + | + | |
| LMG 19240 | ENV | Australia | NT | + | − | ||
| CEP0868/LMG 21461 | CF | Argentina | NT | + | + | ||
| R161 | CF | Canada | + | + | + | ||
| R452 | CF | Canada | + | + | + | ||
| R750 | CF | Canada | + | + | + | ||
| R1284 | CF | Canada | + | + | + | ||
| R1285 | CF | Canada | + | + | + | ||
| R1314 | CF | Canada | + | + | + | ||
| R1434 | CF | Canada | + | + | + | ||
| R1619 | CF | Canada | + | + | + | ||
| R1882 | CF | Canada | + | + | + | ||
| R1883 | CF | Canada | + | + | + | ||
| R1884 | CF | Canada | + | + | + | ||
| R2314 | CF | Canada | + | + | + | ||
| S11528 | CF | Canada | + | + | + | ||
| Burkholderia stabilis | LMG 14294T* | CF | Belgium | J | NT | + | + |
| C7322*/LMG 18870* | CF | Canada | NT | + | + | ||
| R450 | CF | Canada | + | + | + | ||
| R2140 | CF | Canada | + | + | + | ||
| R2339 | CF | Canada | + | + | + | ||
| Burkholderia vietnamiensis | LMG 10929T* | ENV | Vietnam | B | NT | + | + |
| PC259*/LMG 18835* | CF | United States | A | NT | + | + | |
| ATCC 29424 | ENV | United States | NT | + | + | ||
| G4 | ENV | United States | A | NT | + | + | |
| Burkholderia dolosa | AU0645T*/LMG 18943T* | CF | United States | Q | NT | + | + |
| CEP021*/LMG 21819* | CF | United States | NT | + | + | ||
| E12*/LMG 21820* | CF | United Kingdom | NT | + | + | ||
| L06 | CF | NT | + | + | |||
| STM1441*/LMG 21443* | ENV | Senegal | NT | + | + | ||
| Burkholderia ambifaria | ATCC 53266*/LMG 17828* | ENV | United States | L | NT | + | + |
| AMMDT*/LMG 19182T* | ENV | United States | N | NT | + | + | |
| CEP0996*/LMG 19467* | CF | Australia | N | NT | + | + | |
| M53 | ENV | United States | NT | + | + | ||
| Burkholderia anthina | W92T*/LMG 20980T* | ENV | United States | T | NT | + | + |
| C1765*/LMG 20983* | CF | United Kingdom | T | NT | + | + | |
| J2552*/LMG 16670* | ENV | United Kingdom | AS | NT | + | + | |
| AU1293*/LMG 21821* | CF | United States | AS | NT | + | + | |
| Burkholderia pyrrocinia | ATCC 15958T*/LMG 14191T* | ENV | Japan | NT | + | + | |
| ATCC 39277*/LMG 21822* | ENV | United States | P | NT | + | + | |
| BC011*/LMG 21823* | ENV | United States | AR | NT | + | + | |
| C1469*/LMG 21824* | CF | United Kingdom | AA | NT | + | + | |
| Burkholderia gladioli | R406 | CF | Canada | NA | + | + | NA |
| R1879 | CF | Canada | NA | + | + | NA | |
| Pseudomonas aeruginosa | R285 | CF | Canada | NA | − | − | NA |
| Herbaspirillum sp. | R740 | CF | Canada | NA | − | − | NA |
| Listeria monocytogenes | R1653 | CF | Canada | NA | − | − | NA |
| Pandoraea sp. | R1717 | CF | Canada | NA | + | − | NA |
| Burkholderia sp. | JS150 | ENV | United States | NA | NT | + | NA |
Abbreviations: RFLP, restriction fragment length polymorphism; CF, CF isolate; ENV, environmental isolate; CF-e, CF epidemic isolate; NA, not applicable; NT, not tested. Strains in boldface type were used to test each of the nine species-specific primer sets. Asterisks indicate strains of the BCC experimental strain panel (10, 22).
DNA preparation and PCR.
Genomic DNA was prepared using a standard protocol (2). Gene amplification was performed in a total volume of 50 μl containing 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 1.5 mM MgSO4, 0.2 mM each deoxynucleoside triphosphate, 50 pmol of each primer, 1.25 units of Taq PCRx DNA polymerase (Invitrogen, Burlington, Ontario, Canada), 5 μl of PCRx enhancer solution (Invitrogen), and ∼10 ng of genomic DNA. PCRs were performed with an Eppendorf Mastercycler gradient DNA thermal cycler (Westbury, NY) under the following conditions: 96°C for 2 min for the first cycle, followed by 30 cycles of 96°C for 1 min, 55°C for 1 min, and 72°C for 1 min, with a final extension step of 72°C for 2 min. PCR products were separated on 0.8% (wt/vol) agarose gels in 1× Tris-acetate-EDTA (pH 8.0).
fur gene sequencing.
The fur gene (located on chromosome 1 [bp 3702141 to 3702734] in B. cenocepacia J2315 [http://www.sanger.ac.uk/Projects/B_cenocepacia]; Sanger Centre) was initially amplified from various BCC strains using primers JD490 and JD491 (Table 2). The DNA sequences were determined by direct sequencing from amplicons purified using a Geneclean II kit (Qbiogene, Irvine, CA) or from amplicons cloned into pJET1.2/blunt (Fermentas, Burlington, Ontario, Canada). Nucleotide sequences were determined at least once on each DNA strand using BigDye Terminator reaction mixtures according to the manufacturer's recommendations (PE Biosystems, Foster City, CA), and the products were separated and collected using an ABI Prism 3100 genetic analyzer (PE Biosystems) using standard sequencing conditions. The sequences were aligned and edited using EditView and AutoAssembler software (PE Biosystems). These sequences were analyzed using BLASTN (NCBI) to verify amplification of the correct product. When aligned, the resulting DNA sequences from different BCC species showed heterogeneity at several sites. These sites were used to design PCR primers that would specifically amplify species-specific products. Primers were designed to possess similar melting and optimum annealing temperatures and to specifically amplify a fur product from each of the BCC species, as shown in Table 2. All primers were purchased from Sigma/Genosys Canada (Oakville, Ontario, Canada). To identify strains that were not amplified by JD490 and JD491, 16S rRNA gene sequences were determined. Primers 27F and 1522R were used to amplify a partial region of the 16S rRNA gene (18). This product was cloned into pJET1.2/blunt (Fermentas) and sequenced as described above.
TABLE 2.
Species-specific primers used for amplification of the BCC fur gene
| Species | Forward primer | Forward primer sequence | Forward primer positions | Reverse primer | Reverse primer sequence | Reverse primer positions | Product length (bp) | BCC species amplified |
|---|---|---|---|---|---|---|---|---|
| B. cepacia/BCC group K | F1 | GGCNGAAGACGTCTACCGG | 102-120 | R1 | TCGAAGTTGCTGCGCGAC | 201-218 | 117 | B. cepacia |
| B. multivorans | F2 | AGCAGAGCCCCGTGCGG | 77-93 | R2 | GGTGGGGGCAGTTTTCGGTG | 399-418 | 342 | B. multivorans |
| B. cenocepacia | F | TGACCAATCCGACCGATCTCA | 2-22 | R3 | ATCGCCTGCTGGCGGCTC | 321-338 | 337 | B. cenocepacia IIIA, IIIB, IIID |
| B. stabilis | F4 | CNACCGTCTATCGCGTGCTC | 155-174 | R | TCAGTGCTTGCGGTGGGG | 412-429 | 275 | B. multivorans, B. cenocepacia, B. stabilis, B. dolosa |
| B. vietnamiensis | F | TGACCAATCCGACCGATCTCA | 2-22 | R5 | CGTGGTGGGAGCCTTCGTTG | 243-262 | 261 | B. vietnamiensis |
| B. dolosa | F6 | CTAAAGGCCACCCTACCGCGG | 34-54 | R | TCAGTGCTTGCGGTGGGG | 412-429 | 396 | B. dolosa |
| B. ambifaria | F7 | CCCNGTGCGTCACCTGACT | 84-102 | R7 | CGTGGTGCGAACCTTCATTCAA | 241-262 | 179 | B. ambifaria |
| B. anthina | F | TGACCAATCCGACCGATCTCA | 2-22 | R8 | CAGGTGACGCACGGGGCTC | 81-99 | 98 | B. multivorans, B. cenocepacia, B. stabilis, B. anthina, B. pyrrocinia (1 strain) |
| B. pyrrocinia | F1 | GGCNGAAGACGTCTACCGG | 102-120 | R9 | ATCGCCTGCTGGCGGCC | 322-338 | 237 | B. pyrrocinia |
| JD490 | ATGACCAATCCGACCGATCTCAA | 1-23 | JD491 | TCAGTGCTTGCGITNIGGGCAGTT | 406-429 | 429 | All |
Phylogenetic analysis.
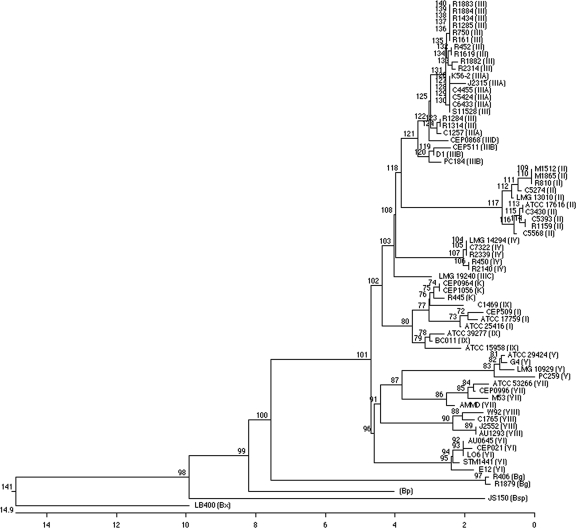
To construct phylogenetic trees of the assembled and edited sequences for each BCC strain, the Clustal V algorithm (15a) was used as part of the alignment software included with the MegAlign program from DNASTAR (Madison, WI). This algorithm groups sequences into clusters first individually and then collectively to produce an overall alignment. At each alignment stage, a two-sequence alignment method is utilized using algorithms described previously by Wilbur and Lipman (33a) for input sequences or by Myers and Miller (24a) for ancestral consensus sequences. The general approach is to progressively align groups of sequences according to a branching order in a hypothetical phylogenetic tree, with gaps occurring in earlier alignments preserved throughout later alignments. The final phylogenetic relationships are constructed by applying the neighborhood-joining method of Saitou and Nei to the distance and alignment data (28a). Confidence levels of individual branches within the optimal tree were assessed by pseudoreplicate data set resampling or bootstrap analysis. The resulting rooted phylogenetic tree includes several non-BCC fur sequences as outliers, including B. pseudomallei (GenBank accession number AF117238), Burkholderia sp. strain JS150, and B. xenovorans LB400 (accession number NC_007951). EditSeq (DNASTAR) was used to modify the original sequences to files that could be assembled by MegAlign. Nearest-neighbor interchange analysis with these data using the Hein algorithm (13a) produced a similarly branched tree (data not shown). By examining sequence pairs, this analysis builds a phylogeny represented by a graph of possible alignments where individual sequences entered into the graph retain a relationship with other sequences that allow the algorithm to ignore the exploration of distant pair relationships. The phylogeny created by this process is finally reexamined for the best possible arrangement of ancestral branches. The phylogenetic analysis methods providing the most discriminatory results are presented in Fig. 1.
FIG. 1.
Phylogenetic tree comparing sequences of a 429-bp fur fragment from strains and isolates of the BCC. The phylogenies are rooted due to the assumption of a common ancestor and biological clock. Genetic distance is shown on the scale, with demarcations representing 2% estimated substitutions. The genomovar status (I through IX) of each BCC species is shown in parentheses following the strain or isolate name. Abbreviations are as follows: Bg, Burkholderia gladioli; Bp, Burkholderia pseudomallei; Bsp, Burkholderia species; Bx, Burkholderia xenovorans.
Nucleotide sequence accession numbers.
The fur gene and 16S rRNA gene sequences determined herein have been deposited in the GenBank database under accession numbers EU090823 to EU090895, EU273473, and EU273474.
RESULTS
Analysis of fur gene sequence.
Following chromosome isolation, 73 strains and clinical isolates listed in Table 1 were tested in a PCR protocol using primers JD490 and JD491. A region of approximately 400 bp was amplified from 69 of these bacteria. Each strand of these DNA products was sequenced, producing 429-bp contigs. BLASTN analysis showed that each of these contigs was homologous to the Burkholderia fur gene, indicating that the protocol amplified the correct product in each case. These 69 contigs exhibited sufficient sequence similarity to be organized in a single alignment. Sequences from strains that had been previously identified to the species level were nearly identical within a single BCC species. Among different species, however, characteristic single-nucleotide polymorphisms that differentiated groups of species from each other and, in several cases, differentiated one species from all others were present. DNA from four isolates that could not be amplified using primers JD490 and JD491 was used in a PCR protocol with the 16S rRNA gene-specific primers 27F and 1522R (18). Using these primers, a product of approximately 1,500 bp was obtained for each sample. Sequencing and comparison of these amplified products indicated that these isolates did not belong to the BCC (Table 1). These isolates were positively identified as the gram-negative organisms Pseudomonas aeruginosa, Herbaspirillum sp., and Pandoraea sp. and the gram-positive organism Listeria monocytogenes. Of these four isolates, only Pandoraea sp. was able to grow on BCSA (Table 1).
The 69 aligned fur sequences were used to construct a phylogenetic tree (Fig. 1). The fur gene sequences from B. xenovorans, Burkholderia sp. strain JS150, and B. pseudomallei were included in the tree as outliers. The clinical isolates R406 and R1879, although putatively identified as being BCC species, also appeared to be outliers. To confirm the identities of these two isolates, 16S rRNA gene sequencing was performed. Sequence data showed that these two strains were not part of the BCC but were instead Burkholderia gladioli, a closely related Burkholderia species. Both of these isolates were able to grow on BCSA selective medium (Table 1).
Of the strains that had been previously characterized, 38 of 40 grouped according to species level on the tree (Fig. 1). One exception was C1469, a B. pyrrocinia strain that appears to be more closely related to BCC recA group K isolates than to other B. pyrrocinia strains. A second exception was LMG 19240, a B. cenocepacia strain that belongs to the recA IIIC subgroup. The fur gene sequence of LMG 19240 is substantially different from the sequences of the other B. cenocepacia strains and isolates tested. As such, it forms an isolated B. cenocepacia branch on the tree (Fig. 1). Despite these two exceptions, the strong correlation between the position of any BCC strain's fur sequence on the tree and its species designation suggested that the addition of fur gene sequences from unidentified strains would allow unambiguous identification at the species level. As predicted, the 21 uncharacterized clinical isolates clustered on the tree among the previously characterized strains. The largest cluster of these isolates, 13 of 21 (62%), was B. cenocepacia based on their position in the tree. The next largest cluster was B. multivorans, a group in which 4 of the 21 (19%) isolates belonged. These values are consistent with previous reports showing that the majority of BCC clinical isolates are either B. cenocepacia or B. multivorans isolates (28). The remainder of the uncharacterized clinical isolates belonged to Burkholderia stabilis (three isolates) and BCC group K (one isolate). The fur gene sequence from isolate R445 was more closely related to BCC group K strains (33) than to prototypical B. cepacia strains (21) (Fig. 1).
With the exception of C1469 and LMG 19240, fur gene sequence analysis separates strains and isolates of a single species into a single cluster on the phylogenetic tree. However, like recA analysis, subgroups are present within these clusters (Fig. 1). For example, B. multivorans strains and isolates branch into two discrete groups, the first including M1512, M1865, R810, C5274, and LMG 13010 and the second including ATCC 17616, C3430, C5393, R1159, and C5568. For previously characterized B. cenocepacia strains, the phylogenetic clusters based on the fur sequence match the groupings assigned by recA analysis. The strains belonging to groups IIIA to IIID all form discrete groups on the tree. The cluster formed by the IIIA strains is the most diffuse. It branches into two groups, one including R1284, R1314, and C1257 and the second including all other IIIA strains and isolates tested. Based on this analysis, all of the uncharacterized B. cenocepacia clinical isolates are most closely related to strains in the IIIA subgroup.
Design and application of species-specific primers.
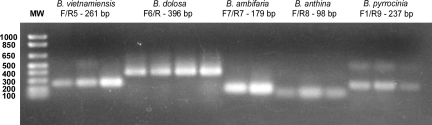
While analysis of the fur gene sequence allowed the BCC strains and isolates to be easily and accurately identified to the species level, a complementary PCR protocol was developed to make the classification process more rapid. There are several sites in the fur gene where a single base pair difference can be used to differentiate strains of one BCC species from others. These polymorphisms were used to design primer sets that would amplify the fur gene in a species-specific fashion. These primers, the expected products, and the species from which the products can be amplified are shown in Table 2. Primers were tested against a panel of nine representative BCC strains (Table 1) in order to verify that the primer sets would result in the synthesis of a product of the expected size for only one of the nine species. The primers designed for B. cepacia/BCC recA group K, B. multivorans, B. cenocepacia, B. vietnamiensis, B. dolosa, B. ambifaria, and B. pyrrocinia were highly specific. When tested with the nine representative BCC strains, these primers amplified a product of the expected size from only a single species. Although a small number of nonspecific products were amplified in some cases, these amplicons could be distinguished from the expected product by their sizes (Fig. 2). Each amplicon of the correct size was sequenced in order to verify amplification of the expected product. In addition to the nine representative BCC strains, these primer sets were subsequently tested with an additional 50 strains and isolates: 5 B. cepacia and BCC group K strains/isolates for primer set F1/R1, 9 B. multivorans strains/isolates for primer set F2/R2, 23 B. cenocepacia strains/isolates for primer set F/R3, 3 B. vietnamiensis strains for primer set F/R5, 4 B. dolosa strains for primer set F6/R, 3 B. ambifaria strains for primer set F7/R7, and 3 B. pyrrocinia strains for primer set F1/R9. In each reaction, a product of the expected size was amplified for each of the strains, except for LMG 19240, which lacks the fur gene polymorphism used to design the F/3R primer set.
FIG. 2.
Gel electrophoresis of fur PCR products for the BCC species B. vietnamiensis, B. dolosa, B. ambifaria, B. anthina, and B. pyrrocinia. PCR products were electrophoresed on a 0.8% agarose gel prior to visualization with ethidium bromide. Molecular weight standards (MW) are shown in lane 1, with the corresponding base pair sizes shown at the left of the gel. Shown below each BCC species name is the fur primer set used and the size of the obtained PCR product (in base pairs) with the following template DNAs: lane 2, PC259/LMG 18835; lane 3, G4; lane 4, ATCC 29424; lane 5, STM1441/LMG 21443; lane 6, CEP021/LMG 21819; lane 7, E12/LMG 21820; lane 8, L06; lane 9, CEP0996/LMG 19467; lane 10, M53; lane 11, J2552/LMG 16670; lane 12, C1765/LMG 20983; lane 13, AU1293/LMG 21821; lane 14, ATCC 39277/LMG 21822; lane 15, BC011/LMG 21823; lane 16, C1469/LMG 21824.
The primers designed for B. stabilis and B. anthina were less specific, as the polymorphisms used to design these two primer sets are each present in four species (Table 2). More specific primer sets could not be designed for either species because of the relatively low number of heterogeneous base pairs in the fur gene specific to each species. However, discrimination was still possible by designing F4/R to amplify B. multivorans, B. cenocepacia, B. stabilis, and B. dolosa, while F/R8 was designed to amplify B. multivorans, B. cenocepacia, B. stabilis, and B. anthina. In addition, this primer set has the potential to amplify internal fur gene products from B. pyrrocinia as demonstrated with strain BC011. For each of these sets of species, the primer pairs were highly specific, amplifying only single products of the expected sizes. For example, using DNA from a B. stabilis isolate will give an amplicon product with primer set F4/R but no PCR product with primer set F6/R, whereas B. dolosa genomic DNA will produce a product in both reactions. Similarly, B. anthina DNA will produce an amplicon using primer set F/R8 but not F1/R9, whereas B. pyrrocinia DNA may or may not produce a PCR product with primer set F/R8 but will produce an amplicon with primer set F1/R9. An additional seven strains and isolates were tested (four B. stabilis strains/isolates for F4/R and three B. anthina strains for F/R8), and for each strain, a product of the correct size was amplified. Together, these nine primer sets allow the rapid discrimination of BCC species from one another.
DISCUSSION
Although the BCC was originally isolated as a pathogen of onions, it has recently gained notoriety as a serious threat to patients with the heritable genetic disease CF. BCC species cause severe infections that (in 20% of cases) result in the development of a fatal necrotizing pneumonia referred to as cepacia syndrome in 20% of cases (9, 17). BCC species are easily transmitted among a susceptible population by both direct and indirect contact and are highly resistant to a wide range of antibiotics (26, 28). Because of the devastating potential impact of a false-positive or false-negative diagnosis, especially since some BCC species are of a greater clinical concern than others, BCC infections must be identified both rapidly and accurately in a clinical setting (29). Unfortunately, there are various problems with current diagnostic tests for BCC species, leading to unacceptably high misidentification rates (23). As such, the further development of diagnostic tests that are able to differentiate BCC species is required.
We have developed a simple, specific, and accurate procedure to identify BCC isolates to the species level based on the sequence of the gene encoding the ferric uptake regulator, or fur. As shown in Fig. 1, for 97% of the strains and isolates tested, members of a single species cluster together on a phylogenetic tree based on their fur gene sequences. To identify the species of an uncharacterized strain or isolate with relative certainty, one can PCR amplify and sequence its fur gene for comparison to those of other BCC species. One of the main benefits of this procedure is its simplicity. Another benefit of this protocol is that PCR with primers JD490 and JD491 will be unsuccessful if the isolate is not of the genus Burkholderia. As such, this step permits rapid discrimination between Burkholderia and non-Burkholderia samples. In our analysis of 27 previously uncharacterized clinical isolates, negative JD490/JD491 PCR amplification results were the first indication that four of the samples did not represent Burkholderia strains (Table 1). Growth on BCSA was an unreliable measure in this case, as one of the four strains (Pandoraea sp.) was able to grow on this medium.
In order to take advantage of the single-base polymorphisms present in the fur gene, a PCR protocol that would allow the rapid determination of BCC species status was designed. The base pair changes in fur tend to be consistent among strains of a single BCC species, indicating that this gene is an appropriate target for species-specific PCR. The percent sequence diversity of fur is 18%, which is intermediate to that of other genetic loci used for similar purposes, such as atpD (13.1%) and recA (26.7%) (3). Testing of an unknown strain in a PCR protocol with fur gene-based primer sets can identify the species to which a strain or isolate belongs using only one set of nine PCRs. The protocol was designed such that the sequencing data, which are highly accurate but take some time to collect, can be used in concert with the PCR, which is less specific but more rapid. It therefore fulfils the need for a diagnostic procedure that is both effective and efficient at identifying clinical and environmental BCC samples.
In practical use, the procedure was successful in classifying 27 clinical isolates from CF patients putatively identified as being BCC species. As predicted, the majority of the isolates were either B. cenocepacia or B. multivorans, with a lesser number identified as being either B. stabilis or BCC group K isolates. This system was also able to determine that there was a misidentification of 6 of the 27 isolates. These isolates were identified as being B. gladioli, Pseudomonas aeruginosa, Herbaspirillum sp., Pandoraea sp., or Listeria monocytogenes isolates. As shown in Table 1, three of these six isolates were able to grow on BCSA selective medium. These results further underscore the need for a new, more accurate practical method for examining clinical specimens. Similar ambiguous results using conventional testing protocols have been observed recently by another laboratory (31). In this case, the cable pilus gene was used as an additional molecular marker for the ET12 lineage to further differentiate BCC isolates grouped by recA cluster analysis.
The fur gene protocol has a number of advantages over those developed previously, as it does not rely on biochemical measures; it can identify strains to the species level (unlike 16S rRNA gene sequencing); with few exceptions, it identifies members of a single species as part of a single group (unlike recA); and it uses a single gene target (unlike multilocus restriction typing and MLST). The PCR can be completed rapidly with relatively inexpensive reagents and equipment and does not require special training to complete. This fur gene protocol quickly separates the more important clinical isolates B. multivorans and B. cenocepacia from those species that are less able to cause acute disease or to undergo epidemic spread in a susceptible population. This protocol was unable to classify the recA IIIC isolate tested as part of the B. cenocepacia cluster. This result suggests that B. cenocepacia IIIC may be significantly different at other loci besides fur and that further testing should be carried out in order to confirm its phylogenetic position among BCC strains. All IIIC strains characterized to date are environmental isolates, suggesting that this lineage may not be prevalent clinically (32). If necessary, sufficient fur gene polymorphisms exist to allow the design of a IIIC subgroup-specific primer (data not shown). This protocol also demonstrates a previously observed subdivision between B. multivorans strains that is almost as significant as the subdivision of B. cenocepacia species into recA phylotypes (IIIA, IIIB, and IIID). Again, further sampling will serve to reinforce this assertion.
One potential limitation of all PCR-based classification systems is that a mutation of a single base will prevent amplification by the correct primer set or allow amplification by an incorrect primer set. However, companion DNA sequencing and comparison of the complete fur gene sequence will position the strain correctly in the BCC fur phylogenetic tree. Only in instances where there is substantial similarity between fur gene sequences of different BCC species will the PCR test results conflict with fur gene sequence analysis. This appears to be the case for the B. cepacia and B. pyrrocinia clade. Although B. cepacia strains and isolates are specifically amplified using B. cepacia-specific primer set F1/R1, and B. pyrrocinia strains are specifically amplified by primer set F1/R9, because of the high homology between fur gene sequences of these two species, B. pyrrocinia strain C1469 clusters with B. cepacia strains on the fur phylogenetic tree. Further analysis shows that the B. cepacia strains that cluster with B. pyrrocinia strain C1469 are different from prototypical B. cepacia (type strain ATCC 25416) and type as group K variants by recA analysis (33). Therefore, the fur gene alignment is sensitive enough to recognize recA-based differences between subspecies, even though the PCR assay correctly assigns strains to either B. cepacia or B. pyrrocinia.
The observed homology between fur alleles of B. cepacia/ B. pyrrocinia and B. anthina/B. stabilis is reminiscent of the homology observed with recA alleles of B. cepacia, B. cenocepacia, and B. ambifaria (33). Although the sensitivity and specificity of B. cepacia recA PCR diagnostics could undergo improvement, it bears reminding that the B. cepacia complex is just that: a group of closely related bacterial species with relatively plastic genomes. It is not surprising, then, that some BCC species possess genes that are relatively unchanged from those of other BCC species, perhaps through the mechanism of recombination, which makes the discovery of a single gene-based diagnostic classification system all the more difficult. To overcome this limitation, multigene-based diagnostic systems such as MLST have been developed (3). Although these types of analyses are beyond the capability of most clinical laboratories, they can unequivocally identify all existing BCC species and differentiate strains and isolates from various sources. The BCC MLST scheme utilizes seven conserved loci including recA, which provides the potential for distinguishing approximately 1013 different BCC genotypes (3). However, our analysis of the fur gene from the 66 BCC strains and isolates that we examined indicates that fur has a nucleotide sequence diversity that is higher than those of at least some of the BCC MLST loci used previously (3). This suggests that the fur gene sequence is conserved well enough to be accurate in PCR-based BCC diagnostics but still variable enough to be used as an ideal gene locus candidate in a BCC MLST scheme.
In summary, we have developed an effective method of differentiating BCC strains and isolates based on their fur gene sequences. Although further large-scale sensitivity and specificity testing is required to validate the clinical utility of this method, it represents a potentially very useful advance in rapid BCC diagnostics. This protocol can be used in both clinical and research laboratories to quickly and accurately classify BCC isolates. This BCC fur gene-based method permits unambiguous identification at the species level and, as evidenced by the resulting phylogenetic tree, creates a population structure that coincides with current species assignments for the BCC. It is able to resolve unknown clinical isolates into specific BCC species or subspecies, thereby providing a comprehensive framework for discriminating strain classification and epidemiological evaluation.
Acknowledgments
J.J.D. gratefully acknowledges financial support from the Canadian Cystic Fibrosis Foundation for an operating research grant. K.H.L. is indebted to the Natural Sciences and Engineering Research Council of Canada for a CGS-M award.
We thank Pamela Sokol, David Speert, and Deb Henry of the Canadian Burkholderia cepacia Complex Research and Referral Repository for providing bacterial strains. We also gratefully acknowledge Robert Rennie and LeeAnn Turnbull of the University of Alberta Hospitals Cystic Fibrosis Clinic for providing clinical isolates.
Footnotes
Published ahead of print on 5 December 2007.
REFERENCES
- 1.Aaron, S. D., W. Ferris, D. A. Henry, D. P. Speert, and N. E. MacDonald. 2000. Multiple combination bactericidal antibiotic testing for patients with cystic fibrosis infected with Burkholderia cepacia. Am. J. Respir. Crit. Care Med. 1611206-1212. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seldman, J. A. Smith, and K. Struhl. 1991. Current protocols in molecular biology. Greene Publishing Associates, New York, NY.
- 3.Baldwin, A., E. Mahenthiralingam, K. M. Thickett, D. Honeybourne, M. C. J. Maiden, J. R. Govan, D. P. Speert, J. J. Lipuma, P. Vandamme, and C. G. Dowson. 2005. Multilocus sequence typing scheme that provides both species and strain differentiation for the Burkholderia cepacia complex. J. Clin. Microbiol. 434665-4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berriatua, E., I. Ziluaga, C. Miguel-Virto, P. Uribarren, R. Juste, S. Laevens, P. Vandamme, and J. R. W. Govan. 2001. Outbreak of subclinical mastitis in a flock of dairy sheep associated with Burkholderia cepacia complex infection. J. Clin. Microbiol. 39990-994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown, A. R., and J. R. W. Govan. 2007. Assessment of fluorescent in situ hybridization and PCR-based methods for rapid identification of Burkholderia cepacia complex organisms directly from sputum samples. J. Clin. Microbiol. 451920-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burkholder, W. H. 1950. Sour skin, a bacterial rot of onion bulbs. Phytopathology 40115-117. [Google Scholar]
- 7.Chan, C., D. E. Stead, and R. H. A. Coutts. 2003. Development of a species-specific recA-based PCR test for Burkholderia fungorum. FEMS Microbiol. Lett. 224133-138. [DOI] [PubMed] [Google Scholar]
- 8.Coenye, T., T. Spilker, A. Martin, and J. J. LiPuma. 2002. Comparative assessment of genotyping methods for epidemiologic study of Burkholderia cepacia genomovar III. J. Clin. Microbiol. 403300-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coenye, T., P. Vandamme, J. R. W. Govan, and J. J. Lipuma. 2001. Taxonomy and identification of the Burkholderia cepacia complex. J. Clin. Microbiol. 393427-3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coenye, T., P. Vandamme, J. J. LiPuma, J. R. W. Govan, and E. Mahenthiralingam. 2003. Updated version of the Burkholderia cepacia complex experimental strain panel. J. Clin. Microbiol. 412797-2798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corey, M., and V. Farewell. 1996. Determinants of mortality from cystic fibrosis in Canada, 1970-1989. Am. J. Epidemiol. 1431007-1017. [DOI] [PubMed] [Google Scholar]
- 12.Dalmastri, C., L. Pirone, S. Tabacchioni, A. Bevivino, and L. Chiarini. 2005. Efficacy of species-specific recA PCR tests in the identification of Burkholderia cepacia complex environmental isolates. FEMS Microbiol. Lett. 24639-45. [DOI] [PubMed] [Google Scholar]
- 13.Engledow, A. S., E. G. Medrano, E. Mahenthiralingam, J. J. LiPuma, and C. F. Gonzalez. 2004. Involvement of a plasmid-encoded type IV secretion system in the plant tissue watersoaking phenotype of Burkholderia cenocepacia. J. Bacteriol. 1866015-6024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13a.Hein, J. J. 1990. Unified approach to alignment and phylogenies. Methods Enzymol. 183626-645. [DOI] [PubMed] [Google Scholar]
- 14.Henry, D. A., M. E. Campbell, J. J. LiPuma, and D. P. Speert. 1997. Identification of Burkholderia cepacia isolates from patients with cystic fibrosis and use of a simple new selective medium. J. Clin. Microbiol. 35614-619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry, D. A., E. Mahenthiralingam, P. Vandamme, T. Coenye, and D. P. Speert. 2001. Phenotypic methods for determining genomovar status of the Burkholderia cepacia complex. J. Clin. Microbiol. 391073-1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15a.Higgins, D. G., and P. M. Sharp. 1989. Fast and sensitive multiple sequence alignments on a microcomputer. Comput. Appl. Biosci. 5151-153. [DOI] [PubMed] [Google Scholar]
- 16.Holmes, A., R. Nolan, R. Taylor, R. Finley, M. Riley, R. Z. Jiang, S. Steinbach, and R. Goldstein. 1999. An epidemic of Burkholderia cepacia transmitted between patients with and without cystic fibrosis. J. Infect. Dis. 1791197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Isles, A., I. Maclusky, and M. Corey. 1984. Pseudomonas cepacia infection in cystic fibrosis: an emerging problem. J. Pediatr. 104206-210. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, J. 1994. Similarity analysis of rRNAs, p. 683-700. In P. Gerhardt, R. G. E. Murray, W. A. Wood, and N. R. Krieg (ed.), Methods for general and molecular bacteriology. ASM Press, Washington, DC.
- 19.LiPuma, J. J. 1998. Burkholderia cepacia: management issues and new insights. Clin. Chest Med. 19473-486. [DOI] [PubMed] [Google Scholar]
- 20.LiPuma, J. J., B. J. Dulaney, J. D. McMenamin, P. W. Whitby, T. L. Stull, T. Coenye, and P. Vandamme. 1999. Development of rRNA-based PCR assays for identification of Burkholderia cepacia complex isolates recovered from cystic fibrosis patients. J. Clin. Microbiol. 373167-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahenthiralingam, E., J. Bischof, S. K. Byrne, C. Radomski, J. E. Davies, Y. Av-Gay, and P. Vandamme. 2000. DNA-based diagnostic approaches for identification of Burkholderia cepacia complex, Burkholderia vietnamiensis, Burkholderia multivorans, Burkholderia stabilis, and Burkholderia cepacia genomovars I and III. J. Clin. Microbiol. 383165-3173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahenthiralingam, E., T. Coenye, J. W. Chung, D. P. Speert, J. R. W. Govan, P. Taylor, and P. Vandamme. 2000. Diagnostically and experimentally useful panel of strains from the Burkholderia cepacia complex. J. Clin. Microbiol. 38910-913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McMenamin, J. D., T. M. Zaccone, T. Coenye, P. Vandamme, and J. J. LiPuma. 2000. Misidentification of Burkholderia cepacia in US cystic fibrosis treatment centers: an analysis of 1,051 recent sputum isolates. Chest 1171661-1665. [DOI] [PubMed] [Google Scholar]
- 24.Moore, J. E., B. C. Millar, J. Xu, M. Crowe, A. O. B. Redmond, and J. S. Elborn. 2002. Misidentification of a genomovar of Burkholderia cepacia by recA restriction fragment length polymorphism. J. Clin. Pathol. 55309-311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Myers, E. W., and W. Miller. 1988. Optimal alignments in linear space. Comput. Appl. Biosci. 411-17. [DOI] [PubMed] [Google Scholar]
- 25.Payne, G. W., P. Vandamme, S. H. Morgan, J. J. LiPuma, T. Coenye, A. J. Weightman, T. H. Jones, and E. Mahenthiralingam. 2005. Development of a recA gene-based identification approach for the entire Burkholderia genus. Appl. Environ. Microbiol. 713917-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Prince, A. 1986. Antibiotic resistance of Pseudomonas species. J. Pediatr. 108830-834. [DOI] [PubMed] [Google Scholar]
- 27.Ramette, A., J. J. LiPuma, and J. M. Tiedje. 2005. Species abundance and diversity of Burkholderia cepacia complex in the environment. Appl. Environ. Microbiol. 711193-1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saiman, L., and J. Siegel. 2004. Infection control in cystic fibrosis. Clin. Microbiol. Rev. 1757-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28a.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic tress. Mol. Biol. Evol. 4406-425. [DOI] [PubMed] [Google Scholar]
- 29.Speert, D. P. 2002. Advances in Burkholderia cepacia complex. Paediatr. Respir. Rev. 3230-235. [DOI] [PubMed] [Google Scholar]
- 30.Speert, D. P., D. Henry, P. Vanadamme, M. Corey, and E. Mahenthiralingam. 2002. Epidemiology of Burkholderia cepacia complex in patients with cystic fibrosis, Canada. Emerg. Infect. Dis. 8181-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turton, J. F., N. Arif, D. Hennessy, M. E. Kaufmann, and T. L. Pitt. 2007. Revised approach for identification of isolates within the Burkholderia cepacia complex and description of clinical isolates not assigned to any of the known genomovars. J. Clin. Microbiol. 453105-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vandamme, P., B. Holmes, T. Coenye, J. Goris, E. Mahenthiralingam, J. J. LiPuma, and J. R. W. Govan. 2003. Burkholderia cenocepacia sp. nov. —a new twist to an old story. Res. Microbiol. 15491-96. [DOI] [PubMed] [Google Scholar]
- 33.Vermis, K., T. Coenye, E. Mahenthiralingam, H. J. Nelis, and P. Vandamme. 2002. Evaluation of species-specific recA-based PCR tests for genomovar level identification within the Burkholderia cepacia complex. J. Med. Microbiol. 51937-940. [DOI] [PubMed] [Google Scholar]
- 33a.Wilbur, W. J., and D. J. Lipman. 1983. Rapid similarity searches of nucleic acid and protein data banks. Proc. Natl. Acad. Sci. USA 80726-730. [DOI] [PMC free article] [PubMed] [Google Scholar]