Abstract
Molecular evidence is limited for the hypothesis that humans, dogs, and cats can become colonized and infected with similar virulent Escherichia coli strains. To further assess this possibility, archived E. coli O6 isolates (n = 130) from humans (n = 55), dogs (n = 59), and cats (n = 16), representing the three main H (flagellar) types within serogroup O6 (H1, H7, and H31), were analyzed, along with selected reference strains. Isolates underwent PCR-based phylotyping, multilocus sequence typing, PCR-based detection of 55 virulence-associated genes, and XbaI pulsed-field gel electrophoresis (PFGE) profiling. Three major sequence types (STs), which corresponded closely with H types, accounted for 99% of the 130 O6 isolates. Each ST included human, dog, and cat isolates; two included reference pyelonephritis isolates CFT073 (O6:K2:H1) and 536 (O6:K15:H31). Virulence genotypes overlapped considerably among host species, despite statistically significant differences between human and pet isolates. Several human and dog isolates from ST127 (O6:H31) exhibited identical virulence genotypes and highly similar PFGE profiles, consistent with cross-species exchange of specific E. coli clones. In conclusion, the close similarity in the genomic backbone and virulence genotype between certain human- and animal-source E. coli isolates within serogroup O6 supports the hypothesis of zoonotic potential.
Escherichia coli is a major cause of urinary tract infections (UTI) and other extraintestinal infections in humans, dogs, and cats (1, 19, 25). Several cross-sectional surveys have demonstrated similarities among clinical or fecal E. coli isolates from humans, dogs, and cats with respect to genomic background and virulence-associated accessory traits (virulence factors [VFs]), particularly within serogroups O6 and O4, suggesting possible zoonotic (whether animal-to-human or human-to-animal) transmission (3, 5, 6, 9, 11, 13, 15, 16, 20, 26, 30-33). Consistent with this possibility, in two longitudinal surveillance studies involving the E. coli flora of human household members and their canine or feline pets, pets were found to be intermittently colonized with the same virulent-appearing E. coli clones that colonized multiple humans and caused acute cystitis in the women (8, 22).
However, these studies examined only modest numbers of isolates and accessory traits and/or relied on somewhat imprecise phylogenetic methods such as multilocus enzyme electrophoresis, outer membrane protein profiling, or random amplified polymorphic DNA analysis (3, 5, 6, 8, 9, 11, 13, 15, 16, 20, 22, 26, 30-33). Accordingly, they leave uncertainty as to the extent of commonality among human- and pet-derived E. coli isolates.
To examine more rigorously the question of human-pet commonality with respect to extraintestinal pathogenic E. coli (ExPEC) clones, we used contemporary molecular methods to characterize a large collection of archival E. coli isolates of serotypes O6:H1, O6:H7, and O6:H31 from humans, dogs, and cats, obtained from the E. coli Reference Center at Pennsylvania State University. Specifically, we assessed broad phylogenetic relationships by using PCR-based phylotyping and multilocus sequence typing (MLST), determined accessory trait profiles through PCR-based detection of 55 virulence-associated genes and variants, and defined clonal identity by pulsed-field gel electrophoresis (PFGE). We then assessed the extent to which these traits segregated by host species or were shared among host species, especially between humans and pets.
MATERIALS AND METHODS
Strains.
One-hundred thirty E. coli O6 isolates from humans (n = 55), dogs (n = 59), and cats (n = 16) that exhibited the three most prevalent H types within serogroup O6 (i.e., H1, H7, and H31) were selected from the strain collection of the E. coli Reference Center, Pennsylvania State University, University Park. As many as 30 isolates, as available, for each H type-host species combination were selected randomly for analysis. To avoid potential duplicates, only 1 isolate per H type-host species combination, as received on a given day from a particular sender, was used. Isolates had been submitted between 1981 and 2005. Most were from either urine or feces from individuals with UTI. Many of the human-source isolates were from females. The isolates were all from the United States and represented 16 different states, namely, California, the District of Columbia, Florida, Illinois, Louisiana, Massachusetts, Maryland, Michigan, Minnesota, North Carolina, New York, Ohio, South Dakota, Texas, Virginia, and Washington.
Serotyping.
O typing was performed as described by Orskov and Orskov (23). H typing was performed using a fliC PCR-restriction fragment length polymorphism method (21).
Phylogenetic analysis and MLST.
The major E. coli phylogenetic group (A, B1, B2, or D) was determined by a multiplex PCR assay (4). Closer phylogenetic relationships were assessed according to maximum parsimony, using PAUP* (28), based on the partial DNA sequence (∼500 bp per locus) for fumC (all isolates) or a concatenated sequence including adk, fumC, gyrB, icd, mdh, purA, and recA (several representatives of each fumC allele, selected for diversity of H types and host species) (12). Additionally, designations for alleles at each of these loci, and sequence types (STs) reflecting specific allele combinations, were assigned according to the Achtman MLST system (http://web.mpiib-berlin.mpg.de/mlst/dbs/Ecoli). Strains MG1655 (K-12; group A), 536 (O6:K15:H31; group B2), and CFT073 (O6:K2:H1; group B2) and Escherichia fergusonii were included for reference (12).
Virulence genotypes.
Fifty-five ExPEC-associated VF genes and variants were detected by using established multiplex PCR-based assays (10, 14, 17) (see Table S1 in the supplemental material). A dendrogram based on extended VF profiles was inferred by using the unweighted-pair group method with arithmetic averaging (UPGMA) (27).
PFGE.
Animal and human isolates that belonged to the same ST and exhibited identical VF profiles underwent PFGE analysis using XbaI according to a standardized protocol (24a). Dendrograms based on Dice similarity coefficients were constructed from the resulting profiles according to UPGMA. Isolates with ≥94% similar profiles (corresponding approximately with a ≥3-band difference) were considered to represent the same pulsotype, or clone (29).
Statistical methods.
Two multivariate ordination techniques, correspondence analysis (CA) and principal-coordinates analysis (PCA), were used to visualize the arrangement, or “ordering,” of data units (either variables or cases) along gradients.
Correlations among variables (host species, ST, H type, and individual VFs) were assessed by using CA. A special case of canonical correlation, CA is an eigenanalysis ordination technique for visualizing the associations in contingency tables (7). It produces a display of two-way contingency table cells as points in a low-dimensional space. The positions of the points provide a spatial map reflecting their associations within the table and enabling a global view of the data that is useful for interpretation. The computation determines a plane defined by two principal axes of the analysis. The first axis, F1, accounts for most of the variance, and the second axis, F2, orthogonal to F1, accounts for the largest part of the variance not accounted for by F1. Here, CA was conducted from a two-way table that had 130 rows (1 per E. coli isolate) and 59 columns (1 per variable).
Similarity relationships among the individual isolates with respect to VF profiles were assessed by using PCA. Also known as metric multidimensional scaling, PCA is a distance-based ordination technique that allows one to plot similarities on underlying dimensions characterizing a multivariate data set, e.g., multiple loci and multiple samples, in a low-dimensional space where distances between points are directly related to their similarity (18). Using GenAlEx6 (24), PCA was applied to the VF data set as a way to collapse the multiple VFs for simplified among-group comparisons. Values for each isolate from the first two PCA axes, which are the axes that capture most of the variance within the data set, were used in a one-way multivariate analysis of variance to test for pairwise between-group (ST or host species) differences. The values also were plotted to depict spatially the degree of separation or overlap among groups on the axis 1-axis 2 plane.
Comparisons of proportions were tested using Fisher's exact test. In assessing the distribution of categorical traits among multiple groups, an initial overall Fisher exact test screen was used to identify the presence of a significant among-group difference. The criterion for statistical significance was a P value of <0.05.
RESULTS
Phylogenetic relationships.
The 130 serogroup O6 study isolates were from humans (n = 55), dogs (n = 59), and cats (n = 16) and represented H types H1 (n = 41), H7 (n = 24), and H31 (n = 65). According to PCR-based phylotyping, all but 1 (99%) derived from group B2. The sole exception, a human H31 isolate, ostensibly represented group A.
Maximum-parsimony analysis of partial fumC sequence data supported this inference (Fig. 1). The 129 group B2 study isolates segregated into three distinct groups, largely by H type. According to the Achtman MLST system, the three groups corresponded with fumC alleles 24 (n = 39) (92% H1), 43 (n = 22) (82% H7), and 14 (n = 68) (91% H31). Three strains (a cat H1, a dog H1, and a cat H7 strain) exhibited fumC allele 24 except for a 6-bp in-frame deletion, so they were analyzed with allele 24.
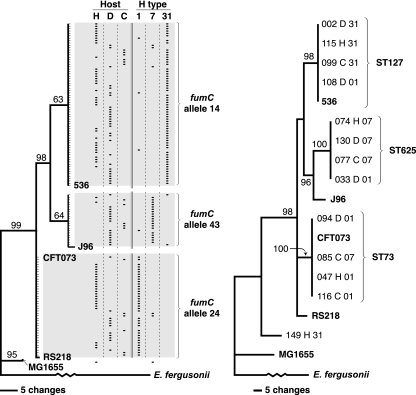
FIG. 1.
Phylogeny of 130 Escherichia coli isolates of serogroup O6 from humans, dogs, and cats according to fumC (left) (total population) or adk, fumC, gyrB, icd, mdh, purA, and recA (right) (representatives of each fumC allele). Trees were inferred using maximum parsimony. Bootstrap values of >60% (from 1,000 iterations) are shown. In the fumC tree (left), fine tick marks indicate individual study isolates with identical sequences. In the composite tree (right), each study isolate's unique identification number and (7-locus) sequence type (ST) are given. In both trees, study isolates are labeled with the host species (H, human; D, dog; C, cat) and H type (1, 7, or 31). According to the Achtman scheme, fumC alleles 14 (91% H31), 24 (92% H1), and 43 (82% H7) correspond to STs 127, 73, and 625, respectively. Reference strains 536, J96, CFT073, RS218, and MG1655 are included for comparison.
Each host species was multiply represented for each of the three main fumC alleles, accounting for 9% to 68% of isolates per allele. Human and animal isolates were approximately equally represented for fumC alleles 24 and 14 (O6:H1 and O6:H31, respectively), whereas animal isolates predominated for fumC allele 43 (O6:H7) (see Table S1 in the supplemental material). The O6 reference strains CFT073 (O6:K2:H1) and 536 (O6:K15:H31) clustered with O6 study isolates of the corresponding H types; the other (non-O6) reference strains were placed separately (Fig. 1).
To corroborate the fumC phylogeny, 4 study isolates each per major fumC allele, selected to reflect the diversity of hosts and H types encountered for that allele, underwent partial sequence analysis for six additional housekeeping genes, as did the putative group A O6 isolate. A maximum-parsimony tree based on concatenated sequences across all seven loci exhibited much the same overall conformation as did the fumC tree, but with greater group separation (Fig. 1). Within each of the three main fumC groups, all isolates tested exhibited identical sequences across the seven loci, evidence that the fumC alleles correspond with 7-locus STs. According to the Achtman MLST scheme, the three main STs observed among the O6 isolates were ST73 (H1), ST625 (H7), and ST127 (H31). Reference strains CFT073 (ST73) and 536 (ST127) clustered within STs corresponding to their respective H types, whereas other reference strains represented distinct STs. Only for one study isolate did 7-locus MLST significantly alter the phylogenetic inference based on analysis of fumC alone. This was the putative group A O6 strain, which according to MLST was a chimera that exhibited ST127-like alleles of icd, gyrB, mdh, purA, and recA, a K-12 (ST98)-like allele of fumC, and a distinct (i.e., non-K12, non-ST127) adk allele.
CA.
To explore associations among bacterial characteristics in relation to the host species and ST, CA was done. The F1/F2 plane accounted for 30% of total variance, with factors F1 and F2 accounting for 16% and 14%, respectively (Fig. 2). The other factors (not shown) accounted for lower percentages of the variance than did F1 and F2 and did not further improve the interpretation of the data.
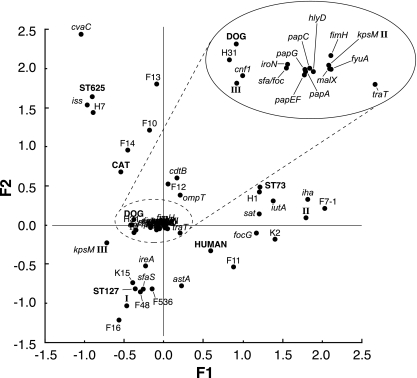
FIG. 2.
CA for bacterial traits and host species among 130 Escherichia coli serogroup O6 isolates. Shown is a projection of the 55 VFs (named as in Table S1 in the supplemental material) characterized for the 130 E. coli isolates plus the three H types, three STs, and three host species (human, dog, and cat), on the F1/F2 plane, as computed from the CA. The loading score of each variable for factors F1 and F2, respectively, can be inferred from its x and y coordinates on the F1/F2 plane. I, II, and III refer to the corresponding papG (P fimbrial adhesin) alleles.
The F1/F2 plane allowed the positioning of the variables according to their coordinates on each of these factors (Fig. 2). Cat, dog, and human were placed fairly near one another, toward the center of the plot, with dog in an intermediate position, but closer to cat than to human. The three major STs (and their associated H types) were placed farther from the center of the plot, with ST73 (H1) closest to human, ST625 (H7) closest to cat, and ST127 (H31) closest to dog. Finally, the various VFs were scattered widely across the plot, both singly and in groups. Certain VFs clustered near a particular ST (e.g., F13 and iss near ST625; sat, foc, K2, iutA, iha, papG allele II, and F7-1 near ST73; and ireA, K15, F48, F536, F48, and F16 near ST127) or host species (e.g., F11 near human, F14 near cat, and papG III, cnf1, iroN, and sfa/foc near dog), whereas others occupied indeterminate positions relative to the STs and host species.
These inferences regarding the associations of VFs with host species and STs were supported by univariate comparisons of proportions, which showed more numerous and statistically stronger associations of individual VFs with STs than with host species (see Tables S1 and S2 in the supplemental material). Notably, no VF was associated categorically with a single host species except for cvaC and iss, associated with cats (P = 0.048 and P = 0.008, respectively), or with humans versus dogs and cats combined except for the F13 papA allele (P < 0.001) (see Table S2 in the supplemental material).
PCA.
PCA was used to analyze jointly the 55 VFs for among-population comparisons involving host species and STs. The first two axes of the PCA collectively accounted for 64% of total variance, with axes 1 and 2 accounting for 43% and 21%, respectively. Based on each isolate's values on these two axes, pairwise comparisons were made between different host species and, separately, between different STs, by using a one-factor multivariate analysis of variance.
In the analysis by host species, human isolates differed significantly from dog and cat isolates (P < 0.001 for each comparison), whereas dog and cat isolates did not differ significantly from each other. However, this stratification of the PCA results yielded an eta-squared value of only 0.14. Likewise, in the plot of the axis 1-axis 2 plane, human, dog, and cat isolates overlapped considerably, despite some apparent segregation (Fig. 3).
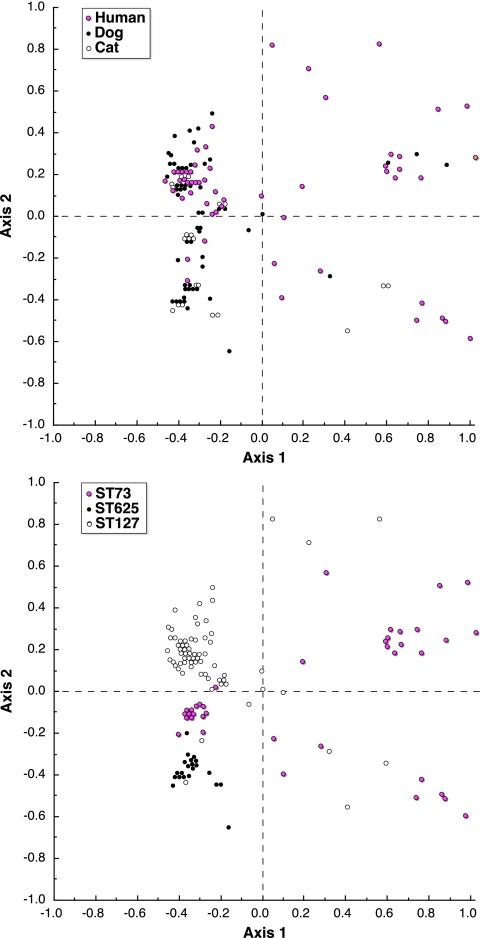
FIG. 3.
Distribution of 130 Escherichia coli serogroup O6 isolates from humans, dogs, and cats on the axis 1-axis 2 PCA plane. Data used were extended virulence genotypes (55 traits). Top and bottom plots contain identical data (one point per isolate), with points colored to indicate either host species (top) or ST (bottom). Axis 1 (positive values to the right and negative values to the left of the dashed vertical line) accounted for 43% of total variance. Axis 2 (positive values above and negative values below the dashed horizontal line) accounted for 22% of total variance. The axes are dimensionless, i.e., they have no units.
In contrast, in the analysis of the same data by ST, all three STs differed significantly from one another (P < 0.001 for each comparison). Moreover, this stratification of the PCA results yielded an eta-squared value of 0.38, nearly three times greater than that for the by-host stratification. Likewise, in the plot of the axis 1-axis 2 plane, isolates from the three STs were well separated, with little overlap among STs (Fig. 3).
These conclusions were supported by analysis of a similarity dendrogram based on extended VF profiles, which exhibited seven prominent clusters, each representing a distinct VF profile group (see Fig. S4 in the supplemental material). The three host species were extensively intermingled within the seven VF profile clusters, whereas the three STs segregated largely by cluster.
Similarity of human and animal isolates by PFGE.
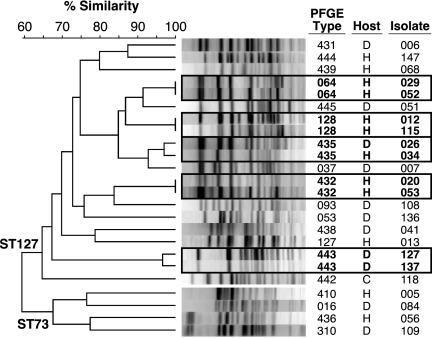
To identify possible cross-species clonal commonality, human and animal isolates from the same ST that also exhibited identical VF profiles underwent comparative PFGE analysis. Multiple PFGE profiles were encountered per ST, evidence of greater discriminating power for PFGE than for MLST (Fig. 4). In the resulting PFGE-based dendrogram, isolates from ST74 (H1) and ST127 (H31) were resolved as separate clusters (Fig. 4). Within each cluster, certain animal isolates were closer to one or more human isolates than to other animal isolates, and vice versa. In one instance, a dog-human isolate pair (isolates 26 and 34) exhibited the same PFGE profile (97% similarity; PFGE type 435) and hence presumably represented the same clone.
FIG. 4.
PFGE profiles of Escherichia coli serogroup O6 isolates from humans (H), dogs (D), and cats (C) with identical STs and virulence profiles. The dendrogram was inferred according to UPGMA based on Dice similarity coefficients as calculated from PFGE band positions. Profiles exhibiting ≥94% similarity (boxed) were regarded as representing the same PFGE type. Isolates D026 and H034 (PFGE type 435; ST127) were submitted in 1985. They were isolated, respectively, from a dog in Florida and a woman in New York, each with a UTI.
DISCUSSION
In this molecular-ecological analysis of E. coli O6:H1, O6:H7, and O6:H31 isolates from humans, dogs, and cats, we found that human and animal isolates occurred together within each of the three major sequence-based clonal groups (i.e., STs), exhibited overlapping virulence profiles, and in certain instances had similar or indistinguishable PFGE profiles. These findings provide strong molecular support for the hypothesis of cross-species transmission of pathogens between humans and their household pets and hence of a zoonotic potential (in either direction) for such strains.
The three main STs (as resolved similarly by fumC sequence analysis and 7-locus MLST) corresponded closely with the three H (flagellar) types studied: H1, H7, and H31. However, each ST included several isolates that exhibited alternate H types, evidence of lateral transfer of fliC alleles among lineages. This finding confirms, using a more robust comparison standard than that used by Cherifi et al., these investigators’ suggestion that within serogroup O6 the H antigen is a useful clonal marker (3). However, it also demonstrates that H typing has limitations in this role.
Although some segregation of host species by ST was apparent, the three main STs each included isolates from all three host species, suggesting that the STs represent broad-host-range clonal groups. Of note, ST73 and ST127 appear to correspond, respectively, with clones 1 and 2 of Cherifi et al. (3) and with multilocus enzyme electrophoretic types 1 and 21 of Whittam and colleagues (9, 31). These represent two prominent clonal groups within E. coli serogroup O6 that are common causes of extraintestinal infections in humans, dogs, and cats. Underscoring the pathogenic importance of ST73 and ST127, these two clonal groups include, respectively, reference strains CFT073 and 536, two archetypal ExPEC strains from humans with pyelonephritis that have been extensively studied to elucidate causal mechanisms in UTI and other extraintestinal E. coli infections (12).
We also found that human and animal isolates overlapped considerably according to aggregate VF profiles and individual VFs, notwithstanding some statistically significant differences. Although it is conceivable that unrecognized host group-specific VFs were omitted from our analysis, the present findings suggest that similar VFs and VF profiles are relevant for infections in multiple host species, further supporting concerns regarding the possible zoonotic potential of such strains.
To more stringently test the hypothesis of cross-species commonality of virulent E. coli clones, we used PFGE analysis to resolve finer clonal relationships among animal and human isolates from the same ST that exhibited identical VF profiles. The close similarity of PFGE profiles observed among several human and animal isolates, including one 97% similar dog-human isolate pair, further supports the possibility of pet-human exchange of virulent E. coli clones. This is consistent with the results of two recent longitudinal household surveillance studies in which multiple humans and their pets were found to be colonized with the same E. coli clones, including two cystitis-causing strains (8, 22). Notably, in one such study the most extensively shared clone, which colonized both human subjects and the family cat and caused an episode of acute cystitis in the woman, belonged to serogroup O6 and, based on the VF profile and random amplified polymorphic DNA analysis, likely derived from ST127 (22).
The present study supersedes previous similar work (3, 5, 6, 8, 9, 11, 13, 15, 16, 20, 22, 26, 30-33) in several respects. It involves more isolates and a more extensive battery of VFs than did previous studies and is the first to use sequence-based phylogenetic analysis. It also is the first to demonstrate such a close PFGE profile match between epidemiologically unrelated human and pet E. coli isolates. Limitations include the undefined clinical background of the isolates, the absence of epidemiological evidence regarding cross-species transmission, and the lack of attention to minor sequence variation within virulence genes that might confer host specificity.
In summary, we found extensive commonality among human, dog, and cat E. coli isolates of serogroup O6 with respect to phylogenetic background, VF profiles, and clonal identity. Although some segregation by host species was apparent, the findings support the hypothesis that humans and their domestic pets can be colonized and infected with similar E. coli strains and may serve as reservoirs of potential pathogens for one another. Thus, in some instances extraintestinal E. coli infections in humans, dogs, and cats may represent animal-to-human or human-to-animal zoonoses.
Supplementary Material
Acknowledgments
This material is based on work supported by the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and the National Institutes of Health.
Dave Prentiss (VA Medical Center) prepared the figures.
Footnotes
Published ahead of print on 14 November 2007.
Supplemental material for this article may be found at http://jcm.asm.org/.
REFERENCES
- 1.Beutin, L. 1999. Escherichia coli as a pathogen in dogs and cats. Vet. Res. 30285-298. [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention. Reference deleted.
- 3.Cherifi, A., M. Contrepois, B. Picard, P. Oullet, I. Orskov, F. Orskov, and J. De Rycke. 1991. Clonal relationships among Escherichia coli serogroup O6 isolates from human and animal infections. FEMS Microbiol. Lett. 64225-230. [DOI] [PubMed] [Google Scholar]
- 4.Clermont, O., S. Bonacorsi, and E. Bingen. 2000. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 664555-4558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Féria, C., J. Machado, J. D. Correia, J. Goncalves, and W. Gaastra. 2001. Virulence genes and P fimbriae PapA subunit diversity in canine and feline uropathogenic Escherichia coli. Vet. Microbiol. 8281-89. [DOI] [PubMed] [Google Scholar]
- 6.Garcia, E., H. E. N. Bergmans, J. F. van den Bosch, I. Orskov, B. A. M. van der Zeijst, and W. Gaastra. 1988. Isolation and characterization of dog uropathogenic Escherichia coli strains and their fimbriae. Antonie van Leeuwenhoek 54149-163. [DOI] [PubMed] [Google Scholar]
- 7.Greenacre, M. 1992. Correspondence analysis in medical research. Stat. Methods Med. Res. 197-117. [DOI] [PubMed] [Google Scholar]
- 8.Johnson, J. R., and C. Clabots. 2006. Sharing of virulent Escherichia coli clones among household members of a woman with acute cystitis. Clin. Infect. Dis. 43e101-e108. [DOI] [PubMed] [Google Scholar]
- 9.Johnson, J. R., P. Delavari, A. L. Stell, T. S. Whittam, U. Carlino, and T. A. Russo. 2001. Molecular comparison of extraintestinal Escherichia coli isolates from the same electrophoretic lineages from humans and domestic animals. J. Infect. Dis. 183154-159. [DOI] [PubMed] [Google Scholar]
- 10.Johnson, J. R., A. Gajewski, A. J. Lesse, and T. A. Russo. 2003. Extraintestinal pathogenic Escherichia coli as a cause of invasive nonurinary infections. J. Clin. Microbiol. 415798-5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson, J. R., T. T. O'Bryan, D. A. Low, G. Ling, P. Delavari, C. Fasching, T. A. Russo, U. Carlino, and A. L. Stell. 2000. Evidence of commonality between canine and human extraintestinal pathogenic Escherichia coli strains that express papG allele III. Infect. Immun. 683327-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson, J. R., K. L. Owens, C. R. Clabots, S. J. Weissman, and S. B. Cannon. 2006. Phylogenetic relationships among clonal groups of extraintestinal pathogenic Escherichia coli as assessed by multi-locus sequence analysis. Microbes Infect. 81702-1713. [DOI] [PubMed] [Google Scholar]
- 13.Johnson, J. R., A. Stell, and P. Delavari. 2001. Canine feces as a reservoir of extraintestinal pathogenic Escherichia coli. Infect. Immun. 691306-1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181261-272. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, J. R., A. L. Stell, P. Delavari, A. C. Murray, M. Kuskowski, and W. Gaastra. 2001. Phylogenetic and pathotypic similarities between Escherichia coli isolates from urinary tract infections in dogs and extraintestinal infections in humans. J. Infect. Dis. 183897-906. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, J. R., A. L. Stell, N. Kaster, C. Fasching, and T. T. O'Bryan. 2001. Novel molecular variants of allele I of the Escherichia coli P fimbrial adhesin gene papG. Infect. Immun. 692318-2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. R., A. L. Stell, F. Scheutz, T. T. O'Bryan, T. A. Russo, U. B. Carlino, C. C. Fasching, J. Kavle, L. van Dijk, and W. Gaastra. 2000. Analysis of F antigen-specific papA alleles of extraintestinal pathogenic Escherichia coli using a novel multiplex PCR-based assay. Infect. Immun. 681587-1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kruskal, J. B., and M. Wish. 1978. Multidimensional scaling. Sage Publications, Beverly Hills, CA.
- 19.Ling, G. V. 1995. Urinary tract infections. Mosby, St. Louis, MO.
- 20.Low, D. A., B. A. Braaten, G. V. Ling, D. L. Johnson, and A. L. Ruby. 1988. Isolation and comparison of Escherichia coli strains from canine and human patients with urinary tract infections. Infect. Immun. 562601-2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machado, J., F. Grimont, and P. A. Grimont. 2000. Identification of Escherichia coli flagellar types by restriction of the amplified fliC gene. Res. Microbiol. 151535-546. [DOI] [PubMed] [Google Scholar]
- 22.Murray, A. C., M. A. Kuskowski, and J. R. Johnson. 2004. Virulence factors predict Escherichia coli colonization patterns among human and animal household members. Ann. Intern. Med. 140848-849. [DOI] [PubMed] [Google Scholar]
- 23.Orskov, F., and I. Orskov. 1984. Serotyping of Escherichia coli. Methods Microbiol. 1443-111. [Google Scholar]
- 24.Peakall, R., and P. E. Smouse. 2006. GENALEX 6: genetic analysis in Excel. Population genetic software for teaching and research. Mol. Ecol. Notes 6288-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Ribot, E. M., M. A. Fair, R. Gautom, D. N. Cameron, S. B. Hunter, B. Swaminathan, and T. J. Barrett. 2006. Standardization of pulsed-field gel electrophoresis protocols for the subtyping of Escherichia coli O157:H7, Salmonella, and Shigella for PulseNet. Foodborne Pathog. Dis. 359-67. [DOI] [PubMed] [Google Scholar]
- 25.Russo, T. A., and J. R. Johnson. 2003. Medical and economic impact of extraintestinal infections due to Escherichia coli: an overlooked epidemic. Microbes Infect. 5449-456. [DOI] [PubMed] [Google Scholar]
- 26.Senior, D. F., P. deMan, and C. Svanborg. 1992. Serotype, hemolysin production, and adherence characteristics of strains of Escherichia coli causing urinary tract infection in dogs. Am. J. Vet. Res. 53494-498. [PubMed] [Google Scholar]
- 27.Sokal, R. R., and P. H. A. Sneath. 1963. Construction of a taxonomic system. W. H. Freeman, San Francisco, CA.
- 28.Swofford, D. L. 2002. PAUP*: phylogenetic analysis using parsimony (* and other methods). Sinauer Associates, Sunderland, MA.
- 29.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 332233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Westerlund, B., A. Pere, T. K. Korhonen, A.-K. Järvinen, A. Siitonen, and P. H. Williams. 1987. Characterization of Escherichia coli strains associated with canine urinary tract infections. Res. Vet. Sci. 42404-406. [PubMed] [Google Scholar]
- 31.Whittam, T. S., M. L. Wolfe, and R. A. Wilson. 1989. Genetic relationships among Escherichia coli isolates causing urinary tract infections in humans and animals. Epidemiol. Infect. 10237-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yuri, K., K. Nakata, H. Katae, T. Tsukamoto, and A. Hasegawa. 1999. Serotypes and virulence factors of Escherichia coli strains isolated from dogs and cats. J. Vet. Med. Sci. 6137-40. [DOI] [PubMed] [Google Scholar]
- 33.Yuri, K., K. Nakata, H. Katae, S. Yamamoto, and A. Hasegawa. 1998. Distribution of uropathogenic virulence factors among Escherichia coli strains isolated from dogs and cats. J. Vet. Med. Sci. 60287-290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.