Abstract
There is a high incidence of cervical cancer in South African women. No large studies to assess human papillomavirus virus (HPV) infection or HPV type 16 (HPV-16) exposure have occurred in the region, a requirement for policy making with regards to HPV screening and the introduction of vaccines. Control women (n = 1,003) enrolled in a case control study of hormonal contraceptives and cervical cancer were tested for 27 cervical HPV types by reverse line blot analysis. The seroprevalence of HPV-16 immunoglobulin G (IgG) and IgA antibodies was assessed by a virus-like particle-based enzyme-linked immunoassay of 908 and 904 control women, respectively, and of 474 women with cervical cancer. The cervical HPV prevalence was 26.1%. The HPV-16 IgG seroprevalence was 44.4% and the HPV-16 IgA seroprevalence was 28.7% in control women, and these levels were significantly higher (61.8% and 52.7%, respectively) for women with cervical cancer (odds ratio [OR], 2.1 and 2.8, respectively). The cervical HPV prevalence showed an association with cervical disease, and the HPV-16 IgG prevalence decreased while the HPV-16 IgA prevalence increased with increasing age (P < 0.05). The prevalence of oncogenic HPV types (including HPV-16) decreased with age, whereas nononcogenic HPV types showed limited association with age. Multivariate analysis revealed cervical HPV infection to be associated with herpes simplex virus type 2 infection (OR, 1.7) and increasing years of education (OR, 1.9). HPV-16 IgG antibodies were inversely associated with current smoking status (OR, 0.6), and the presence of HPV-16 IgA antibodies was inversely associated with the use of alcohol (OR, 2.1) and inversely associated with the use of oral contraceptives (OR, 0.6). High levels of exposure to HPV, and particularly HPV-16, were evident in this population. The apparent increase of serum HPV-16 IgA with increasing age requires further investigation.
Human papillomavirus (HPV) is the etiological agent in cervical cancer and also is implicated in other cancers of anogenital and oropharyngeal origins (12, 30). In South Africa, there is a high incidence of cervical cancer. Cancer of the cervix is the second most common cancer among South African women, with an age-standardized incidence rate of 30.5 per 100,000 per year (26). The role of specific HPV types in the pathogenesis of cervical cancer and its precursors and in other anogenital cancers has been well established (33). Oncogenic HPV types are associated with cervical cancer, and nononcogenic HPV types are associated with genital warts and low-grade cervical disease. HPV is recognized as being a necessary, but not sufficient, cause of cervical cancer (3). A number of cofactors have been suggested, including the use of oral contraceptives; parity; tobacco smoking; genital tract infections, including those with human immunodeficiency virus type 1 (HIV-1), Chlamydia trachomatis, and herpes simplex virus type 2 (HSV-2); and immune suppression. These cofactors may increase viral persistence and/or cause direct tissue damage, increasing the infection rate. There are more than 40 known HPV types that may infect the female genital tract and at least 16 HPV types that are considered oncogenic and are implicated in cervical cancer (9). The most prevalent type worldwide is HPV type 16 (HPV-16), and the geographical distribution of HPV-16 appears to be worldwide, but this is not the case for other HPV types (8).
Little is currently known about the natural history of HPV in South Africa and the individual HPV types associated with cervical disease. There has been no study of the prevalence of HPV in a general female population in which one would expect the majority to have normal cervixes. Cervical HPV infection may be considered as either an infection with a low viral load with no sign of cervical abnormalities or an infection with a high viral load with macroscopic changes and possible precancer development (19), meaning that only a small minority of women with detectable HPV DNA will display cervical abnormalities. Among asymptomatic women in a general population, the prevalence of HPV may range from 2 to 44% (29), and most cervical infections will be cleared (7). Published accounts of the prevalence of cervical HPV types among small groups of South African women with cervical neoplasia and cervical cancer found HPV-16 to be the predominant HPV type (15).
Cervical HPV DNA detection has limitations, in that it does not assess any past HPV exposure or infection at another anatomical site. HPV serology using HPV virus-like particles (VLPs) has the ability to assess prior and present exposures to HPV at all possible anatomical sites. Notably, though, transient HPV infections do not elicit seroconversion, and some women with persistent infection fail to seroconvert (5). Within Africa, a Ugandan study found HPV-16 antibodies significantly associated with cancer of the cervix (20). The present study constituted the first assessment in southern Africa, and possibly the rest of Africa, of cervical HPV infection, as well as HPV-16 antibody prevalence and associated risk factors, in a large population (>1,000).
MATERIALS AND METHODS
Study population and enrollment.
Participants were black women and women of mixed race recruited from hospitals or clinics as controls in a case control study to assess the association between hormonal contraceptives and cervical cancer (24). No such association was found. Informed consent details, sampling methods, and participant demographic data were described in Shapiro et al. (24). Control women were matched with cases of cervical cancer for age, ethnic group, and residential area. They had attended the health services for conditions such as trauma or respiratory infections that were judged to be independent of hormone contraceptive use or cervical cancer risk. Incident cases of histologically confirmed squamous carcinoma or adenocarcinoma of the cervix (n = 524) were identified at two tertiary-care hospitals. The 1,491 control women were aged between 18 and 59 years (median age, 44), and all lived within 150 km of Cape Town. Cervical Pap smears from control women were categorized as normal cytology, abnormal squamous cells of unknown significance (ASCUS), low-grade squamous intraepithelial lesions (LSIL), and high-grade squamous intraepithelial lesions (HSIL).
Tests for HPV DNA and antibodies.
Cervical samples were assessed for type-specific HPV DNA, and serum samples were assessed for HPV-16 antibodies. The total number of women whose samples were tested varied depending on the amount of cervical tissue and serum available. Tissue for HPV typing was not available from cancer patients. Due to financial constraints, cervical scrapings from the first numerical 1,003 of 1,491 control women were tested for HPV types. HPV DNA was extracted and purified from cervical cells by using a QIAamp DNA blood minikit (Qiagen). Extracted samples were assayed for 18 oncogenic HPV types (16, 18, 26, 31, 33, 35, 39, 45, 51, 52, 53, 56, 58, 59, 66, 68, 73, and 82) and 9 nononcogenic types (6, 11, 40, 42, 54, 55, 57, 83, and 84) using the reverse line blot assay (Roche).
Serum samples were tested for immunoglobulin A (IgA) and IgG antibodies to HPV-16 VLPs (supplied by MedImmune Inc.) by VLP-based enzyme linked immunosorbent assay (ELISA). HPV-16 IgG determinations were possible on sera from 908 of the 1,003 women tested for HPV types, and HPV-16 IgA determinations were possible on 904 women's sera. Of 524 women with cervical cancer, 474 were tested for HPV-16 serum antibodies. The ELISA was performed according to the method described by Studentsov et al. (27), with certain modifications. Briefly, ELISA plate (Maxisorp C; Nunc) wells were coated overnight at 4°C with 100 μl HPV-16 VLP diluted to a concentration of 0.7 μg/ml in phosphate-buffered saline, pH 7.4 (PBS). The plates were blocked (300 μl/well) with 0.5% (wt/vol) polyvinyl alcohol (PVA) (molecular weight, 30,000 to 70,000 [Sigma]) in PBS supplemented with 0.5% PVA at room temperature for 2 h. The plates were washed six times with PBS after the blocking step and after each incubation step. Serum samples were diluted 1:100 in 0.5% PVA, and 100 μl was added to duplicate wells and incubated at 37°C for 1 h. After the samples were washed, antigen-bound immunoglobulin was detected with horseradish peroxidase-conjugated anti-human IgA or IgG (Dako, Sweden) diluted 1:4,000 and 1:6,000, respectively, in 0.5% PVA, 0.8% (wt/vol) polyvinylpyrrolidone (molecular weight, 360,000; Sigma) in PBS. After 30 min at 37°C, wells were again washed six times before the addition of 100 μl 1,2-phenylenediamine (diluted according to the manufacturer's instructions; Dako) with 0.006% hydrogen peroxide. The color reaction was stopped after 30 min by the addition of 100 μl of 0.5 M sulfuric acid, and the absorbances were read at 492 nm to determine the optical densities (OD). For each serum, the mean OD of duplicate antigen-coated wells was calculated. To control for plate-to-plate and day-to-day variability, high-positive, low-positive, and negative control sera were included on each plate. Any variation of >15% in the serum duplicates and/or control sera on a plate resulted in the sera undergoing repeat testing. Data were examined using different cutoff points for seropositivity. This was achieved by calculating the means and standard deviations (SD) of the absorbance (OD) values obtained from 42 children's sera after reactivity with antigen and eliminating those sera with values greater than the means plus 2 SD (i.e., outliers who could be HPV-16 antibody positive) (17). This was repeated until none of the remaining sera were excluded, and the OD assessed at the means plus 3 SD were selected for the cutoff values.
Statistical analysis.
The prevalence of cervical HPV and the HPV antibodies were described according to sociodemographic characteristics, sexual activity, contraceptive use, and certain lifestyle factors. Odds ratios (OR) and 95% confidence intervals (95% CIs) were calculated for HPV DNA and HPV antibodies according to cervical pathology using unconditional logistic regression analysis. In addition, age-adjusted and multivariate-adjusted odds ratios (AOR) with 95% CIs were estimated for categories of the different risk factors. Variables showing a strong association with the outcome in the age analysis, as well as those showing significant associations in multivariate stepwise procedures, were included in the final multivariate model. Tests for linear trends across categories of exposure variables were performed by regression analysis. Statistical analyses were performed using STATA, version 8.0.
(Sections of this study were presented at the 22nd International HPV Conference, Vancouver, Canada, 1 to 6 May 2005.)
RESULTS
In this study, cervical HPV infection and serum HPV-16 antibodies and associated risk factors were investigated in a South African population. It should be noted that the findings of this study can be generalized only to black women and women of mixed race (1,003) below the age of 60 years. The majority were diagnosed with normal cervixes (848), and there were 82 with ASCUS, 40 with LSIL, and 33 with HSIL.
HPV prevalence according to cervical cytology.
The cervical HPV prevalence, as assessed in all (1,003) of the control women, was found to be 26%. The most prevalent cervical types were HPV-16 (3.3%), HPV-53 (3.3%), and HPV-52 (3.2%), all of which are oncogenic HPV types. Of the total number of women with cervical HPV infections (261), 12.6% (33/261) were infected with HPV-16. Of all infections, 73.6% were infections with a single HPV type, whereas 26.4% were infections with multiple (>1) HPV types. There were, in total, 360 HPV isolations from the 261 HPV-infected women. The majority, 76.1% (274/360), were oncogenic HPV types, and 23.9% (86/360) were nononcogenic HPV types.
Among the women with a normal cervical cytology, 20.4% were HPV positive. A comprehensive study of the prevalence of cervical HPV type infection in the control women has been conducted (see reference 1). The HPV prevalence increased significantly with the severity of cytological abnormalities (Table 1). The cervical HPV prevalence in the women with ASCUS was 42.7% (OR, 2.9; 95% CI, 1.7 to 4.8), 70.0% in the women with LSIL (OR, 9.0; 95% CI, 4.2 to 19.4), and 75.8% in the women with HSIL (OR, 12.2; 95% CI, 5.4 to 27.5).
TABLE 1.
Unadjusted OR for HPV infection and HPV-16 serology in control women according to cervical pathology
| Cervical disease status | No. of women in group | % of women positive for HPV DNA | OR (95% CI) | No. (%) of women positive for HPV-16 IgG | OR (95% CI) | No. (%) of women positive for HPV-16 IgA | OR (95% CI) |
|---|---|---|---|---|---|---|---|
| Control women (no cancer) | |||||||
| Normal | 848 | 20.4 | 1.0 | 772 (43.8) | 1.0 | 767 (27.1) | 1.0 |
| ASCUS | 82 | 42.7 | 2.9 (1.7-4.8) | 70 (44.3) | 1.0 (0.6-1.7) | 71 (45.1) | 2.2 (1.3-3.6) |
| LSIL | 40 | 70.0 | 9.0 (4.2-19.4) | 36 (63.9) | 2.3 (1.1-4.6) | 36 (25) | 0.9 (0.4-1.9) |
| HSIL | 33 | 75.8 | 12.2 (5.4-27.5) | 30 (26.7) | 0.5 (0.2-1.1) | 30 (33.3) | 1.3 (0.6-2.9) |
| Total | 1,003 | 26.1 | 908 (44.1) | 904 (28.7) | |||
| Cancer | 474 | NDa | 474 (61.8) | 2.1 (1.6-2.6) | 474 (52.7) | 2.8 (2.2-3.5) |
ND, not determined (tissue for HPV typing was not available from cancer patients).
The prevalence of HPV-16 IgA and IgG serum antibodies among controls stratified according to disease and women with cervical cancer.
In the assessment of HPV-16 serum antibodies, it was found that 44.1% of the control women were HPV-16 IgG seropositive, and 28.7% were HPV-16 IgA seropositive. When stratified according to cervical cytology, the presence of HPV-16 IgG was found to be associated with LSIL (OR, 2.3; 95% CI, 1.13 to 4.55), and the presence of HPV-16 IgA was associated with ASCUS (OR, 2.2; 95% CI, 1.3 to 3.6) (Table 1). Of the 474 women with cervical cancer, 61.8% were seropositive for HPV-16 IgG (OR, 2.1; 95% CI, 1.6 to 2.6) and 52.7% for IgA (OR, 2.8; 95% CI, 2.2 to 3.5); these rates are significantly higher if all control women are considered. Women were more likely to have cervical cancer if they were seropositive for either HPV-16 IgA (OR, 1.7; 95% CI, 1.2 to 2.4) or IgG individually (OR, 1.4; 95% CI, 1.1 to 1.95) than were seronegative women, and they were more likely to have cervical cancer if they were seropositive for both IgA and IgG (OR, 4.8; 95% CI, 3.6 to 6.4).
The prevalence of cervical HPV infection and HPV-16 serum antibodies in control women according to sociodemographic, smoking, and alcohol use characteristics.
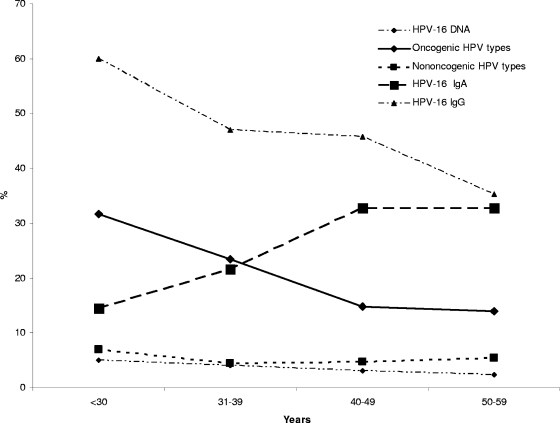
The prevalence of HPV infection (oncogenic and nononcogenic) and HPV-16 serum antibodies was assessed according to age for the 1,003 control women. In an unadjusted assessment for women <30 years, cervical HPV infection was found in 41.8%; this decreased for women 30 to 44 years of age to a low of 18.6% at 45 to 49 years. The HPV prevalence then increased slightly for women older than 50 or between 55 and 59 years (23.5 and 21.4%, respectively) (Table 2). The assessment of oncogenic HPV infection according to age revealed a similarly decreasing pattern, reflecting the majority-oncogenic content of HPV types found in the participants of this study (Fig. 1). There were 31.7, 23.4, 14.8, and 14.0% of women showing oncogenic HPV infection in the four age categories of <30 years, 30 to 39 years, 40 to 49 years, and >50 years, respectively (Fig. 1). Nononcogenic HPV types appeared in 7% of women <30 years old, 4.5% in women 30 to 39 years old, and 4.7% in women 40 to 49 years old, but the rate increased to 5.4% in the women >50 years old (Fig. 1). The HPV-16 IgG antibody prevalence simulated the HPV-16 and oncogenic HPV DNA prevalence, decreasing with increasing age (Fig. 1, Table 2). Conversely, the prevalence of HPV-16 IgA increased with increasing age, being most prevalent in the older women of 50 to 54 years (36.9%), whereas the prevalence was 14.5% in the women <30 years of age (Fig. 1, Table 2). In the age-adjusted analysis, significantly more black women than women of mixed race displayed serum HPV-16 IgG (51.9 and 41.3%, respectively; OR, 1.5) (Table 2), while fewer black women had HPV-16 IgA serum antibodies (23.9 and 30.4%, respectively; OR, 0.7). There also was reduced HPV-16 IgA seroprevalence with a higher level of education. Alcohol use was found to be associated with an increased prevalence of HPV infection and HPV-16 IgA. Past and current cigarette smoking were associated with reduced levels of HPV-16 IgG antibodies, and current smoking was positively associated with increased levels of HPV-16 IgA antibodies.
TABLE 2.
Prevalence of cervical HPV infection and HPV-16 IgG and IgA antibodies in control women according to sociodemographic, smoking, and alcohol use characteristics
| Variable | Prevalence ofb:
|
|||||
|---|---|---|---|---|---|---|
| HPV DNA
|
HPV-16 IgG
|
HPV-16 IgA
|
||||
| % Positive (n = 1,002a) | OR (95% CI) | % Positive (n = 908) | OR (95% CI) | % Positive (n = 904) | OR (95% CI) | |
| Age | ||||||
| <30 | 41.8 | 2.6 (1.4-4.9) | 60 | 2.9 (1.6-5.3) | 14.5 | 0.4 (0.2-9.7) |
| 30-34 | 37.9 | 2.2 (1.3-4.0) | 40.5 | 1.3 (0.74-2.3) | 15.9 | 0.5 (0.3-1.0) |
| 35-39 | 27.7 | 1.4 (0.8-2.5) | 51.6 | 2.1 (1.2-3.4) | 25.0 | 0.9 (0.5-1.5) |
| 40-44 | 25.1 | 1.2 (0.7-2.1) | 50.3 | 2.0 (1.2-3.2) | 31.6 | 1.2 (0.7-2.0) |
| 45-49 | 18.6 | 0.8 (0.5-1.5) | 41.5 | 1.4 (0.8-2.2) | 33.9 | 1.3 (0.8-2.2) |
| 50-54 | 23.5 | 1.1 (0.7-2.0) | 36.2 | 1.1 (0.7-1.8) | 36.9 | 1.5 (0.9-2.6) |
| 55-59 | 21.4 | 1.0 | 34.2 | 1.0 | 27.6 | 1.0 |
| Race | ||||||
| Mixed | 24.5 | 1.0 | 41.26 | 1.0 | 30.4 | 1.0 |
| Black | 30.2 | 1.3 (0.9-1.7) | 51.88 | 1.5 (1.1-2.1) | 23.9 | 0.7 (0.5-1.0) |
| Education (yr) | ||||||
| <8 | 23.1 | 1.0 | 42.2 | 1.0 | 32.7 | 1.0 |
| 8-10 | 27.7 | 1.1 (0.8-1.5) | 46.6 | 10 (0.8-1.4) | 25.9 | 0.8 (0.6-1.1) |
| >10 | 39.1 | 1.5 (0.9-2.5) | 47.4 | 1.0 (0.6-1.7) | 10.5 | 0.3 (0.2-0.7) |
| Alcohol use | ||||||
| Never | 29.2 | 1.0 | 43.3 | 1.0 | 21.8 | 1.0 |
| Past | 26.6 | 1.3 (0.8-1.9) | 40 | 0.9 (0.6-1.3) | 31.2 | 1.7 (1.1-2.6) |
| Present | 37.6 | 1.6 (1.2-2.2) | 47.0 | 1.1 (0.8-1.5) | 37.23 | 2.3 (1.7-3.2) |
| Cigarette smoking | ||||||
| Never | 31.9 | 1.0 | 51.6 | 1.0 | 23.8 | 1.0 |
| Past | 20.6 | 0.5 (0.3-1.0) | 41.4 | 0.7 (0.5-1.2) | 28.3 | 1.1 (0.7-1.9) |
| Present | 34.3 | 1.2 (0.9-1.6) | 38.4 | 0.6 (0.4-0.8) | 32.8 | 1.5 (1.1-2.1) |
There were no demographic data available for one woman; hence, there are 1,002 and not 1,003 controls, as was the case in Table 1.
With the exception of OR pertaining to the age variable, the OR are age adjusted.
FIG. 1.
Prevalence of serum antibodies, cervical oncogenic and nononcogenic HPV types, and HPV-16 infection according to age. Also shown are the prevalence of serum HPV-16 IgG and IgA, oncogenic cervical HPV infection, nononcogenic HPV infection, and HPV-16 infection stratified according to the age of control women. The HPV-16 IgG 95% CIs for the various prevalence rates by age are the following: <30 years, 47.6 to 71.3; 30 to 39 years, 40.2 to 53.9; 40 to 49 years, 40.4 to 51.1; >50 years, 29.7 to 41.4. The HPV-16 IgA 95% CIs for the various prevalence rates by age are the following: <30 years, 7.5 to 25.5; 30 to 39 years, 16.2 to 27.5; 40 to 49 years, 28 to 38; >50 years, 27.3 to 38.9. For nononcogenic HPV types, the 95% CIs for the various prevalence rates by age are the following: <30 years, 4.8 to 19.5; 30 to 39 years, 5.5 to 13; 40 to 49 years, 4.8 to 10.1; >50 years, 5.7 to 12.5. For oncogenic HPV types, the 95% CIs for the various prevalence rates by age are the following: <30 years, 28.6 to 50.9; 30 to 39 years, 22.1 to 33.6; 40 to 49 years, 13.6 to 21.3; >50 years, 13.1 to 22. For HPV-16 DNA, the 95% CIs for the various prevalence rates by age are the following: <30 years, 1.6 to 13.2; 30 to 39 years, 2.1 to 7.6; 40 to 49 years, 1.7 to 5.5; >50 years, 1.1 to 5.1.
Cervical HPV infection and HPV-16 serum antibodies among control women according to sexual and reproductive characteristics and sexually transmitted infection (STI) status.
In the age-adjusted assessment, cervical HPV infection and HPV-16 IgG prevalence increased with increasing numbers of sexual partners, as did the HPV-16 IgA prevalence, although to a lesser degree (Table 3). There was no difference in cervical HPV infection in women according to the age of their first sexual intercourse, injectable or oral contraceptive use, or parity (all 95% CIs intersect at a value of 1) (Table 3). However, there was evidence of an increase in both HPV-16 serum antibodies in women having their first intercourse at a younger age but a reduction in HPV-16 IgA in those who had used oral or injectable contraceptives (Table 3).
TABLE 3.
Prevalence of cervical HPV infection and HPV-16 IgG and IgA antibodies in control women according to their sexual and reproductive characteristics and STI status
| Variable | Prevalence ofb:
|
|||||
|---|---|---|---|---|---|---|
| HPV DNA
|
HPV-16 IgG
|
HPV-16 IgA
|
||||
| % Positive (n = 1,002a) | OR (95% CI) | % Positive (n = 908) | OR (95% CI) | % Positive (n = 904) | OR (95% CI) | |
| Sexual partners (n) | ||||||
| 1 | 19.55 | 1.0 | 38.78 | 1.0 | 23.39 | 1.0 |
| 2 | 26.6 | 1.5 (1.0-2.2) | 42.44 | 1.2 (0.8-1.6) | 30.94 | 1.5 (1.0-2.2) |
| 3 | 29.0 | 1.8 (1.1-2.7) | 45 | 1.3 (0.8-1.9) | 31.84 | 1.5 (1.00-2.3) |
| >4 | 31.6 | 1.8 (1.2-2.9) | 54.5 | 1.9 (1.2-2.8) | 29.3 | 1.4 (0.9-2.2) |
| Age of first sex (yr) | ||||||
| <16 | 26.5 | 1.1 (0.7-1.6) | 48.45 | 1.4 (1.0-2.09) | 32.87 | 1.7 (1.1-2.7) |
| 16-19 | 26.1 | 1.1 (0.7-1.7) | 42.39 | 1.1 (0.7-1.5) | 29.28 | 1.4 (1.0-2.2) |
| >19 | 24.2 | 1.0 | 40.57 | 1.0 | 22.41 | 1.0 |
| Oral contraceptive use | ||||||
| Never | 26.9 | 1.0 | 45.64 | 1.0 | 33.3 | 1.0 |
| Ever | 24.0 | 0.9 (0.7-1.2) | 41.73 | 0.8 (0.6-1.1) | 21.8 | 0.5 (0.4-0.7) |
| Injectable contraceptive use | ||||||
| Never | 24.7 | 1.0 | 39.15 | 1.0 | 35.1 | 1.0 |
| Ever | 26.5 | 0.9 (0.6-1.3) | 45.55 | 1.0 (0.7-1.5) | 26.7 | 0.7 (0.5-1.0) |
| Parityc | ||||||
| Nulliparous | 23.7 | 1.0 | 40.82 | 1.0 | 27.66 | 1.0 |
| 1-2 | 32.4 | 1.5 (0.8-2.9) | 44.56 | 1.2 (0.8-1.6) | 26.62 | 1.0 (0.5-1.9) |
| 3-4 | 24.8 | 1.3 (0.7-2.4) | 47.08 | 1.4 (0.7-2.6) | 24.56 | 0.7 (0.3-1.4) |
| >4 | 20.2 | 1.1 (0.5-2.2) | 39.46 | 1.2 (0.6-2.4) | 37.84 | 1.2 (0.6-2.4) |
| HIV-1 status | ||||||
| Negative | 24.4 | 1.0 | 43.1 | 1.0 | 28.2 | 1.0 |
| Positive | 50.0 | 2.9 (1.5-6.0) | 70.6 | 2.0 (1.0-4.0) | 29.4 | 1.1 (0.5-2.3) |
| Syphilis status | ||||||
| Negative | 24.9 | 1.0 | 43.1 | 1.0 | 27.3 | 1.0 |
| Positive | 33.3 | 1.4 (0.6-3.1) | 56.7 | 1.6 (0.8-3.4) | 33.3 | 1.4 (0.6-3.1) |
| HSV-2 status | ||||||
| Negative | 18.6 | 1.0 | 38.6 | 1.0 | 23.3 | 1.0 |
| Positive | 28.2 | 1.9 (1.3-2.6) | 46.4 | 1.4 (1.0-1.9) | 31.4 | 1.5 (1.1-2.0) |
There were no demographic data available for one woman; hence, there are 1,002 and not 1,003 controls, as was the case in Table 1.
The OR are age adjusted.
Parity values represent numbers of viable children control women have borne, excluding abortions and early miscarriages but including stillbirths. Nulliparous, never having borne a fetus.
For associations with STI status, the frequency of the presence of HPV infection, HPV-16 IgG, and HPV-16 IgA increased for women who were positive for HSV-2, but this was not so for women who were positive for syphilis (Table 3). HIV-1-infected women were more likely to have HPV infection (50.0%) and HPV-16 IgG antibodies (70.6%) than HIV-1-seronegative women (24.4 and 43.1%, respectively). Although the association between HPV infection, HPV-16 IgG seropositivity, and HIV-1 infection was weaker in the multivariate analysis (Table 4), the high percentages of HPV infection and of HPV-16 IgG antibodies for the HIV-1-seropositive women are notable and are suggestive of a high level of exposure to HPV for these women.
TABLE 4.
AOR for risk factors for HPV infection and HPV-16 IgA and IgG antibodies among control women
| Variable | HPV DNA infectiona
|
HPV-16 IgGb
|
HPV-16 IgAc
|
|||
|---|---|---|---|---|---|---|
| AOR (95% CI) | P trend | AOR (95% CI) | P trend | AOR (95% CI) | P trend | |
| Age (yr) | ||||||
| <30 | 1.6 (0.8-3.4) | 0.026 | 2.7 (1.4-5.2) | 0.001 | 0.6 (0.2-1.3) | 0.001 |
| 30-34 | 1.8 (0.9-3.5) | 1.2 (0.7-2.2) | 0.4 (0.2-0.9) | |||
| 35-39 | 1.1 (0.6-2.1) | 2.0 (1.2-3.5) | 0.9 (0.5-1.7) | |||
| 40-44 | 1.2 (0.7-2.2) | 1.9 (1.1-3.2) | 1.3 (0.8-2.3) | |||
| 45-49 | 0.7 (0.4-1.3) | 1.1 (0.7-1.9) | 1.3 (0.8-2.4) | |||
| 50-54 | 1.1 (0.6-2.1) | 1.1 (0.6-1.9) | 1.6 (0.9-2.8) | |||
| 55-59 | 1.0 | 1.0 | 1.0 | |||
| Race | ||||||
| Mixed | Not included | 1.0 | 1.0 | |||
| Black | 0.9 (0.6-1.3) | 0.6 (0.4-1.0) | ||||
| Education (yr) | ||||||
| <8 | 1.0 | 0.057 | Not included | 1.0 | 0.371 | |
| 8-10 | 1.3 (0.9-1.8) | 1.0 (0.7-1.4) | ||||
| >10 | 1.9 (1.0-3.4) | 0.6 (0.2-1.3) | ||||
| Alcohol use | ||||||
| Never | 1.0 | Not included | 1.0 | |||
| Past | 1.7 (1.1-2.8) | 1.5 (0.96-2.5) | ||||
| Present | 1.3 (0.9-1.9) | 2.1 (1.4-3.0) | ||||
| Cigarette smoking | ||||||
| Never | 1.0 | 1.0 | 1.0 | |||
| Past | 0.5 (0.2-1.0) | 0.7 (0.5-1.2) | 0.9 (0.5-1.7) | |||
| Present | 1.2 (0.8-1.8) | 0.6 (0.4-0.8) | 1.0 (0.7-1.5) | |||
| Sexual partners (n) | ||||||
| 1 | 1.0 | 0.069 | 1.0 | 0.078 | 1.0 | 0.680 |
| 2 | 1.4 (0.93-2.4) | 1.2 (0.8-1.7) | 1.2 (0.8-1.9) | |||
| 3 | 1.6 (0.97-2.6) | 1.2 (0.8-1.9) | 1.2 (0.8-2.0) | |||
| >4 | 1.6 (0.96-2.6) | 1.5 (0.97-2.5) | 1.1 (0.7-1.9) | |||
| Age of first sex (yr) | ||||||
| <16 | Not included | 1.4 (0.9-2.2) | 0.126 | 1.3 (0.8-2.1) | 0.399 | |
| 16-19 | 1.0 (0.7-1.5) | 1.3 (0.8-2.1) | ||||
| >19 | 1.0 | 1.0 | ||||
| Oral contraceptive use | ||||||
| Never | Not included | Not included | 1.0 | |||
| Ever | 0.6 (0.4-0.8) | |||||
| HIV-1 status | ||||||
| Negative | 1.0 | 1.0 | Not included | |||
| Positive | 1.9 (0.93-3.9) | 1.7 (0.78-3.81) | ||||
| HSV-2 status | ||||||
| Negative | 1.0 | 1.0 | 1.0 | |||
| Positive | 1.7 (1.12-2.5) | 1.3 (0.95-1.80) | 1.3 (0.94-1.9) | |||
The HPV DNA AOR was adjusted for age, education, cigarette and alcohol use, number of sexual partners, HIV status, and HSV-2 status.
The HPV-16 IgG AOR was adjusted for age, ethnicity, smoking, number of sexual partners, age at first sex, HIV status, and HSV-2 status.
The HPV-16 IgA AOR was adjusted for age, ethnicity, oral contraceptive use, cigarette and alcohol use, number of sexual partners, age at first sex, and HSV-2 status. Entries for which the P values were <0.05 are in boldface.
Multivariate analysis.
The strength of the association of cervical HPV with each age category was reduced after a multivariate assessment was performed; however, the linear trend of association with decreasing age remained significant. The association of cervical HPV with HSV-2 infection, past alcohol use, and higher education levels was significant. A reduced prevalence of cervical HPV was associated with past smoking (OR, 0.5). In the multivariate model, the risk factors remaining associated with HPV-16 IgG seroprevalence were ages of <30 years (OR, 2.7), 35 to 39 years (OR, 2.0), and 40 to 44 years (OR, 1.9), with a significant linear trend with decreasing age. The HPV-16 IgG seroprevalence retained a borderline association for women who had had more than four sexual partners (OR, 1.5) and who were positive for HSV-2 infection (OR, 1.3) (Table 4). A reduced HPV-16 IgG seroprevalence was associated with current cigarette smoking (OR, 0.6). For the HPV-16 IgA seroprevalence, the linear trend associated with increasing age remained significant, and this trend is in the opposite direction of that of the associations between age and HPV infection or HPV-16 IgG antibodies. The risk factor that remained significant in the multivariate analysis for HPV-16 IgA was current alcohol use (OR, 2.1). There was a strong reduction in HPV-16 IgA levels among users of oral contraceptives (OR, 0.6).
The prevalence of HPV-16 serum antibodies in control women according to the type of cervical HPV infection.
There was no significant difference in the seroprevalence of HPV-16 IgG or IgA in the control women stratified according to the presence of cervical HPV infection or the type of cervical HPV infection. HPV-16 IgG and IgA antibody seroprevalences were high even in the women with no cervical HPV (43.9 and 28.0%, respectively) and could be related to a past infection or an infection at another site. The HPV-16 IgG and IgA antibody seroprevalences were somewhat lower in those with cervical HPV-16 infection (37.9 and 24.1%, respectively). Most (17/29) of the HPV-16 DNA-positive women whose sera were tested for antibodies had normal cervical pathology and negative viral load estimates, as measured in Digene Hybrid Capture light units (1a), indicating a newly acquired or transient infection and, thus, possibly an infection that had not had the required time or level of HPV infection to induce detectable levels of antibody (5). Also, women with HPV-16 cervical infection formed a small group (29), making statistical comparisons unreliable.
DISCUSSION
This epidemiological study was the first of its magnitude in Africa and is the most substantial cervical HPV infection estimate and HPV antibody investigation in a large representative population on the continent. These are findings for South African women, the majority of whom had normal cervical cytology. We were able to strengthen observations from previous smaller studies and identified some novel findings, most notably the increasing HPV-16 IgA prevalence in control women with increasing age. The prevalence of cervical HPV infection among the control women (26.1%) was high but was in accordance with data from other countries with similarly high cervical cancer incidence rates, such as Costa Rica (14) and Nigeria (28). The increasing prevalence of HPV infection in women with an increasing severity of cervical neoplasia shown in this study has been well documented. An increased prevalence of HPV infection was seen in HIV-1-seropositive women. It has been shown that cervical abnormalities are high in these HIV-1-seropositive women (53%), significantly higher than the 13.6% found in HIV-1-seronegative women (OR, 7.2; 95% CI, 3.4 to 15.3), indicating a need for frequent cervical surveillance of HIV-1-infected women (18).
The knowledge that HPV-16 antibody prevalence as an indication of HPV-16 exposure is 44% in this population of women will be important data for the introduction of HPV-16/HPV-18 vaccines to the region. An age-specific HPV prevalence, highest in women <30 years of age and decreasing with increasing age, has been described previously (7), and a high or increasing prevalence in older women also is not uncommon in Africa (14) but is higher than elsewhere in the world (11). The age-specific prevalence found elsewhere in Africa (in Nigeria) in the International Agency for Research on Cancer HPV prevalence survey (11) was similar to that of the present study, with a high HPV prevalence across all age groups. Indications are that the increase of HPV prevalence with age could be lost when adjusted for confounding factors (4), but this increase was retained in the present study for women older than 50 years in the multivariate model, albeit weakly. HPV oncogenic types showed the reverse J-shaped age-related prevalence curve, as described by Ferreccio et al. and Castle et al. for oncogenic HPV types (7, 10). The distribution of nononcogenic types in the present study did not change significantly with age. The reason for the increased prevalence of cervical HPV in older women (>50 years), when the infection rate previously had been decreasing with increasing age, has been hypothesized to be due to the increased persistence of HPV infection and possible age-related immune senescence. In light of the findings in the present study, women older than 50 years were significantly more likely to display serum HPV-16 IgA responses than younger women, indicating that even if the cellular immune response has senesced, a humoral immune response had not. However, the increased persistence of HPV infection in older women could be due to cellular immune senescence. A decline in immune function in the elderly has been described with an accompanying increase in levels of total serum IgA in women (16). This possibly could account for the increased prevalence of anti-HPV-16 serum IgA in the women of the present study.
An HPV IgA serum response is indicative of a more recent or prevalent infection, with IgA antibodies not lasting as long as IgG antibodies (22) after HPV DNA clearance. Persistent HPV infection would be expected to induce and maintain HPV-specific serum IgA responses (32), and the duration of HPV-16 DNA in genital mucosa has been shown to be a necessary factor for the production of serum IgA. We postulate that the increase of HPV-16 IgA with increasing age found in this study could reflect cervical HPV persistence, but this could not be substantiated by a concordant increase in HPV-16 DNA. A striking increase of cervical HPV persistence with age has been described. The curve obtained for HPV-16 IgA responses with age (Fig. 1) obtained in the present study closely resembles the persistence curve described for HPV-16 with age by Castle et al., supporting this theory (7). Whether the majority of older women with persistent HPV infection do have HPV serum IgA will have to be elucidated. The increased prevalence of HPV-16 serum IgA with increasing age has been described previously (32), as has the association of serum IgA with HPV persistence (23). However, another study showed serum IgA to be associated with HPV clearance (2). In the present study, the HPV-16 serum IgA response showed an increasing prevalence with increasing disease severity (LSIL to HSIL), which supports the premise that the IgA response is an indicator of HPV persistence. The present study showed very different HPV-16 IgA and IgG age-related prevalence rates. This could indicate that the IgA and IgG responses act independently of one another. Despite this, there was a significant increase of both HPV-16 IgA and IgG antibodies in women with cervical cancer, a possible further indication of persistence.
The seroprevalence of HPV-16 IgG was high (44.1%) but was consistent with the broad range found in previous reports (13, 21, 25, 34), and it was similar to the HPV prevalence rates found in Costa Rican studies. HPV-16 IgG seropositivity was found to be associated with a lifetime number of sexual partners, as has been described in multiple studies (6, 35), and is as expected for an STI.
The very moderate agreement between HPV-16 IgG and IgA seropositivity and cervical DNA found in this study has been reported previously (31, 32) and could be due to the small number of women with HPV-16 infection, the cross-sectional character of the study, and the transient nature of some infections. Also, the long lag time from HPV infection to antibody seroconversion (5), with only 64.7% of women acquiring serum IgG after 18 months and 47.7% of women acquiring serum IgA in the same time, could explain this disparity. In the present study, most of the 29 HPV-16-infected women showed no cervical disease, indicating the early or transient nature of the infection with antibodies possibly not at detectable levels.
In conclusion, there is evidence of significant exposure to HPV-16 in the study population. The prevalence of other cervical HPV types is discussed in an accompanying report (1). The significant finding that the serum HPV-16 IgA response increases with increasing age warrants further investigation. The premise that this IgA response reflects the persistence of HPV infection is appealing but requires confirmation in longitudinal studies.
Acknowledgments
We are grateful to Janet Kornegay from Roche Diagnostics for providing the reverse line blot kits for HPV typing, and MedImmune (Gaithersburg, MD) for the HPV-16 VLPs.
We gratefully acknowledge Bristol-Myers Squibb Company “Secure the Future” for funding this project. The original study was funded by the U.S. National Institutes of Health.
Footnotes
Published ahead of print on 12 December 2007.
REFERENCES
- 1.Allan, B., D. J. Marais, M. Hoffman, S. Shapiro, and A.-L. Williamson. 2008. Cervical human papillomavirus (HPV) infection in South African women: implications for HPV screening and vaccine strategies. J. Clin. Microbiol. 46•••-•••. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Allan, B. R., D. J. Marais, L. Denny, M. Hoffman, S. Shapiro, and A. L. Williamson. 2006. The agreement between cervical abnormalities identified by cytology and detection of high-risk types of human papillomavirus. S. Afr. Med. J. 961186-1190. [PubMed] [Google Scholar]
- 2.Bontkes, H. J., T. D. de Gruijl, J. M. Walboomers, J. T. Schiller, J. Dillner, T. J. Helmerhorst, R. H. Verheijen, R. J. Scheper, and C. J. Meijer. 1999. Immune responses against human papillomavirus (HPV) type 16 virus-like particles in a cohort study of women with cervical intraepithelial neoplasia. II. Systemic but not local IgA responses correlate with clearance of HPV-16. J. Gen. Virol. 80409-417. [DOI] [PubMed] [Google Scholar]
- 3.Bosch, F. X., M. M. Manos, N. Munoz, M. Sherman, A. M. Jansen, J. Peto, M. H. Schiffman, V. Moreno, R. Kurman, K. V. Shah, et al. 1995. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J. Natl. Cancer Inst. 87796-802. [DOI] [PubMed] [Google Scholar]
- 4.Burchell, A. N., R. L. Winer, S. S. de Sanjose, and E. L. Franco. 2006. Epidemiology and transmission dynamics of genital HPV infection. Vaccine 24(Suppl. 3)S52-S61. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus types 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 1811911-1919. [DOI] [PubMed] [Google Scholar]
- 6.Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174927-936. [DOI] [PubMed] [Google Scholar]
- 7.Castle, P. E., M. Schiffman, R. Herrero, A. Hildesheim, A. C. Rodriguez, M. C. Bratti, M. E. Sherman, S. Wacholder, R. Tarone, and R. D. Burk. 2005. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J. Infect. Dis. 1911808-1816. [DOI] [PubMed] [Google Scholar]
- 8.Clifford, G. M., J. S. Smith, M. Plummer, N. Munoz, and S. Franceschi. 2003. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br. J. Cancer 8863-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Villiers, E. M., C. Fauquet, T. R. Broker, H. U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 32417-27. [DOI] [PubMed] [Google Scholar]
- 10.Ferreccio, C., R. B. Prado, A. V. Luzoro, S. L. Ampuero, P. J. Snijders, C. J. Meijer, S. V. Vaccarella, A. T. Jara, K. I. Puschel, S. C. Robles, R. Herrero, S. F. Franceschi, and J. M. Ojeda. 2004. Population-based prevalence and age distribution of human papillomavirus among women in Santiago, Chile. Cancer Epidemiol. Biomarkers Prev. 132271-2276. [PubMed] [Google Scholar]
- 11.Franceschi, S., R. Herrero, G. M. Clifford, P. J. Snijders, A. Arslan, P. T. Anh, F. X. Bosch, C. Ferreccio, N. T. Hieu, E. Lazcano-Ponce, E. Matos, M. Molano, Y. L. Qiao, R. Rajkumar, G. Ronco, S. de Sanjose, H. R. Shin, S. Sukvirach, J. O. Thomas, C. J. Meijer, and N. Munoz. 2006. Variations in the age-specific curves of human papillomavirus prevalence in women worldwide. Int. J. Cancer 1192677-2684. [DOI] [PubMed] [Google Scholar]
- 12.Gillison, M. L., W. M. Koch, R. B. Capone, M. Spafford, W. H. Westra, L. Wu, M. L. Zahurak, R. W. Daniel, M. Viglione, D. E. Symer, K. V. Shah, and D. Sidransky. 2000. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl. Cancer Inst. 92709-720. [DOI] [PubMed] [Google Scholar]
- 13.Hagensee, M. E., J. Slavinsky III, C. M. Gaffga, J. Suros, P. Kissinger, and D. H. Martin. 1999. Seroprevalence of human papillomavirus type 16 in pregnant women. Obstet. Gynecol. 94653-658. [DOI] [PubMed] [Google Scholar]
- 14.Herrero, R., P. E. Castle, M. Schiffman, M. C. Bratti, A. Hildesheim, J. Morales, M. Alfaro, M. E. Sherman, S. Wacholder, S. Chen, A. C. Rodriguez, and R. D. Burk. 2005. Epidemiologic profile of type-specific human papillomavirus infection and cervical neoplasia in Guanacaste, Costa Rica. J. Infect. Dis. 1911796-1807. [DOI] [PubMed] [Google Scholar]
- 15.Kay, P., R. Soeters, J. Nevin, L. Denny, C. M. Dehaeck, and A. L. Williamson. 2003. High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. J. Med. Virol. 71265-273. [DOI] [PubMed] [Google Scholar]
- 16.Listì, F., G. Candore, M. A. Modica, M. Russo, L. G. Di, M. Esposito-Pellitteri, G. Colonna-Romano, A. Aquino, M. Bulati, D. Lio, C. Franceschi, and C. Caruso. 2006. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann. N.Y. Acad. Sci. 1089487-495. [DOI] [PubMed] [Google Scholar]
- 17.Marais, D., R. C. Rose, and A. L. Williamson. 1997. Age distribution of antibodies to human papillomavirus in children, women with cervical intraepithelial neoplasia and blood donors from South Africa. J. Med. Virol. 51126-131. [DOI] [PubMed] [Google Scholar]
- 18.Moodley, J. R., M. Hoffman, H. Carrara, B. R. Allan, D. D. Cooper, L. Rosenberg, L. E. Denny, S. Shapiro, and A. L. Williamson. 2006. HIV and pre-neoplastic and neoplastic lesions of the cervix in South Africa: a case-control study. BMC Cancer 6135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moscicki, A. B., M. Schiffman, S. Kjaer, and L. L. Villa. 2006. Updating the natural history of HPV and anogenital cancer. Vaccine 24(Suppl. 3)S42-S51. [DOI] [PubMed] [Google Scholar]
- 20.Newton, R., L. Bousarghin, J. Ziegler, D. Casabonne, V. Beral, E. Mbidde, L. Carpenter, D. M. Parkin, H. Wabinga, S. Mbulaiteye, H. Jaffe, A. Touze, and P. Coursaget. 2004. Human papillomaviruses and cancer in Uganda. Eur. J. Cancer Prev. 13113-118. [DOI] [PubMed] [Google Scholar]
- 21.Nonnenmacher, B., K. S. Kruger, E. I. Svare, J. D. Scott, N. L. Hubbert, A. J. van den Brule, R. Kirnbauer, J. M. Walboomers, D. R. Lowy, and J. T. Schiller. 1996. Seroreactivity to HPV16 virus-like particles as a marker for cervical cancer risk in high-risk populations. Int. J. Cancer 68704-709. [DOI] [PubMed] [Google Scholar]
- 22.Onda, T., J. J. Carter, L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2003. Characterization of IgA response among women with incident HPV 16 infection. Virology 312213-221. [DOI] [PubMed] [Google Scholar]
- 23.Sasagawa, T., H. Yamazaki, Y. Z. Dong, S. Satake, M. Tateno, and M. Inoue. 1998. Immunoglobulin-A and -G responses against virus-like particles (VLP) of human papillomavirus type 16 in women with cervical cancer and cervical intra-epithelial lesions. Int. J. Cancer 75529-535. [DOI] [PubMed] [Google Scholar]
- 24.Shapiro, S., L. Rosenberg, M. Hoffman, J. P. Kelly, D. D. Cooper, H. Carrara, L. E. Denny, B. R. Allan, I. A. Stander, and A. L. Williamson. 2003. Risk of invasive cancer of the cervix in relation to the use of injectable progestogen contraceptives and combined estrogen/progestogen oral contraceptives (South Africa). Cancer Causes Control 14485-495. [DOI] [PubMed] [Google Scholar]
- 25.Shields, T. S., L. A. Brinton, R. D. Burk, S. S. Wang, S. J. Weinstein, R. G. Ziegler, Y. Y. Studentsov, M. McAdams, and M. Schiffman. 2004. A case-control study of risk factors for invasive cervical cancer among U.S. women exposed to oncogenic types of human papillomavirus. Cancer Epidemiol. Biomarkers Prev. 131574-1582. [PubMed] [Google Scholar]
- 26.Sitas, F., D. Blaauw, M. Terblanche, J. Madhoo, and H. Carrara. 1998. Incidence of histological diagnosed cancer in South Africa, 1992. National Cancer Registry of South Africa, South African Institute of Medical Research, Johannesburg, South Africa.
- 27.Studentsov, Y. Y., G. Y. Ho, M. A. Marks, R. Bierman, and R. D. Burk. 2003. Polymer-based enzyme-linked immunosorbent assay using human papillomavirus type 16 (HPV16) virus-like particles detects HPV16 clade-specific serologic responses. J. Clin. Microbiol. 412827-2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thomas, J. O., R. Herrero, A. A. Omigbodun, K. Ojemakinde, I. O. Ajayi, A. Fawole, O. Oladepo, J. S. Smith, A. Arslan, N. Munoz, P. J. Snijders, C. J. Meijer, and S. Franceschi. 2004. Prevalence of papillomavirus infection in women in Ibadan, Nigeria: a population-based study. Br. J. Cancer 90638-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Trottier, H., and E. L. Franco. 2006. The epidemiology of genital human papillomavirus infection. Vaccine 24(Suppl. 1)S1-S15. [DOI] [PubMed] [Google Scholar]
- 30.van Houten, V. M., P. J. Snijders, M. W. van den Brekel, J. A. Kummer, C. J. Meijer, F. Denkers, L. E. Smeele, G. B. Snow, and R. H. Brakenhoff. 2001. Biological evidence that human papillomaviruses are etiologically involved in a subgroup of head and neck squamous cell carcinomas. Int. J. Cancer 93232-235. [DOI] [PubMed] [Google Scholar]
- 31.Viscidi, R. P., L. Hdieh-Grant, B. Clayman, K. Fox, L. S. Massad, S. Cu-Uvin, K. V. Shah, K. M. Anastos, K. E. Squires, A. Duerr, D. J. Jamieson, R. D. Burk, R. S. Klein, H. Minkoff, J. Palefsky, H. Strickler, P. Schuman, E. Piessens, and P. Miotti. 2003. Serum immunoglobulin G response to human papillomavirus type 16 virus-like particles in human immunodeficiency virus (HIV)-positive and risk-matched HIV-negative women. J. Infect. Dis. 187194-205. [DOI] [PubMed] [Google Scholar]
- 32.Viscidi, R. P., L. Hdieh-Grant, M. F. Schneider, B. Clayman, L. S. Massad, K. M. Anastos, R. D. Burk, H. Minkoff, J. Palefsky, A. Levine, and H. Strickler. 2003. Serum immunoglobulin A response to human papillomavirus type 16 virus-like particles in human immunodeficiency virus (HIV)-positive and high-risk HIV-negative women. J. Infect. Dis. 1881834-1844. [DOI] [PubMed] [Google Scholar]
- 33.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Munoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 18912-19. [DOI] [PubMed] [Google Scholar]
- 34.Wang, S. S., M. Schiffman, T. S. Shields, R. Herrero, A. Hildesheim, M. C. Bratti, M. E. Sherman, A. C. Rodriguez, P. E. Castle, J. Morales, M. Alfaro, T. Wright, S. Chen, B. Clayman, R. D. Burk, and R. P. Viscidi. 2003. Seroprevalence of human papillomavirus-16, -18, -31, and -45 in a population-based cohort of 10000 women in Costa Rica. Br. J. Cancer 891248-1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wideroff, L., M. H. Schiffman, R. Hoover, R. E. Tarone, B. Nonnenmacher, N. Hubbert, R. Kirnbauer, C. E. Greer, A. T. Lorincz, M. M. Manos, A. G. Glass, D. R. Scott, M. E. Sherman, J. Buckland, D. Lowy, and J. Schiller. 1996. Epidemiologic determinants of seroreactivity to human papillomavirus (HPV) type 16 virus-like particles in cervical HPV-16 DNA-positive and -negative women. J. Infect. Dis. 174937-943. [DOI] [PubMed] [Google Scholar]