Abstract
The malachite green microtube (MGMT) susceptibility assay was performed directly on sputum specimens (n = 80) and indirectly on Mycobacterium tuberculosis clinical isolates (n = 60). The technique is based on the malachite green dye, which changes color in response to M. tuberculosis growth. The MGMT assay is simple and rapid and does not require expensive instruments.
Recent advances in technology have introduced many rapid and reliable methods to differentiate between susceptible and resistant Mycobacterium tuberculosis strains (7, 10, 12, 16). However, due to their high cost and equipment requirement, these new methods are not feasible in the clinical laboratories of developing countries in the diagnosis of tuberculosis (TB). Instead, these countries use the proportional method, which is very time-consuming (2, 11). Consequently, physicians base their diagnosis of TB on microscopy results. Therefore, supplementary rapid and reliable methods are highly needed for clinical laboratories with limited resources. In 1995, Yajko et al. (15) used an oxidation-reduction indicator, Alamar blue, which changes color in response to the growing bacteria. In 2004, we demonstrated the viability of M. tuberculosis in sputum specimens of TB patients using malachite green indicator dye, a compound routinely used in Löwenstein-Jensen (LJ) medium (3, 4). Malachite green is a triphenylmethane dye and has a dark green color, which becomes colorless during M. tuberculosis metabolism (6).
In the present study, the malachite green indicator dye was used to assess the susceptibility of M. tuberculosis clinical isolates against first- and second-line anti-TB drugs. The test was also performed directly on sputum specimens of patients with pulmonary TB. In total, 80 sputum specimens from TB patients and 30 sputum specimens from patients without mycobacterial infection (e.g., with lung cancer or asthma) were collected. Furthermore, the malachite green microtube (MGMT) assay was tested indirectly on 60 M. tuberculosis clinical isolates and the M. tuberculosis H37RV ATCC 27294 reference strain (American Type Cell Culture Collection, Rockville, MD). The accuracy and feasibility of the MGMT assay were compared to those of the standard LJ culture method for drug susceptibility testing.
Sputum specimens were digested and decontaminated by the Petroff method with a 2% final concentration of NaOH. Following digestion for 15 min, the samples were centrifuged at 3,000 × g for 30 min and decanted, leaving 1 to 2 ml of sediment (2, 11). The remaining sediments were reconstituted in 2 ml of sterile phosphate-buffered saline. Two hundred microliters of this suspension was inoculated onto each LJ culture slant, and 100 μl was inoculated into each microtube containing100 μl of 7H9GC broth with or without added drugs (4). All cultures were incubated at 37°C for up to 8 weeks. The drugs and their final concentrations in the malachite green microtubes were as follows: isonizid (INH), 0.2 μg/ml; streptomycin,10 μg/ml; ethambutol, 2 μg/ml; rifampin (RF), 40 μg/ml; caperomycin, 10 μg/ml; ciprofloxacin, 2 μg/ml; cycloserine, 30 μg/ml; ethionamide, 20 μg/ml; kanamycin, 20 μg/ml; and ofloxacin, 2 μg/ml (14). The drug concentrations were chosen according to the protocols of the World Health Organization (14) and Franzblau et al. (5). As the length of incubation needed for sufficient metabolic activity to occur can vary from strain to strain, three control microtubes were included in each test run. The samples were tested after 7, 14, and 21 days of incubation by adding 50 μl of a 0.02-μg/ml solution of malachite green (Merck, Germany) to the control tubes and documenting whether a color change occurred. If the green color disappeared (tube contents became colorless), it meant that sufficient metabolic activity had occurred to allow the test to be read (4). At this point, malachite green was added to each of the drug-containing microtubes, and the color of each tube was recorded. Before reporting the test results, a smear was prepared from the concentrated sediments of each malachite green susceptibility test tube for Ziehl-Neelsen acid-fast staining.
For indirect MGMT susceptibility testing, colonies from the surface of LJ medium were transferred into sterile test tubes containing six to eight glass beads and 3.0 ml of 7H9GC broth. The turbidity was adjusted to a McFarland standard of 1, and the suspension was diluted in 7H9 broth (1:5 in broth). As described above, 100 μl of this suspension inoculated into malachite green susceptibility test tubes contained 100 μl of diluted 7H9GC broth with or without drugs. The final concentration of mycobacteria in the susceptibility test tubes was approximately 6 × 105 CFU/ml. All the microtubes were incubated at 37°C. As soon as the color change from dark green to the loss of color was observed in the drug-free control tubes, 50 μl of the 0.02-mg/ml solution of malachite green was added to the drug-containing test tubes. Tubes were incubated for another 12 to 24 h at 37°C before the test results were recorded.
Out of 80 sputum specimens, 38 (47.5%) patients had a positive smear microscopy. Culture on LJ medium recovered 50 (62.5%) M. tuberculosis-positive specimens, while MGMT yielded 42 (52.5%) positive specimens. In the control subjects (n = 30), no mycobacterial growth was detected. The overall contamination rates for LJ and MGMT in the TB patients and control subjects were 3.6% and 6.3%, respectively. The sensitivity and specificity of MGMT for M. tuberculosis detection were 84% and 95.9%, respectively. Out of the 42 positive-culture media, 21 were susceptible, 8 were multidrug resistant (MDR) (i.e., resistant to INH and RF), 2 were extensively drug-resistant TB (XDR-TB) {i.e., resistant to fluoroquinolones and to at least one of the three injectable second-line drugs in addition to INH and RF [13]), and 4 had other resistance patterns by both methods (Table 1). We observed discrepancies between the MGMT assay and the standard LJ culture system for seven isolates; two isolates were falsely interpreted as susceptible, and for five isolates (11.9%), no reaction occurred in the MGMT assay. Microscopy examination of these samples revealed negative smear results. It is possible that some technical errors occurred during inoculation procedures or that the number of bacilli in tubes were not sufficient to reduce the malachite green dye. The mean time from inoculation to interpretation of results was 15 days (8 to 20 days) for direct MGMT testing compared to a mean of 70 days (17 to 42 days) for the indirect proportional method (P < 0.001). The overall sensitivity of the direct MGMT assay for drug susceptibility testing was 87% with a specificity of 80%. Drug susceptibility results using indirect MGMT for the H37RV strain and the 60 M. tuberculosis clinical isolates were available within 6 to 17 days (median, 12 days). The correlations between the tests are illustrated in Table 2. As shown, for the most important first-line drugs, INH and RIF, as well as for the key second-line drug (ciprofloxacin), the results were excellent with a sensitivity and specificity of 100%. For 9 of the 12 drugs tested, we saw a high specificity of at least 94%. For SM, ETH, and ETB, the specificities (below 90%) were not as good. The sensitivity of the indirect MGMT assay was 80.5% with a specificity of 95%. To our knowledge, this study represents the first evaluation of the malachite green indicator for drug susceptibility testing of the first- and second-line anti-TB drugs.
TABLE 1.
Direct susceptibility testing by MGMT assay and comparison with indirect proportional method in the same clinical specimens
| M. tuberculosis straina | No. of strains with the following test result:b
|
% Agreement | |||
|---|---|---|---|---|---|
| Susceptible by both methods | Resistant by both methods | Sensitive by MG, resistant by LJ | No growth by MG, sensitive by LJ | ||
| Susceptible strains (n = 26) | 21 | 5 | 80 | ||
| MDR strains (resistant to INH and RF) | |||||
| Susceptible to all second-line drugs (n = 3) | 3 | ||||
| Resistant to SM also (n = 1) | 1 | 100 | |||
| Resistant to SM, ETB, and AM also (n = 2) | 2 | ||||
| Resistant to SM and KAN also (n = 1) | 1 | ||||
| Resistant to ETB and PAZ also (n = 1) | 1 | ||||
| XDR strains resistant to INH, RF, CIP, OF, KAN, AM, ETH, and DC (n = 2) | 2 | 100 | |||
| Strains with other resistance patterns | |||||
| Resistant to only ETB (n = 2) | 1 | 1 | 66 | ||
| Resistant to ETB and SM (n = 3) | 2 | 1 | |||
| Resistant to ETB, RF, and AM (n = 1) | 1 | ||||
Abbreviations: AM, ampicillin; CIP, ciprofloxacin; DC, doxycycline; ETB, ethambutol; ETH, erythromycin; INH, isonizid; KAN, kanamycin; OF, ofloxacin; PAZ, pyrazinamide; RF, rifampin; SM, streptomycin.
MG, malachite green microtube susceptibility assay; LJ, Löwenstein-Jensen culture medium.
TABLE 2.
Comparison of malachite green microtube assay results with proportional method results (60 stock cultures)
| Druga | No. of strains with the following resultb:
|
% Agreement in isolated strains | Sensitivity (%)c | Specificity (%)d | |||
|---|---|---|---|---|---|---|---|
| Susceptible by both | Resistant by both | Resistant by MG, sensitive by LJ | Sensitive by MG, resistant by LJ | ||||
| SM | 25 | 29 | 3 | 4 | 90 | 87 | 89 |
| INH | 30 | 30 | 0 | 0 | 100 | 100 | 100 |
| RF | 30 | 30 | 0 | 0 | 100 | 100 | 100 |
| ETB | 24 | 28 | 5 | 3 | 86 | 90 | 82 |
| CIP | 53 | 7 | 0 | 0 | 100 | 100 | 100 |
| DC | 55 | 3 | 1 | 1 | 96 | 75 | 98 |
| OF | 53 | 3 | 3 | 93 | 75 | 94 | |
| CAP | 55 | 3 | 1 | 1 | 96.6 | 75 | 98 |
| ETH | 49 | 3 | 6 | 2 | 86 | 60 | 89 |
| PAZ | 55 | 3 | 1 | 1 | 96 | 75 | 98 |
| KAN | 54 | 4 | 1 | 1 | 96 | 80 | 98 |
| AM | 51 | 3 | 3 | 3 | 90 | 50 | 94 |
Abbreviations: AM, ampicillin; CAP, caperomycin; CIP, ciprofloxacin; DC, doxycycline; ETB, ethambutol; ETH, erythromycin; INH, isonizid; KAN, kanamycin;OF, ofloxacin; PAZ, pyrazinamide; RF, rifampin; SM, streptomycin.
MG, malachite green microtube susceptibility assay; LJ, Löwenstein-Jensen culture medium.
The average sensitivity for all drugs studied was 80.5%.
The average specificy for all drugs studied was 95%.
Nevertheless, since there were few strains (Tables 1 and 2) resistant to second-line drugs in our study sample, further studies are suggested for these substances. For many decades, TB laboratories have used malachite green to prevent contamination in LJ culture medium (6). Here, we used malachite green as an indicator to determine drug susceptibility. The mechanism behind malachite green is similar to other colorimetric methods, i.e., Alamar blue, or Rarazurim salts (1, 8, 9). Upon inoculation, malachite green is in an oxidized state and has a dark green color. When there is bacterial growth, typically after a few days, the green color of the dye disappears (tube contents become colorless) (Fig. 1). Compared to the other dye methods, malachite green is less expensive ($2.50 for 12-drug susceptibility testing per strain) and is more easily implemented in TB laboratories. Furthermore, the ingredients (7H9 broth medium plus malachite green powder) are already available in TB laboratories of developing countries. In addition, the microtube format of MGMT has an advantage over colorimetric microplates with respect to biosafety (1, 15). Our results also showed a rapid and fast recovery of M. tuberculosis from smear-negative TB cases by the MGMT assay. As presented in Table 3, 59.5% of smear-negative specimens yielded a positive MGMT culture and were confirmed by the LJ culture medium (100%). A method like MGMT where simultaneous detection and susceptibility testing of clinical samples is possible thus has clear clinical advantages and is worth the effort and cost required. In conclusion, the MGMT assay is easily implemented, reliable, and inexpensive and allows for rapid identification of MDR or XDR-TB in patients. If implemented, this assay would help clinicians prescribe an effective treatment directly and be a tool in preventing the transmission of MDR or XDR-TB. Last, this method eliminates the need for expensive diagnostic procedures, which is a prerequisite for TB laboratories in developing countries.
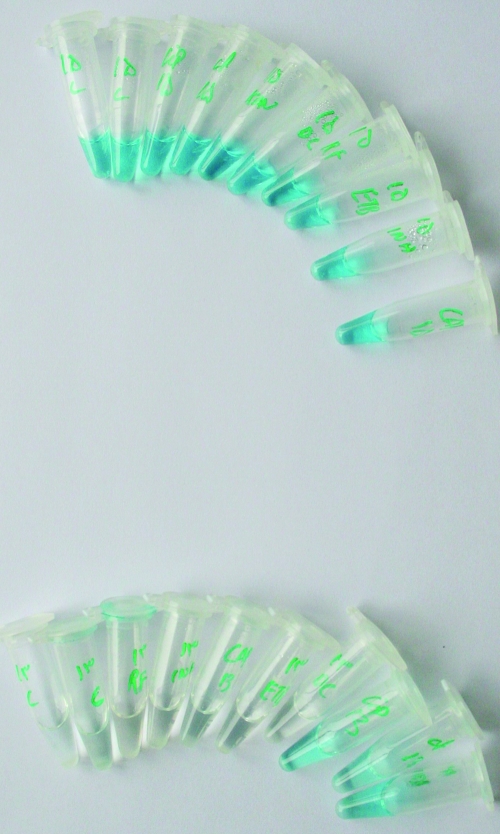
FIG. 1.
In response to bacterial growth, the malachite green indicator changes in color from green to the total loss of color.
TABLE 3.
Rate of recovery of Mycobacterium tuberculosis from sputum specimens using malachite green and LJ culture medium
| Specimen and microscopic finding | No. of specimens with the following culture resulta:
|
No. of specimens showing contamination
|
||||
|---|---|---|---|---|---|---|
| Positive by both MG and LJ | Negative by both MG and LJ | Negative by MG, positive by LJ | Positive by MG, negative by LJ | LJ | MG | |
| TB cases (n = 80) | ||||||
| Smears with the following no. of positive results (11): | ||||||
| 3 (n = 11) | 4 | 2 | 0 | 2 | 1 | 2 |
| 2 (n = 6) | 4 | 2 | 0 | 0 | 0 | 0 |
| 1 (n = 12) | 5 | 3 | 1 | 0 | 1 | 2 |
| Few bacilli (n = 9) | 4 | 3 | 2 | 0 | 0 | 0 |
| Negative (n = 42) | 25 | 11 | 5 | 0 | 1 | 0 |
| Control subjects (n = 30), negative | 0 | 26 | 0 | 0 | 1 | 3 |
| Total specimens (n = 110) (%) | 42 (38.1) | 47 | 8 (7.2) | 2 (1.8) | 4 (3.6) | 7 (6.3) |
MG, malachite green microtube susceptibility assay; LJ, Löwenstein-Jensen culture medium.
Footnotes
Published ahead of print on 19 December 2007.
REFERENCES
- 1.Bastian, I., L. Rigouts, J. C. Palomino, and F. Portaels. 2001. Kanamycin susceptibility testing of Mycobacterium tuberculosis using Mycobacterium Growth Indicator Tube and a colorimetric method. Antimicrob. Agents Chemother. 451934-1936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Canetti, G., W. Fox, A. Khomenko, H. T. Mahler, N. K. Menon, D. A. Mitchison, N. Rist, and N. A. Smelev. 1969. Advances in techniques of testing mycobacterial drug sensitivity, and the use of sensitivity tests in tuberculosis control programmes. Bull. W. H. O. 29565-578. [PMC free article] [PubMed] [Google Scholar]
- 3.Farnia, P., F. Mohammadi, M. Mirsaedi, A. Z. Zarifi, J. Tabatabee, M. Bahadori, A. A. Velayati, and M. R. Masjedi. 2004. Application of oxidation-reduction assay for monitoring treatment of patients with pulmonary tuberculosis. J. Clin. Microbiol. 423324-3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farnia, P., F. Mohammadi, M. Mirsaedi, Z. Zarifi, J. Tabatabee, M. Bahadori, A. A. Velayati, and M. R. Masjedi. 2004. Bacteriological follow-up of pulmonary tuberculosis treatment: a study with a simple colorimetric assay. Microbes Infect. 6972-976. [DOI] [PubMed] [Google Scholar]
- 5.Franzblau, S. G., R. S. Witzig, J. C. McLaughlin, P. Torres, G. Madico, A. Hernandez, M. T. Degnan, M. B. Cook, V. K. Quenzer, R. M. Ferguson, and R. H. Gilman.1998. Rapid, low-technology MIC determination with clinical Mycobacterium tuberculosis isolates by using the microplate Alamar Blue assay. J. Clin. Microbiol. 36362-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones, J. J., and J. O. Falkinham III. 2003. Decolorization of malachite green and crystal violet by waterborne pathogenic mycobacteria. J. Clin. Microbiol. 472323-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaul, K. L. 2001. Molecular detection of Mycobacterium tuberculosis: impact on patient care. Clin. Chem. 471553-1558. [PubMed] [Google Scholar]
- 8.Martin, A., H. Takiff, P. Vandamme, J. Swings, J. C. Palomino, and F. Portaels. 2006. A new rapid and simple colorimetric method to detect pyrazinamide resistance in Mycobacterium tuberculosis using nicotinamide. J. Antimicrob. Chemother. 58327-331. [DOI] [PubMed] [Google Scholar]
- 9.Martin, A., F. Portaels, and J. C. Palomino. 2006. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J. Antimicrob. Chem. 59175-183. [DOI] [PubMed] [Google Scholar]
- 10.Mohammadi, F., P. Farnia, and A. Z. Zarifi. 2002. Recovery of Mycobacterium from clinical specimens and assessing drug susceptibility test of M. tuberculosis specimens by MGIT. Tanaffos 335-44. [Google Scholar]
- 11.Rieder, H. L., T. M. Chonde, and H. Myking. 1998. The Public Health Service National Tuberculosis Reference Laboratory and the National Laboratory Network: minimum requirements, role and operation in low-income country. International Union Against Tuberculosis and Lung Diseases, Paris, France.
- 12.Rüsch-Gerdes, S., C. Domehl, G. Nardi, M. R. Gismondo, H.-M. Welscher, and G. E. Pfyffer. 1999. Multicenter evaluation of the Mycobacteria Growth Indicator Tube for testing susceptibility of Mycobacterium tuberculosis to first-line drugs. J. Clin. Microbiol. 3745-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah, N. S., A. Wright, G. H. Bai, L. Barrera, F. Boulahbal, N. Martín-Casabona, F. Drobniewski, C. Gilpin, M. Havelková, R. Lepe, R. Lumb, B. Metchcok, F. Portaels, M. F. Rodrigues, S. Rusch-Gerdes, A. Van Deun, V. Vincent, K. Laserson, C. Wells, and J. P. Cegielski. 2007. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg. Infect. Dis. 13380-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Health Organization. 2001. Guidelines for drug susceptibility testing for second-line anti-tuberculosis drugs for DOTS-plus. WHO/CDS/TB/2001.288. World Health Organization, Geneva, Switzerland.
- 15.Yajko, D. M., J. J. Madej, M. V. Lancaster, C. A. Sanders, V. L. Cawthon, B. Gee, A. Babst, and W. H. Hadley. 1995. Colorimetric method for determining MICs of antimicrobial agents for Mycobacterium tuberculosis. J. Clin. Microbiol. 332324-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yates, M. D., A. F. Drobniewski, and S. M. Wilson. 2002. Evaluation of a rapid PCR-based epidemiological typing method for routine studies of Mycobacterium tuberculosis. J. Clin. Microbiol. 40712-714. [DOI] [PMC free article] [PubMed] [Google Scholar]